INTRODUCTION
Hemosiderosis, the accumulation of excessive iron within tissues without structural harm, primarily occurs in patients who undergo repeated red blood cell transfusions and is a cause of death in transfusion-dependent thalassemia (TDT) [1]. Thalassemia is a hereditary disorder caused by mutations and/or deletions that disrupt the synthesis of α/β globin chains, leading to globin chain impairment within hemoglobin’s structure [2,3]. As per the World Health Organization (WHO), this condition represents 5% of the global hereditary disease burden and is the most prevalent hereditary disorder in Indonesia [4,5]. The management of thalassemia is routine blood transfusions [6]. However, these transfusions induce iron overload (IO), as evidenced by elevated serum ferritin, transferrin, and evidence of excess iron in various tissues, including the heart [7,8]. Cardiac siderosis can instigate heart failure, a prominent cause of mortality in severe thalassemia cases [8,9]
The elevated ferrous iron (Fe2+) levels lead to the formation of nontransferrin-bound iron (NTBI), triggering the generation of rseactive oxygen species (ROS) through Fenton and Haber-Weiss reactions. ROS subsequently forms hydroxyl radicals (OH•) capable of damaging lipid membranes, proteins, DNA, and causing tissue injury [10,11]. Lipid peroxidation of the cell membrane results in the production of malondialdehyde (MDA), known for its toxic and mutagenic attributes stands as the primary pathogenesis of heart diseases arising from IO [11,12]. NTBI infiltrates cardiomyocytes through L-type Ca2+ channels and divalent metal transporter 1, leading to cardiomyopathy. The sensitivity of cardiac excitation to cellular redox alterations prompts diastolic (relaxation) dysfunction, a distinctive feature of IO-induced cardiomyopathy [13]. Elevated oxidative stress additionally induces aging due to structural macromolecular damage. ROS-driven cellular senescence is regulated through pathways like p16INK4a/pRB and p53/p21, culminating in the cessation of the cell cycle during early-stage human fibroblast cell culture aging [14,15]. Cardiomyocyte senescence results in diminished heart function, resulting in the upsurge of SUR2A, a regulator of the K-ATP channel subunit [16,17]. Moreover, the plasma concentration of brain natriuretic peptide (BNP) escalates due to cardiac aging and conditions such as atrial enlargement, heightened afterload, and diastolic dysfunction [18]
Thalassemia patients with IO requires iron chelation therapy, one of which is deferiprone (DFP), which has lipophilicity that allows it to permeate myocytes and bind with iron molecules in a 1:3 iron-chelator complex [19–22]. Nevertheless, DFP administration frequently precipitates severe side effects, with agranulocytosis manifesting in approximately 63% of cases within the initial 6 months of treatment. The regular administration of DFP carries a considerable financial burden [23–25]. Phaleria macrocarpa is a renowned medicinal plant enriched with compounds, including saponins, alkaloids, polyphenols, phenols, flavonoids, lignans, and tannins. The fruit of P. macrocarpa has been found to contain icariside C3, mangiferin, and gallic acid [26,27]. Mangiferin in P. macrocarpa extract showcases robust antioxidant attributes through iron complexation and free radical activity, mitigating iron levels in the heart and liver, thereby averting heart failure stemming from IO [28]. Administering P. macrocarpa extract at a dose of 100 mg/kgBW can increase mangiferin levels in the heart organ, which is equivalent to administering the mangiferin compound at a dose of 50 mg/kgBW [29]. The administration of 50 mg/kgBW of mangiferin to rats subjected to IO for a 4-week period decreased iron levels in the liver and heart [30]. This research aims to assess the efficacy of the combination of ethanol extract from Phaleria macrocarpa and DFP in diminishing excessive iron levels while minimizing adverse effects and substantiating cardiac damage through cardiac biomarker parameters and antioxidant activities in rats subjected to IO.
MATERIALS AND METHODS
Materials
Thirty-six male Sprague Dawley rats (obtained from Indonesia National Agency of Drug and Food Control) 8 weeks old, P. macrocarpa fruits simplisia (PT Flozindo Purwokerto) was extracted by maceration using 70% ethanol, iron dextran solution (Sigma D8517), DFP 500 mg tab, BNP enzyme-linked immunosorbent assay (ELISA) kit (Elabscience), ketamine HCl injection (Bernofarm), xylazine HCl 200 mg/ml injection for veterinary use only (NexGen)
Study design
This research constitutes an in vivo experimental study with a parallel design involving male Sprague Dawley rats induced with IO.
