INTRODUCTION
The most common glycosaminoglycans (GAGs) include chondroitin sulphate (CS), dermatan sulphate, heparan sulphate, and keratin sulphate (KS). GAGs are large polysaccharides, consisting of repeating disaccharide units of amino sugar either N-acetyl D-galactosamine (D-GalNAc) or N-acetyl D-glucosamine (D-GlcNAc), and uronic acid, either glucuronic acid or iduronic acid. KS consists of galactose instead of uronic acid [1–3]. CS proteoglycans (CSPGs) consist of core proteins covalently bonded to CS-GAG. CSPGs abundant in the extracellular matrix, play roles in neurological diseases. Elevated CSPGs impede repair and support cancer progression. Chondroitinase ABC, a bacterial enzyme from Proteus vulgaris, offers a promising approach to degrade CSPGs and address associated conditions [1,2,4–8]. In this study, for the first time, we have identified a bacterial strain Bacillus velezensis B26 which produces chondroitinase enzyme. In addition to chondroitinase activity, B. velezensis B26 strain exhibited chitinase, glucanase, amylase, and gelatinase activities. Chitinases enzymes belonging to a diverse group, are capable of digesting large chitin polymers into their constituent acetyl glucosamine units. These enzymes exhibit variability in their catalytic mechanisms, structures, and substrate specificities [9]. Several bacterial isolates have been identified as chitinase producers, underscoring the significant potential of chitin degradation in diverse applications such as waste management, biocontrol, and even osteoarthritis treatment [10]. Glucanases are hydrolytic enzymes that degrade the polysaccharide chains of glucans. They are primarily classified based on the specific linkage they target, including β-1,3, β-1,4, or α-1,3 glucanases. Bacterial glucanases hold immense biotechnological importance, finding extensive applications in animal feed and the brewing industry [11,12].
Even though fungal diseases affect a significant population worldwide that is estimated to be around 1 billion annually, there is a general lack of awareness with respect to the threat posed by them in public [13]. An increase in co-morbid conditions such as cancer, AIDS, and diabetes also leads to higher susceptibility to fungal infections. Overall, Candida spp. is one of the most prevalent fungal pathogens causing both superficial and invasive diseases. With the limited number of drug classes available and the rising emergence of infections, there is an urgent necessity to start exploring novel targets against the pathogens [14]. In this research work, for the first time, we have identified that B. velezensis B26 strain exhibits several enzymatic activities, and importantly showed antifungal activity against Candida albicans (C. albicans) and Saccharomyces cerevisiae (S. cerevisiae).
MATERIAL AND METHODS
Materials
Nutrient Broth, NB (M002-100G), Luria Bertani broth, LB (M1725-500G), Sabouraud Dextrose Broth (MH033-100G), MR-VP broth (M070S-100G), Simmon’s Citrate Agar ((M099-100G), Urea Broth base (M111-100G), Meat Extract (RM002-500G), Skim Milk Agar (M763-500G), SIM medium (M181-500G), Agar powder (GRM026P-500G), CS A (TC040-5G), Bovine Serum Albumin Factor V (TC194-5G), Glucose (TC130-100G), Gelatin (TC041-100G), Starch (GRM198-500G), and N-acetyl D glucosamine (NAG) (TC081-10G), carboxy methyl cellulose (CMC) (MB138-100G), and Chitin (GRM1356-100G) all the compounds listed were purchased from HiMedia Laboratories, India.
Starch agar plate (Starch agar medium-2.5 g in 100 ml distilled water); Nutrient gelatin medium (Peptone-0.5 g, Beef extract-0.3 g, Gelatin-12 g, andAgar-1.5 g in 100 ml distilled water); Skim milk agar (Tryptone-5 g, Yeast extract-2.5 g, Glucose-1 g, SM powder-28 g, and Agar-15 g in 1,000 ml purified/distilled water).
Media composition
Colloidal chitin minimal salt (CCMS) (Colloidal chitin-10 g, yeast extract-1 g, Ammonium sulphate-2.5 g, Dipotassium phosphate-0.7 g, Monopotassium phosphate-0.3 g, Magnesium sulphate pentahydrate-0.5 g, sodium chloride-1 g, ferrous sulphate heptahydrate-0.01 g, zinc sulphate-0.001 g, manganese (III) phosphate-0.001 g, Calcium chloride-0.4 g, Urea; 0.3 g, Agar; 20 g and finally pH is adjusted to 7 [15].