Methods
Thirty-six male Sprague Dawley rats were randomly divided into six groups: normal control (C), IO, DFP 462.5 mg/kg BW (D), P. macrocarpa fruit extract 100 mg/kg BW (PM), DFP 462.5 mg/kg BW + P. macrocarpa extract 100 mg/kg BW (DPM1), and DFP 231.25 mg/kg BW + P. macrocarpa extract 100 mg/kg BW (DPM2). The DFP dose is 25 mg/kgBW given 3 times a day, thus the total administration is 75 mg/kgBW [24]. The dose was converted into an animal dose based on the human equivalent dose formula and obtained a dose of 75 mg/kgBW in humans, equivalent to 462.5 mg/kgBW in Sprague Dawley rats. Based on previous research by Lindayana, 2022, it was found that administration of P. macrocarpa extract at a dose of 100 mg/kgBW was able to reduce iron levels in the heart organ significantly (p = 0.048) compared to controls [29]. Therefore, this study refers to this dose as the standard dose for using P. macrocarpa extract.
The study protocol was approved by the Research Ethics Committee of Faculty of Medicine, University of Indonesia-Cipto Mangunkusumo Hospital, Jakarta, Indonesia (Approval No: KET964/UN2.F1/ETIK/PPM.00.02/2022). Iron dextran was injected twice a week for 3 weeks and continued until week eight along with the specified treatments. Blood samples were collected from the retro-orbital sinus vein and placed in lithium heparin tubes. The heart samples of each rat were excised following decapitation and stored at −80°C.
Phaleria macrocarpa fruit ethanolic extraction
Dried P. macrocarpa fruit was obtained from PT. Flozindo Nature Anugrah which has been certified. A total of 1,000 g of dried fruit was ground into powder and then soaked in 70% ethanol and 30% air. The mixture was left for 24 hours in a closed container and stirred every 8 hours. The macerate was taken after three filters, and then thickened until a yield was obtained. The liquid extract was dried using a rotary evaporator until a thick extract was formed.
Heart organ sampling
The hearts were collected immediately after the rat necropsy and weighed using a digital scale. The right ventricle was separated from the left ventricle wall. The left ventricle was then divided into several parts: the distal (apex of the heart) was used to measure cardiac MDA concentration and the upper 1/3 was used to measure heart iron concentration.
Measurement of cardiac iron concentration
Heart tissue weighing 250 mg was destructed using 2 ml of HNO3 and 1–2 drops of perchloric acid to expedite the breakdown of solid tissue. The added acids also reacted with tissue iron. The mixture was left to stand overnight, then heated until it emitted brown-colored vapors, after which 10 ml of distilled water was added. The solution was filtered and more distilled water was added to reach a total volume of 50 ml. The measurement of iron was conducted using atomic absorbance spectrophotometry with a hollow cathode lamp in deuterium background correction mode, at a wavelength of 248.3 nm [28]
Measurement of cardiac MDA concentration
MDA concentration was measured in cardiac tissue to assess oxidative stress in the heart. The heart ventricles were weighed and immersed in PBS (phosphate buffer saline) at a concentration of 0.1g/ml at pH of 7.2. They were rinsed to remove any blood, then homogenized for 3 minutes at a temperature of 0°C–4°C using an ULTRA-TURRAX homogenizer. Subsequently, the homogenate was centrifuged for 5 minutes at 10,000 g, and the supernatant was collected for MDA analysis [31]
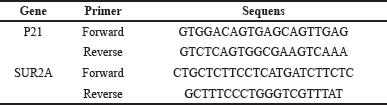 | Table 1. Senescence Genes p21 and SUR2A Primer Design. [Click here to view] |
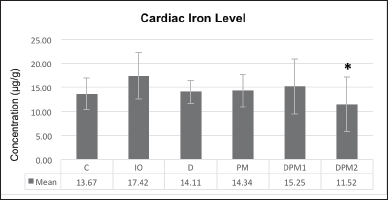 | Figure 1. Cardiac iron concentration difference on week VIII (µg/g). [Click here to view] |
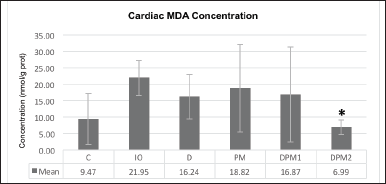 | Table 2. Cardiac iron concentration difference (µg/g). [Click here to view] |
Measurement of BNP levels in plasma
Two milliliters of venous blood were collected and centrifuged for 10 minutes at 4,500 rpm, and the plasma was separated and stored at −20°C. BNP levels were measured using the enzyme-linked immunosorbent assay ELISA method [32]
Measurement of p21 and SUR2A genes expression by PCR
Senescence genes p21 and SUR2A were detected using the RT-qPCR method with three stages: homogenate preparation, RNA and cDNA isolation, and cDNA optimization. Homogenate was prepared from rat heart samples by macerating them with a micropestle. RNA and cDNA isolation was performed following the respective kit procedures: Zymo Research Quick RNA Miniprep Plus and Toyobo Revertra Ace qPCR RT Master Mix with gDNA Remover. cDNA optimization was carried out using an RT-PCR device with BioRad CFX Manager software. The expression results of the p21 gene and SUR2A gene (CT value) were normalized based on the housekeeping gene (β-actin) and quantitative calculations were carried out using the formula of the 2-ΔΔCT method. Then, the relative fold change of the transcription value of each gene was calculated [33]. The primer design was developed within the research facility at the Integrated Laboratory of the Medical Faculty of the University of Indonesia and is provided in Table 1.
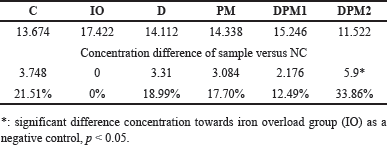 | Figure 2. Cardiac MDA concentration difference (nmol/g prot). [Click here to view] |
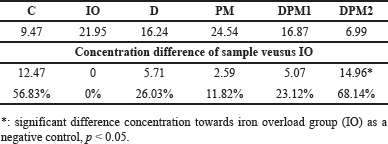 | Table 3. Cardiac MDA concentration difference (nmol/g prot). [Click here to view] |
Statistical analysis
Value expresses the means of each sample. Data analysis used SPSS Statistic 22 software. Statistical analysis used One Way Anova for normal distribution data and paired data used the Paired T-Test analysis.
RESULTS
Cardiac iron concentrations
The cardiac iron concentrations in week VIII were lower in all treatment groups than in the IO group but not significantly different, except for group DMP2 versusgroup IO (p = 0.022, Fig. 1), equivalent to a difference of 33.86% (Table 2).
Cardiac MDA concentration
Cardiac MDA concentration on week VIII showed a nearly twofold difference between the normal group (C) and the IO. Iron induction resulted in an increase in MDA concentration due to the production of ROS which is consistent with iron overload. All treated groups experienced a decrease in MDA levels. The highest decrease in MDA concentration in sequence is as follows: DPM2, DFP, and DPM1. The analysis results indicate a significant difference between the iron-overloaded group (IO) and DPM2 (p = 0.014) while the other groups did not show significant differences (p > 0.05, Fig. 2). The difference in MDA concentration in the DPM2 group was 68,14% lower than IO group (Table 3).
 | Figure 3. Week III and week VIII cardiac BNP concentration difference. [Click here to view] |
Plasma BNP concentration
The plasma BNP concentration measured in weeks III and VIII showed a decrease in all groups. The reduction in plasma BNP concentration was greater and significant in all treatment groups (p < 0.05) than in groups C and IO (Fig. 3). The difference in concentration is provided in Table 4.