Screening for identification of chondroitinase-producing microorganism
Soil specimens from a depth of 3–5 cm of the surface, were collected from 2 locations, one chicken poultry farm and one chicken carcass, from Manipal, Udupi (Location: 13.325922, 74.804554) using sterile tubes. Sample collected from chicken poultry farm labeled as CPF-I. Three samples were collected from chicken carcasses named as CHCS – I, CHCS – II, and CHCS – III. Characteristics of all the soil samples were observed.
Primary screening
Soil samples (1 gram) were screened for organisms capable of utilizing CS based on the rapid plate method [16]. The soil in poultry farm (CPF-I) is powdery, and thus it was directly sprinkled on nutrient agar containing 0.04% w/v CS (filter sterilized with 0.22 μ filter). The three soil samples collected from chicken carcass (CHCS – I, II, III) were suspended in 100 ml sterile water and shaken well for 30 minutes. Further samples were serially diluted and added 1 ml to 18 ml nutrient agar containing 0.04% w/v filter sterilized CS (CNA). All these plates were incubated at 37°C for 24 hours. Each isolate was streaked separately on the surface of CNA containing 0.04% w/v of CS and 1% w/v bovine serum albumin fraction V. The plates were incubated for 3 days at 37°C. Glacial acetic acid (2N) was added to each of the plates and incubated for 10 minutes. The formation of a clear zone around the colony indicates the breakdown of CS, indicating chondroitinase-producing organism. The isolates giving the largest clear zones were selected for the production of chondroitinase. Gram staining was carried out for all the positive isolates.
Identification of the isolate
The identification of isolates was performed at the sequencing facility of the National Centre for Microbial Resource (NCMR), National Centre for Cell Science, Pune. Genomic DNA was extracted using the standard phenol/chloroform extraction method [17], followed by PCR amplification of the 16S rRNA gene using universal primers 16F27 [5’-CCA GAG TTT GAT CMT GGC TCA G-3’] and 16R1492 [5’-TAC GGY TAC CTT GTT ACG ACT T-3’]. The resulting amplification product was purified through PEG-NaCl precipitation and then directly sequenced using ABI® 3730XL automated DNA sequencer (Applied Biosystems, Inc., Foster City, CA) in accordance with the manufacturer’s instructions. Importantly, sequencing was performed bi-directionally to ensure that each position was read at least twice. The assembly of the sequence was performed using a Lasergene package followed by a comparison to sequences from the type material using NCBI BLAST for tentative identification. The amplified gene was sequenced and the identification was performed by using EzBioCloud Database, with the confidence in identification restricted by both the availability and the extent of homology shown by the ~1,200 bp sequence of the sample in comparison to its closest neighbor in the database. The sequencing and identification were performed by NCMR-NCCS Pune, India [18].
Enzymatic activities
Chondroitinase enzyme activity assay
The enzyme activity was assessed by its ability to lyse chondroitin-4-sulphate to produce unsaturated disaccharides. Overnight grown primary culture (Bacillus velezensis B26 strain) was inoculated in the secondary medium, grown overnight at 37ºC for 48 hours in nutrient broth with 0.05% CS, and the colony count was adjusted to 1 × 108 cfu/ml using a 0.5 McFarland solution for the collection of bacterial supernatants. Briefly, varying concentrations of bacterial cell-free supernatant with chondroitinase enzyme and substrate were incubated in 20 mM Tris-HCl buffer, pH 7.5, at 37°C and the relative amount of unsaturated disaccharides was recorded by using spectrophotometer (BioTek Synergy H1 Multimode Reader, Agilent) (UV 232 nm) for 20 minutes at 37ºC. In this assay, EmM, the millimolar absorption coefficient of unsaturated disaccharides 5.1 L/(mol·cm) was used in the calculations. One international unit is defined as the amount of protein needed to form 1 µmol of 4,5-unsaturated uronic acid/minute [19]. Enzyme activity (Units/ml) = (ΔA232nm/minute test)/[(EmM) (0.1)]
Amylase activity
Bacterial cells were spotted on the starch agar plate. The plate was incubated at 37°C for 48 hours following which the plate was flooded with Gram’s iodine using a dropper to check for a zone of clearance around the colony [20,21].
Caseinase activity
The organism was spotted on a skim milk agar plate and incubated at 37°C for 24 hours in order to observe a zone of clearance around the colony [22].