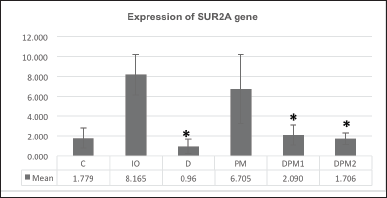 | Figure 4. The difference in SUR2A gene expression on week VIII. [Click here to view] |
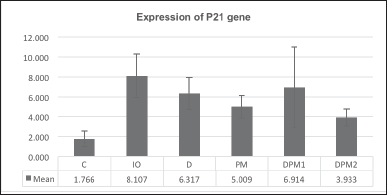 | Figure 5. The difference in p21 gene expression on week VIII. [Click here to view] |
 | Table 4. Plasma BNP concentration difference (pg/ml). [Click here to view] |
Effects of DFP and PM extract combination towards SUR2A gene
The SUR2A gene expression in all treatment groups showed a notable reduction, demonstrating a significant difference when compared to IO (p = 0.045). The administration of DFP (D) and the combination of DFP and PM extract (DPM1 and DPM2) showed significant differences compared to the IO with respective p-values of 0.04, 0.032, and 0.028 (Fig. 4).
Effects of DFP and PM extract combination towards p21 gene
The p21 gene expression exhibited a decreasing trend in all treatment groups, but the observed differences were not statistically significant (p = 0.162, Fig. 5).
DISCUSSION
The iron-chelating effects of P. macrocarpa fruit extract have been previously demonstrated, showing a reduction in iron levels in the hearts of rat models with hemosiderosis, using doses of 100 mg and 200 mg [29]. This research aimed whether DFP (full or half dose) in combination with P. macrocarpa fruit extract could reduce the Fe accumulation in hemosiderosis rat model hearts. Furthermore, the administration of DFP in combination with P. macrocarpa fruit extract could reduce, and it is hoped that the side effects of DFP, e.g. agranulocytosis, will be reduced. The hemosiderosis model utilized male white rats (Rattus norvegicus strain Sprague-Dawley) that were intraperitoneally administered iron dextran. The method for creating hemosiderosis experimental animal models is based on research Papanastasiou et al. [34] dan Estuningtyas et al. [30]. Previous studies involved the administration of iron sucrose over 3 weeks, with intervals of 2–3 times per week, resulting in hemosiderosis with a sixfold increase in liver iron compared to the control group [30]
The treatment for 5 weeks reduced heart iron concentration in all treatment groups compared to the IO group. The most significant reduction in heart iron (p < 0.05) was observed in the DPM2 group, which received a combination of half-dose DFP and P. macrocarpa extract at 100 mg/kgBW. Based on phytochemical screening, the ethanol extract of P. macrocarpa fruit contains naringerin 4,7 dimethyl ether compounds, which enhance mangiferin uptake into cells by reducing P-glycoprotein (Pgp) transporter expression [29,35]. Mangiferin itself has limited gastro-intestinal uptake and thus low bioavailability, the administration of mangiferin-phospholipid complexes results in higher permeability compared to pure mangiferin [35]. The primary pathogenesis of iron overload cardiomyopathy (IOC) occurs when NTBI enters cardiomyocytes through circulation and is catalyzed by Fenton reactions, producing hydroxyl ions that form ROS, disrupting membrane structure, causing cell damage and necrosis, and resulting in myocardial dysfunction [36]. Preventing iron accumulation in the heart is crucial for iron chelation therapy because heart iron deposits have a long half-life, around 19 months or more in humans [37]. DPM2 therapy reduced heart iron levels by 33.86% compared to the IO group, while DFP only reduced heart iron levels by 18.99% compared to IO. Research by Wongjaikam et al. [38] proved that a combination of DFP and N-acetylcysteine (NAC) as an antioxidant was more effective than monotherapy for cardioprotection, by reducing heart iron deposits and improving mitochondrial function in iron-overloaded rats. Therefore, the combination of half-dose DFP and P. macrocarpa extract is considered a potential alternative therapy to reduce the side effects of DFP.
Increased labile iron in cells reacting with superoxide ions (O2-) and hydrogen peroxide (OH-) through Fenton and Haber-Weiss reactions produces toxic hydroxyl radicals, leading to cardiomyopathy. This results in the excessive production of ROS, causing lipid peroxidation of plasma membranes and the formation of toxic aldehydes, particularly MDA [39]
The administration of iron injections for 8 weeks showed a nearly twofold difference in MDA levels between the normal and iron-overloaded groups. This aligns with a meta-analysis by Basu et al. [40], indicating that MDA significantly increased in both TDT and NTDT (nontransfusion-dependent thalassemia), making it a reliable marker for IO and a prognostic biomarker. In another study, Djumhana et al. [11] found no significant difference in MDA levels before and after transfusions. Although blood transfusions increased iron levels in thalassemia patients, there was no increase in median MDA values following transfusion [11]. The results of this study indicate that the treatments in the D and DPM1 groups reduced heart MDA levels but not significantly. However, the DPM2 group significantly reduced heart MDA levels (p < 0.05). This result is consistent with the study by Wongjaikam et al. [38] stating that the combination of DFP and NAC could reduce MDA concentrations to normal or the same as the MDA concentrations in the control group. Permatasari et al. [41] demonstrated a strong correlation between ferritin and MDA concentrations (r = 0.786), where ferritin is widely used as an iron marker in the absence of inflammation [42]. The reduction in MDA concentration is influenced by the phenolic compounds in the ethanol extract of P. macrocarpa fruit, which is a potent antioxidant component, with antioxidant activity results of 86.85% in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) test and 7.47% in the ferric reducing antioxidant power (FRAP) test [43]. Zou et al. [44] demonstrated that DFP at doses of 50 mg/kg/day and 100 mg/kg/day could significantly reduce MDA concentrations (p < 0.05) in rats induced with diabetic cardiomyopathy compared to the control group. Therefore, the combination of DFP and PM can support each other in reducing MDA concentrations as an indicator of ROS.
IOC is characterized by diastolic dysfunction. This leads to the release of BNP and amino terminal BNP (NT-ProBNP) after cardiac stress and volume overload [45]. The results of this study show that all treatment groups exhibited a decrease in plasma BNP levels over 5 weeks. The most significant decrease in plasma BNP levels was observed in the PM group (51.23%), followed by the DPM2 group (45.60%), the DFP group (44.36%), and the DPM1 group (40.89%). The combination of half-dose DFP and PM (DPM2) exceeded the effectiveness of single-dose DFP in reducing plasma BNP levels. Studies have confirmed that NT-proBNP has a positive correlation with ferritin and IO. Therefore, this biomarker can be used to detect the early stage of heart failure when echocardiography and MRI facilities are not available [46,47]. In this study, this correlation is evident through the ability of DPM2 to significantly reduce heart iron levels and plasma BNP levels.
In cases of iron overload, the L-type channels mediate an increase in ferrous iron uptake, leading to excessive ROS production, which triggers excitation-contraction uncoupling and disturbances in both systolic and diastolic functions, hallmarking cardiomyopathy [48]. Accumulation of ROS in cardiomyocytes induces DNA damage by telomere shortening, resulting in cellular senescence [16]. This study reveals an upregulation of the p21 gene expression in the control group (IO), signifying DNA damage due to excessive iron induction. Conversely, the D and DFP-PM groups (DPM1 and DPM2) experience significant downregulation of p21 gene expression compared to the control group. This implies that the administration of DFP or the combination of DFP and PM can suppress DNA damage by preventing ROS accumulation, which is supported by the downward trend in MDA levels. In a study by Chen et al. [49], exposure to ultraviolet B (UVB), which induces DNA damage, led to increased apoptosis, decreased proliferation, and a significant increase in G2 cell cycle arrest. Another study utilizing Basella alba, known for its antioxidant properties, demonstrated the ability to halt the G0/G1 cell cycle by inhibiting pRb and D1 and increasing p21 expression [50]
The expression of the SUR2A gene exhibits a similar trend to that of the p21 gene. The IO group shows a significant increase compared to the normal control group (C), while the expression in all treatment groups decreases compared to the IO group, although not significantly. Cardiac aging is characterized by reduced physical endurance, associated with a decline in cardiac function and adaptive capacity. Cardiac aging is linked to a decrease in the number of ATP-sensitive K(+) channels in the sarcolemma. The quantity of these channels is influenced by the expression of SUR2A, a subunit regulating K(ATP) channels [51]. This suggests that the combination of DFP and PM has the capability to prevent cardiomyopathy associated with aging through p21 and SUR2A gene expression.
CONCLUSION
Administration of DFP and Phaleria macocarpa fruit extract combination in hemosiderosis rat model reduced cardiac iron, MDA, and BNP levels. The combination of DFP with Phaleria macocarpa fruit extract could reduce the senescence gens p21 and SUR2A expression, furthermore could prevent the occurrence of IOC.