Gelatinase activity
The organism from the 24-hour-old culture broth was stab inoculated into the nutrient gelatin medium and incubated at 37°C. After every 24 hours, the test tube was kept in the refrigerator (4-8°C) for 20 minutes and then checked for liquefaction of the medium [20,21].
Chitinase assay and glucanase assay
Preparation of colloidal chitin, chitinase, and glucanase assay was performed as reported [15,23].
Preparation of colloidal chitin
Chitin (5 g) was mixed thoroughly in 50 ml of concentrated HCL by stirring for 30 to 50 minutes. Chitin colloidal suspension was prepared by mixing with 500 ml of ice-cold water with continuous stirring. The colloidal chitin (CC) obtained was filtered using muslin cloth and washed repeatedly until the suspension pH was 6.5. The collected CC was air-dried and stored at 4ºC [15].
Chitinase assay and glucanase assay
Overnight grown primary culture (Bacillus velezensis B26 strain) was inoculated in the secondary medium (CCMS broth) for chitinase production and CCMS broth with CMC (1%) for glucanase production. The cells were maintained at 37ºC and 150 rpm. Samples were collected after 24-hour and enzyme activity was performed by the DNS method. For chitinase enzyme activity, soluble fractions obtained from the bacterial cells were incubated with 1%CC as a substrate and the released glucose molecule was monitored by measuring absorbance at 450 nm. Similarly, for glucanase enzyme activity, soluble fraction obtained from the bacterial cells was incubated with 1% CMC as a substrate and the released glucose molecule was monitored by measuring absorbance at 450 nm. One unit of enzyme was defined as the amount of enzyme required to release 1 µM glucose per minute at 37?C.
Growth curve
A 24-hour culture broth was inoculated in a fresh LB broth to prepare 0.5 McFarland equivalent broth culture. 1 ml of this culture was added to 150 ml of fresh sterile LB broth and incubated at 37°C at 150 rpm. Samples were taken at regular intervals in order to measure their optical density values at 540 nm. A fresh LB broth was used as blank. The samples were taken at 1, 2, 4, 8, 12, 24, 48 and 60 hours, respectively. The assay was done twice and an average of the optical density values were recorded. A graph was plotted with sampling time on the X-axis and optical density on the Y-axis, with the values corresponding to their respective sampling times.
Bacterial cell-free supernatant preparation
Broth cultures were centrifuged at 6,000 rpm for 10 minutes following which the supernatant was collected and cell debris was discarded. The supernatant was then filter sterilized (0.22 µm) to remove any further remaining bacteria. The filtered supernatant was stored at −20ºC until used further.
Antifungal activity
The cell-free supernatant was tested for its antifungal activity against fungi using the cup plate method [24]. The overnight cultures of C. albicans and S. cerevisiae were adjusted to 0.5 Mc Farland and were spread on SDA plates. A borer of 6 mm diameter was used to create 4 wells in the plates. 50 μl of bacterial cell-free supernatant were collected and added to the wells after 1 hour, 6 hours, and 24 hours of incubation, respectively. Additionally, 50 μl of Amphotericin B (100 μg/ml) was added to the fourth well as a control.
0.5 McFarland equivalent suspension of C. albicans and S. cerevisiae in SDB were swabbed individually over SDA plates. Subsequently, following which four wells were made using a sterile cork borer in each plate. 50 µl of cell-free supernatant collected after 1, 6, and 24 hours of incubation were added into the wells of each respective plate. As a control, 50 ul of Amphotericin B (100 µg/ml) was added to one well on every plate. The plates were refrigerated for 30 minutes for the diffusion of suspensions into the media and then incubated at 28ºC for 24 hours.
RESULTS
Isolation and identification of chondroitinase-producing B. velezensis B26 strain from soil samples
The four soil samples, namely CPF-I, CHCS-I, CHCS-II, and CHCS-III were collected from Manipal, Udupi, Karnataka, India. Out of these samples, only 29 morphologically distinct bacterial isolates were obtained using enrichment media. These 29 isolates were checked for chondroitinase production using the rapid plate method [16]. Among these, 8 isolates exhibited clear zones around their growth (Fig. 1a), identifying them as chondroitinase-producing bacterial isolates. The prominent chondroitinase-producing capacity was noticed in the CHCS-IIIe isolate among the 8 isolates, further it was selected for investigation. The isolated CHSCS-IIIe strain was carefully purified by multiple streaking, and individually isolated colonies were designated as B26. This strain, B26, was further subjected to confirm its chondroitinase-producing potential, as evidenced by the presence of a clear zone around the cell growth area (Fig. 1a). B26 cells were grown in nutrient broth supplemented with 0.04% w/v CS at 37ºC for 48 hours. Subsequently, cells were harvested and collected supernatant was subjected to enzyme assay to estimate the amount chondroitinase present in the extracellular soluble fraction. The average activity chondroitinase enzyme activity was found to be 0.235 ± 0.04 U/ml (Table 1). To identify the nature of the organism, gram staining, and biochemical tests were performed (Supplementary Fig. S1, S2; Supplementary Table. S1). To reveal its taxonomic identity, the 16s rRNA gene sequence of the isolated strain was analyzed and identified. The result revealed that the identified strain exhibited 100% similarity with B. velezensis CR-502(T) (Accession no. AY603658) (Table 2). Thus, the strain B26 was identified as B. velezensis.
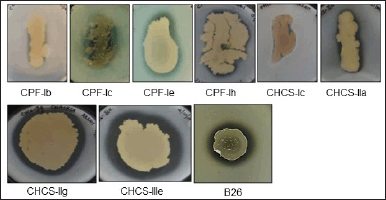 | Figure 1. Identification of chondroitinase-producing bacterial isolates from soil samples. a. Among the four samples-CPF-I, CHCS-I, CHCS-II, and CHCS-III, 8 isolates were found to be producing clear zones around their growth areas. This clear zone serves as an indicator of the chondrotinase production. The isolates were cultured on nutrient agar supplemented with 0.04% w/v of CS and 1% w/v bovine serum albumin, followed by incubation at 37ºC for 3–4 days. [Click here to view] |
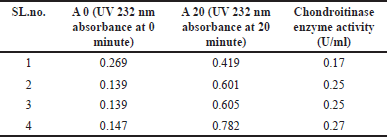 | Table 1. Chondroitinase enzyme activity of B. velezensis B26 strain. [Click here to view] |
Bacillus velezensis B26 exhibits enzymatic activity. To assess the enzymatic activity of B. velezensis B26 cells, we performed an amylase assay using a starch agar medium. The organism was introduced at the center of a starch agar plate and allowed to incubate at 37°C for 48 hours. Subsequently, the plate was flooded with iodine solution. The appearance of a distinct clear zone around the colony served as evidence of the occurrence of starch hydrolysis and the consequent production of the amylase enzyme (Fig. 2a). Similarly, the evaluation of proteolytic activity was conducted through the skim milk agar method. A clear zone around the colony growth suggests that B. velezensis B26 exhibited proteolytic activity (Fig. 2b). In the context of chondroitinase activity, the B. velezensis B26 strain was assessed by introducing it into a medium composed of nutrient agar supplemented with 1% w/v bovine serum albumin and 0.04% w/v CS. The appearance of a clear zone around the colony validated the production of chondroitinase (Fig. 2c). Additionally, an evaluation of gelatinase activity was carried out by observing gelatin medium liquefaction. The selected bacterial cells were stab cultured into the nutrient agar with gelatin and liquefaction of the medium was observed, indicating the gelatinase enzyme production (Fig. 2d). Additionally, for the evaluation of chitinase and glucanase activities, the DNS method was employed [15,23]. In this method, B26 cells were harvested and the collected supernatant was subjected to enzyme assay to estimate the amount of enzymes present in the extracellular soluble fraction. Optical density was measured to calculate the content of sugar present in the sample after its incubation of soluble fraction with the colloidal chitin and carboxymethyl cellulose substrates for chitinase and glucanase assays, respectively. By correlating the optical density of the samples with the standard curve established using standardized glucose concentrations (Fig. 3). Enzyme quantities within the samples were estimated to be 1.06 ± 0.25 U/ml for chitinase and 1.3 ± 0.17 U/ml for glucanase (Table 3). Enzyme activity (μmol/minute ml) or (U/ml) = (Concentration of product of the reaction) (μmol /ml) ×Total Reaction Volume (ml) / [(Reaction time (minute)) × (Enzyme volume(ml))].