ACKNOWLEDGMENT
This study was supported by a research grant “Hibah Publikasi Terindeks Internasional (PUTI)” No.NKB-1220/UN2.RST/HKP.05.00/2022 from Universitas Indonesia.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Research Ethics Committee of Faculty of Medicine, University of Indonesia-Cipto Mangunkusumo Hospital, Jakarta, Indonesia (Approval No: KET964/UN2.F1/ETIK/PPM.00.02/2022).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. de Jongh AD, van Beers EJ, de Vooght KMK, Schutgens REG. Screening for hemosiderosis in patients receiving multiple red blood cell transfusions. Eur J Haematol. 2017;98(5):478–84. CrossRef
2. Fajri P, Estuningtyas A, Louisa M, Freisleben HJ. The preventive effect of mangifera foetida L. leaf extract administered simultaneously to excess iron on markers of iron overload in spraque-dawley rats. Med J Indones. 2017;26(4):246–52. CrossRef
3. Angastiniotis M, Lobitz S. Thalassemias: an overview. Int J Neonatal Screen. 2019;5(1):1–11. CrossRef
4. Sanctis V De, Kattamis C, Canatan D, Soliman AT, Elsedfy H, Karimi M, et al. Mediterranean journal of hematology and infectious diseases β-thalassemia distribution in the old world: an ancient disease seen from a historical standpoint. Mediterr J Hematol Infecttious Dis. 2017;9:1–11.
5. Wahidiyat PA, Sari TT, Rahmartani LD, Setianingsih I, Iskandar SD, Pratanata AM, et al. An insight into Indonesian current thalassaemia care and challenges. ISBT Sci Ser. 2020;15(3):334–41. CrossRef
6. Rujito L. Talasemia Genetik Dasar dan Pengelolaan Terkini. 1st ed. Universitas Jenderal Sudirman. Purwokerto, Indonesia: Unsoed Press; 2019.
7. Kremastinos DT, Farmakis D. Iron overload cardiomyopathy in clinical practice. Circulation. 2011;124(20):2253–63. CrossRef
8. Fleming RE, Ponka P. Iron overload in human disease. N Engl J Med. 2012;366(4):348–59. CrossRef
9. Raghuveerprabhu1 RS, Prabhu3. Iron overload in beta thalassemia—a review. J Biosci Tech. 2009;1(1):20–31.
10. Blesia V, Patel VB, Al-Obaidi H, Renshaw D, Zariwala MG. Excessive iron induces oxidative stress promoting cellular perturbations and insulin secretory dysfunction in MIN6 beta cells. Cells. 2021;10:1–20.
11. Atmakusuma TD, Nasution IR, Sutandyo N. Oxidative stress (Malondialdehyde) in adults beta-thalassemia major and intermedia: comparison between before and after blood transfusion and its correlation with iron overload. Int J Gen Med. 2021;14(October):6455–62. CrossRef
12. Sung HK, Song E, Jahng JWS, Pantopoulos K, Sweeney G. Iron induces insulin resistance in cardiomyocytes via regulation of oxidative stress. Sci Rep. 2019;9(1):1–13. CrossRef
13. Cheng CF, Lian WS. Prooxidant mechanisms in iron overload cardiomyopathy. Biomed Res Int. 2013;2013:740573. CrossRef
14. Russo G, Curcio F, Bulli G, Aran L, Della-morte D, Testa G, et al. Oxidative stress and diseases. Oxidative Stress Dis. 2012;3:757–72. CrossRef
15. Kumari R, Jat P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol. 2021;9(March):1–24. CrossRef
16. Tang X, Li PH, Chen HZ. Cardiomyocyte senescence and cellular communications within myocardial microenvironments. Front Endocrinol (Lausanne). 2020;11(May):1–13. CrossRef
17. Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. Naturally occurring p16 Ink4a-positive cells shorten healthy lifespan. Nature [Internet]. 2016;530(7589):184–9. CrossRef
18. Valle AP Do, Boas PJFV, Vidal EIDO, Fukushima FB. B-type natriuretic peptide in the evaluation of cardiac function. J Bras Patol e Med Lab. 2013;49(4):241–6. CrossRef