 | Table 2. The identification of bacterial sample B26 through analysis of its 16S rRNA gene sequencing. [Click here to view] |
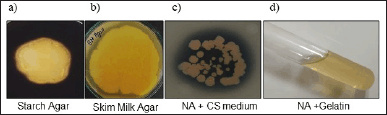 | Figure 2. Bacillus velezensis B26 cells exhibit enzymatic activities. Bacillus velezensis B26 cells were introduced at the center region of different agar plates: a. Starch agar plate, b. Skim milk agar plate, and c. Nutrient Agar plate supplemented with 1% w/v bovine serum albumin and 0.04% w/v CS. Followed by incubation at 37°C for 48 hours, clear zones around the colony growth signifying starch hydrolysis by (a) amylase enzyme, (b) proteolytic enzyme, and (c) chondroitinase activity, (d) For the evaluation of gelatinase activity, B. velezensis B26 cells were stab cultured in a test tube with nutrient agar containing gelatin and incubated at 37°C. After 48 hours of incubation, the nutrient gelatin medium had undergone liquefaction, indicating gelatinase enzyme production. [Click here to view] |
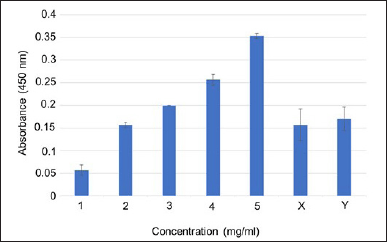 | Figure 3. Estimation of chitinase and glucanase enzyme activities. For chitinase enzyme activity, soluble fractions obtained from the bacterial cells were incubated with colloidal chitin as a substrate and the released glucose molecule was monitored by measuring absorbance at 450 nm. Similarly, for glucanase enzyme activity, soluble fraction obtained from the bacterial cells was incubated with carboxymethyl cellulose as a substrate and the released glucose molecule was monitored by measuring absorbance at 450 nm. Varying glucose concentrations are employed to plot the standard curve. X-axis represents glucose concentration and Y-axis indicates absorbance at 450 nm. Chitinase and glucanase activity is extrapolated from the standard plot. X indicates supernatant obtained from the bacterial cells incubated with colloidal chitin substrate, and Y represents supernatant obtained from the bacterial cells incubated with carboxymethylcellulose. [Click here to view] |
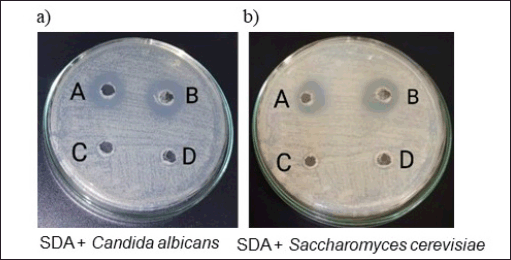 | Figure 4. Antifungal potential of B. velezensis B26 extract. (a) 0.5 McFarland equivalent suspension of C. albicans and (b) S. cerevisiae in SDB were swabbed individually over SDA plates. 50 ul of Amphotericin B (100 µg/ml) was added to one well on every plate (Control). Well A. Amphotericin B (100 µg/ml)—50 µl, Well B. 6-hour supernatant sample—50 µl, Well C. 1-hour supernatant sample—50 µl, Well D—24-hour supernatant sample—50 µl. Following the incubation period, a significant zone of clearance was visibly discerned around both the 6-hour incubated supernatant well and the control well, indicating potential antifungal activity. [Click here to view] |
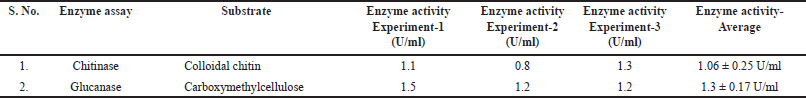 | Table 3. Bacillus velezensis B26 enzyme activity. [Click here to view] |
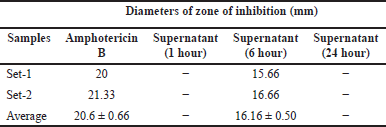 | Table 4. Antifungal activity of B. velezensis B26 against C. albicans. [Click here to view] |
Bacillus velezensis B26 extract exhibits antifungal activity
To assess the antifungal efficacy of the B. velezensis cells, a sample was collected during the lag phase, log phase, and stationary phase (Supplementary Table. S2). The samples were subjected to an antifungal assay against C. albicans and S. cerevisiae (which serve as commonly employed model fungal organisms for experimentation). While no discernible inhibition zones were noted for the samples from the lag and stationary phases, a clear inhibition zone emerged against both fungal organisms in the log phase sample (Fig. 4a, b, and Table 4 and 5). This observation suggests the production of the antifungal component occurs during the intense multiplication stage of cellular growth.
 | Table 5. Antifungal activity of B. velezensis B26 against S. cerevisiae. [Click here to view] |
DISCUSSION
In this study, we show that B. velezensis B26 strain isolated from soil samples exhibited enzymatic and antifungal activity against C. albicans, a predominant human fungal pathogen, as well as S. cerevisiae, an emerging opportunistic pathogen.