19. Irdawati, Syaiful AA, Anis H. Frekuensi Transfusi. J Ber Ilmu Keperawatan. 2021;14(2):73–9.
20. Pinto VM, Forni GL. Management of iron overload in beta-thalassemia patients: clinical practice update based on case series. Int J Mol Sci. 2020;21(22):1–20. CrossRef
21. Saliba AN, Harb AR, Taher AT. Iron chelation therapy in transfusion-dependent thalassemia patients: current strategies and future directions. J Blood Med. 2015;6:197–209. CrossRef
22. Gray JP, Ray SD. Metal antagonists. Side Eff Drugs Annu. 2014;36:323–37. CrossRef
23. Hider RC, Hoffbrand AV. The role of deferiprone in iron chelation. N Engl J Med. 2018;379(22):2140–50. CrossRef
24. EMEA. Scientific discussion. In: Annual report of the European Medicines Agency [Internet]. London, UK: EMEA; 2005. pp. 1–15. Available from: www.emea.eu.int
25. Master S, Mansour RP. Average monthly cost of iron chelator treatment in adult patients with sickle cell disease. Blood. 2016;128(22):4858. CrossRef
26. Altaf R, Asmawi MZ Bin, Dewa A, Sadikun A, Umar MI. Phytochemistry and medicinal properties of Phaleria macrocarpa (Scheff.) Boerl. extracts. Pharmacogn Rev. 2013;7(13):73–80. CrossRef
27. Setiyawan H. Pemberian Ekstrak Buah Mahkota Dewa (Phaleria macrocarpa (Sheff) Boerl) terhadap Struktur Mikroanatomi Hepar. In: Seminar Nasional Rekam Medis & Informasi Kesehatan: Standar Akreditasi Rumah Sakit (SNARS) Terkait Rekam Medis. Yogyakarta; 2020. Vol. 1 pp. 79–84.
28. Estuningtyas A, Zwicker K, Wahyuni T, Fajri P, Wahidiyat PA, Freisleben SKU, et al. Are mangiferin and mangiferin-containing plant extracts helpful for iron-loaded transfusion-dependent and non-transfusion-dependent thalassaemia patients? Biomed Pharmacol J. 2018;11(1):29–43. CrossRef
29. Lindayana. Efek Pemberian Ekstrak Etanol Buah Mahkota Dewa (Phaleria Macrocarpa) Sebagai Kelator Besi Di Organ Jantung Pada Tikus Yang Diberi Besi Berlebih. Jakarta, Indonesia: Universitas Indonesia; 2022.
30. Estuningtyas A, Setiabudy R, Wahidiyat PA, Freisleben HJ. The role of mangiferin in the prevention of experimentally induced iron overload in an animal model. Drug Res (Stuttg). 2018;69(4):234–40. CrossRef
31. Gioda CR, Barreto TDO, Prímola-Gomes TN, De Lima DC, Campos PP, Capettini LDSA, et al. Cardiac oxidative stress is involved in heart failure induced by thiamine deprivation in rats. Am J Physiol Hear Circ Physiol. 2010;298(6):2039–45;
32. Li J, Yin FF, Hou YL. Early diagnosis of rats with acute myocardial infarction by measurement of brain natriuretic peptide. Exp Ther Med. 2013;5(4):1201–5.
33. Bahreyni-Toossi MT, Azimian H, Aghaee-Bakhtiari SH, Mahmoudi M, Sadat-Darbandi M, Zafari N. Radiation-induced DNA damage and altered expression of p21, cyclin D1 and Mre11 genes in human fibroblast cell lines with different radiosensitivity. Mutat Res Fundam Mol Mech Mutagen [Internet]. 2021;823(February):111760.
34. Papanastasiou DA, Vayenas DV, Vassilopoulos A, Repanti M. Concentration of iron and distribution of iron and transferrin after experimental iron overload in rat tissues in vivo: study of the liver, the spleen, the central nervous system and other organs. Pathol Res Pract [Internet]. 2000;196(1):47–54.
35. Weso?owska O, Wi?niewski J, Sroda K, Krawczenko A, Bielawska-Pohl A, Paprocka M, et al. 8-Prenylnaringenin is an inhibitor of multidrug resistance-associated transporters, P-glycoprotein and MRP1. Eur J Pharmacol. 2010;644:32–40.
36. Imran M, Arshad MS, Butt MS, Kwon JH, Arshad MU, Sultan MT. Mangiferin: a natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis. 2017;16(1):1–17.
37. Albakri A. Iron overload cardiomyopathy: a review of literature on clinical status and meta-analysis of diagnostic and clinical management using iron chelators. Intern Med Care. 2018;2(1):1–12.
38. Wongjaikam S, Kumfu S, Khamseekaew J, Sripetchwandee J, Srichairatanakool S, Fucharoen S, et al. Combined iron chelator and antioxidant exerted greater efficacy on cardioprotection than monotherapy in iron-overloaded rats. PLoS One. 2016;11(7):1–19.
39. Detterich J, Leila N, Fred D, Yaniv BC, Paul H, Thomas C, et al. Electrocardiographic consequences of cardiac iron overload in thalassemia major. Am J Hematol. 2012;87(2):139–44.
40. Basu D, Adhya DG, Sinha R, Chakravorty N. Role of malonaldehyde as a surrogate biomarker for iron overload in the β-thalassemia patient: a systematic meta-analysis. Adv Redox Res [Internet]. 2021;3(April):100017.
41. Permatasari TD, Riyanti R, Wisudanti DD. Hubungan antara Kadar Feritin dengan Malondialdehyde pada Pasien Talasemia β Mayor di RSD dr. Soebandi Jember. Pustaka Kesehat. 2020;7(1):52.
42. Tri Kusumastuti FD, Sutaryo S, Mulatsih S. Correlations between hemoglobin, serum ferritin, and soluble transferrin receptor levels in children aged 6-59 months. Paediatr Indones. 2014;54(2):122.
43. Ahmad R, Khairul Nizam Mazlan M, Firdaus Abdul Aziz A, Mohd Gazzali A, Amir Rawa MS, Wahab HA. Phaleria macrocarpa (Scheff.) Boerl.: an updated review of pharmacological effects, toxicity studies, and separation techniques. Saudi Pharm J [Internet]. 2023;31(6):874–88.
44. Zou C, Liu X, Xie R, Bao Y, Jin Q, Jia X, et al. Deferiprone attenuates inflammation and myocardial fibrosis in diabetic cardiomyopathy rats. Biochem Biophys Res Commun. 2017;486(4):930–6.
45. Kautsar A, Advani N, Andriastuti M. N-Terminal-pro-b-type natriuretic peptide levels and cardiac hemosiderosis in adolescent β-thalassemia major patients. Ann Pediatr Cardiol. 2019;12(1):32–7.
46. Dutt A, Kariyappa M, Rangegowda RK. Original article: correlation of N-terminal pro-brain natriuretic peptide (Nt Pro Bnp) with serum ferritin and number of blood transfusions in thalassemia patients-a cross-sectional study. Int J Curr Pharm Res. 2023;15(5):115–8.
47. Balkan C, Tuluce SY, Basol G, Tuluce K, Ay Y, Karapinar DY, et al. Relation between NT-proBNP levels, iron overload, and early stage of myocardial dysfunction in β-thalassemia major patients. Echocardiography. 2012;29(3):318–25.
48. Mancardi D, Mezzanotte M, Arrigo E, Barinotti A, Roetto A. Iron overload, oxidative stress, and ferroptosis in the failing heart and liver. Antioxidants. 2021;10(12):1864.
49. Chen A, Huang X, Xue Z, Cao D, Huang K, Chen J, et al. The role of p21 in apoptosis, proliferation, cell cycle arrest, and antioxidant activity in UVB-irradiated human HaCaT keratinocytes. Med Sci Monit Basic Res. 2015;21:86–95.
50. Sheik A, Kim E, Adepelly U, Alhammadi M, Huh YS. Antioxidant and antiproliferative activity of Basella alba against colorectal cancer. Saudi J Biol Sci [Internet]. 2023;30(4):103609.
51. Sudhir R, Sukhodub A, Du Q, Jovanovi? S, Jovanovi? A. Ageing-induced decline in physical endurance in mice is associated with decrease in cardiac SUR2A and increase in cardiac susceptibility to metabolic stress: therapeutic prospects for up-regulation of SUR2A. Biogerontology. 2011;12(2):147–55.