Previous studies have isolated chondroitinase-producing bacteria from different aquatic and terrestrial environments using different screening methods [25,26]. However, our study for the first time reported isolation of a chondroitinase-producing B. velezensis B26 strain from soil samples. This finding is significant because chondroitinase enzyme exhibits therapeutic potential against several pathological processes [6]. Furthermore, our study revealed that B. velezensis B26 strain exhibits several ranges of enzymatic activities including amylase, gelatinase, chitinase, and glucanase. Amylase and gelatinase are commonly used in the food industry [27]. Chitinase and glucanase are employed in bioconversion of agricultural waste into important by-products [28,29]. Thus, B. velezensis B26 strain underscores its potential applications in both the food and pharmaceutical industries.
Several other studies show that Bacillus species exhibit wide suppression against numerous fungal pathogens, majorly against phytopathogens. Importantly, recent studies suggest the effectiveness of B. velezensis against several fungal pathogens [30–35]. In addition to the production of antifungal metabolites, B. velezensis secretes fungal cell wall-digesting enzymes and bacterial volatiles (metabolites). The synthesis of these diverse compounds by B. velezensis significantly impacts the growth of fungal pathogens [35,36]. Notably, the synthesis of cyclic dipeptides (cyclo-(D-phenylalanyl-D-prolyl) and cyclic tetrapeptide (cyclo-(prolyl-valyl-alanyl-isoleucyl) by B. velezensis CE 100 strain shown to repress the spore germination and mycelial growth of Colletotrichum gloeosporioides [35,37]. Similarly, the B. velezensis CE 100 strain exhibits antifungal activity against phytopathogens that cause root rot disease [38]. Several studies report that chitinase and β-1,3-glucanase inhibit fungal growth, and both hydrolytic enzymes exhibit synergistic increase in antifungal activity.
In this study, we have demonstrated that B. velezensis B26 strain exhibits chondroitinase, chitinase, and glucanase enzyme activities. Chitinases, characterized by their ability to cleave glycosidic bonds within chitin molecules, are induced in plants as a defense mechanism against fungal infections and other stress factors [39,40]. Chitinases obtained from plants and bacterial sources exhibit immense potential as biocontrol agents of plant pathogenic fungi and insects [41–48]. For instance, Bacillus cereus YQ 308, a strain isolated from soil samples secreted chitinase enzyme on induction with shrimp and crab shell powder. The purified chitinase enzyme exhibited antifungal effects against fungi Fusarium oxysporum and Pythium ultimum. The utilization of chitinases and other enzymes for biological control against pathogenic fungi could serve as a viable alternative to chemical agents [49]. Plants secret both β-1,3-glucanase and various isoforms of chitinase to defend against various fungal pathogens. These enzymes work synergistically, hydrolyzing the fungal cell wall and inhibiting fungal growth [39,50]. Given this context, in addition to other antifungal components and metabolites, the synthesis of chitinase, and glucanase by the identified B. velezensis B26 strain might potentially aid in its antifungal activity. The antifungal properities of B. velezensis B26 strain highlight its potential as a biocontrol agent. Thus, B. velezensis B26 might be a promising candidate for developing new antifungal treatments. Further studies could be employed to isolate and characterize the specific compounds responsible for the antifungal activity.
CONCLUSION
This is the first study to identify and report the chondroitinase synthesis from B. velezensis B26. By exploring these novel sources, we hope to contribute to the broader understanding of chondroitinase enzymes and their potential biotechnological applications. We further show that besides chondroitinase activity, the B. velezensis B26 strain exhibits chitinase, glucanase, amylase, and gelatinase enzyme activity. Significantly, we reveal the potent antifungal capabilities of the B. velezensis strain B26 against Candida albicans and Saccharomyces cerevisiae. In conclusion, our findings contribute to the expanding usage of B. velezensis B26 stain as a biocontrol agent and industrial enzyme producer.
ACKNOWLEDGMENT
The authors acknowledge Manipal Academy of Higher Education, Manipal, for providing financial and infrastructural support for the research activities.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
ACCESSION CODES
Bacillus velezensis B26 was received for public access deposit at NCMR allocated the accession number MCC 5370. Whole genome sequence of Bacillus velezensis B26 strain submitted to GenBank (accession number: JAYKOV000000000).
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the journal’s website: Link here [https://japsonline.com/admin/php/uploadss/4449_pdf.pdf].
REFERENCES
1. Kjellén L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–75.
2. Kasinathan N, Volety SM, Josyula VR. Chondroitinase: a promising therapeutic enzyme. Crit Rev Microbiol. 2016 May;42(3):474–84.
3. Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. The FASEB J [Internet]. 2006 Jan 1;20(1):9–22. CrossRef
4. Gallagher JT. The extended family of proteoglycans: social residents of the pericellular zone. Curr Opin Cell Biol. 1989;1(6):1201–18.
5. Bartus K, James ND, Bosch KD, Bradbury EJ. Chondroitin sulphate proteoglycans: key modulators of spinal cord and brain plasticity. Exp Neurol. 2012;235(1):5–17.
6. Muir E, De Winter F, Verhaagen J, Fawcett J. Recent advances in the therapeutic uses of chondroitinase ABC. Exp Neurol. 2019 Nov;321:113032.
7. Bradbury EJ, Moon LDF, Popat RJ, King VR, Bennett GS, Patel PN, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002 Apr;416(6881):636–40.
8. Kumar A, Biswas A, Bojja LS, Kolathur KK, Volety MS. Emerging therapeutic role of chondroitinase (ChABC) in neurological disorders and cancer [Internet]. Curr Drug Therap. 2022;17:160–70. Available from: http://www.eurekaselect.com/article/122099
9. Bhattacharya D, Nagpure A, Gupta RK. Bacterial chitinases: properties and potential. Crit Rev Biotechnol. 2007;27(1):21–8.
10. Hamid R, Khan MA, Ahmad M, Ahmad MM, Abdin MZ, Musarrat J, et al. Chitinases: an update. J Pharm Bioallied Sci. 2013 Jan;5(1):21–9.
11. Planas A. Bacterial 1,3-1,4-beta-glucanases: structure, function and protein engineering. Biochimica et Biophysica Acta. 2000 Dec;1543(2):361–82.
12. Suyotha W, Yano S, Wakayama M. α-1,3-Glucanase: present situation and prospect of research. World J Microbiol Biotechnol [Internet]. 2016;32(2):30. CrossRef
13. Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases—estimate precision. J Fungi (Basel). 2017 Oct 18;3(4):57.
14. Wu S, Song R, Liu T, Li C. Antifungal therapy: novel drug delivery strategies driven by new targets. Adv Drug Deliv Rev [Internet]. 2023;199:114967. Available from: https://www.sciencedirect.com/science/article/pii/S0169409X2300282X
15. Philip NV, Koteshwara A, Kiran GA, Raja S, Subrahmanyam VM, Chandrashekar HR. Statistical optimization for coproduction of chitinase and beta 1, 4-endoglucanase by chitinolytic paenibacillus elgii PB1 having antifungal activity. Appl Biochem Biotechnol [Internet]. 2020;191(1):135–50. CrossRef
16. Smith RF, Willett NP. Rapid plate method for screening hyaluronidase and chondroitin sulfatase-producing microorganisms. Appl Microbiol. 1968;16(9):1434–6.
17. Wilson K. Preparation of genomic DNA from bacteria. Curr Proto Mol Biol [Internet]. 2001 Oct 1;56(1):2.4.1–5. CrossRef
18. Boratyn GM, Camacho C, Cooper PS, Coulouris G, Fong A, Ma N, et al. BLAST: a more efficient report with usability improvements. Nucl Acids Res. 2013 Jul;41(Web Server issue):W29–33.
19. Clemons TD, Fitzgerald M, Dunlop SA, Harvey AR, Iyer KS, Stubbs KA. An improved assay for the spectrophotometric determination of chondroitinase ABC activity. N J Chem. 2013;37(7):1944–9.
20. Clarke PH, Cowan ST. Biochemical methods for bacteriology. J Gen Microbiol. 1952 Feb;6(1–2):187–97.
21. dela Cruz TEE, Torres JMO. Gelatin hydrolysis test protocol. Am Soc Microbiol [Internet]. 2012;(November 2012):1–10. Available from: http://www.asmscience.org/content/education/protocol/protocol.3776
22. Hussein NNA, Ibrahim AI, Kamar FH, Nechifor AC. Caseinase production and media optimization from Bacillus subtilis. Rev de Chim. 2020;71:1–9.
23. Gonfa TG, Negessa AK, Bulto AO. Isolation, screening, and identification of chitinase-producing bacterial strains from riverbank soils at Ambo, Western Ethiopia. Heliyon. 2023 Nov;9(11):e21643.
24. El-Sayed SE, Abdelaziz NA, Ali AA, Alshahrani MY, Aboshanab KM, El-Housseiny GS. Identification, characterization, and production optimization of 6-methoxy-1H-indole-2-carboxylic acid antifungal metabolite produced by Bacillus toyonensis isolate OQ071612. Microorganisms. 2023;11:2835.
25. Manabu K, Young-Zoon L. Chondroitinase-producing bacteria in natural habitats. Appl Microbiol [Internet]. 1975 Mar 1;29(3):414–21. CrossRef
26. Su J, Wang X, Yin C, Li Y, Wu H, Yu W, et al. Identification and biochemical characterization of a surfactant-tolerant chondroitinase VhChlABC from vibrio hyugaensis LWW-1. Marine Drugs. 2021 Jul;19(7):399.
27. Raveendran S, Parameswaran B, Ummalyma SB, Abraham A, Mathew AK, Madhavan A, et al. Applications of microbial enzymes in food industry. Food Technol Biotechnol. 2018 Mar;56(1):16–30.
28. Ravindran R, Hassan SS, Williams GA, Jaiswal AK. A review on bioconversion of agro-industrial wastes to industrially important enzymes. Bioengineering (Basel, Switzerland). 2018 Oct;5(4):93.
29. Taokaew S, Kriangkrai W. Chitinase-assisted bioconversion of chitinous waste for development of value-added chito-oligosaccharides products. Biology. 2023 Jan;12(1):689.
30. Wang C, Ye X, Ng TB, Zhang W. Study on the biocontrol potential of antifungal peptides produced by Bacillus velezensis against fusarium solani that infects the passion fruit Passiflora edulis. J Agri Food Chem. 2021 Feb;69(7):2051–61.
31. Cao Y, Pi H, Chandrangsu P, Li Y, Wang Y, Zhou H, et al. Antagonism of two plant-growth promoting Bacillus velezensis Isolates against ralstonia solanacearum and Fusarium oxysporum. Sci Rep. 2018 Mar;8(1):4360.
32. Maung CEH, Lee HG, Cho JY, Kim KY. Antifungal compound, methyl hippurate from Bacillus velezensis CE 100 and its inhibitory effect on growth of Botrytis cinerea. World J Microbiol Biotechnol. 2021 Aug;37(9):159.
33. Wang GF, Meng JF, Tian T, Xiao XQ, Zhang B, Xiao YN. Endophytic Bacillus velezensis strain B-36 is a potential biocontrol agent against lotus rot caused by Fusarium oxysporum. J Appl Microbiol. 2020 Apr;128(4):1153–62.
34. Lim SM, Yoon MY, Choi GJ, Choi YH, Jang KS, Shin TS, et al. Diffusible and volatile antifungal compounds produced by an antagonistic Bacillus velezensis G341 against various phytopathogenic fungi. Plant Pathol J. 2017 Oct;33(5):488–98.
35. Kim TY, Hwang SH, Noh JS, Cho JY, Maung CEH. Antifungal potential of Bacillus velezensis CE 100 for the control of different colletotrichum species through isolation of active dipeptide, cyclo-(D-phenylalanyl-D-prolyl). Int J Mol Sci. 2022 Jul;23(14):7786.
36. Wang E, Liu X, Si Z, Li X, Bi J, Dong W, et al. Volatile organic compounds from rice rhizosphere bacteria inhibit growth of the pathogen rhizoctonia solani. Agriculture. 2021;11:368.
37. Choub V, Ajuna HB, Won S-J, Moon J-H, Choi S-I, Maung CE, et al. Antifungal Activity of Bacillus velezensis CE 100 against Anthracnose Disease (Colletotrichum gloeosporioides) and Growth Promotion of Walnut (Juglans regia L.) Trees. Vol. 22, International Journal of Molecular Sciences. 2021.
38. Moon JH, Won SJ, Maung CE, Choi JH, Choi SI, Ajuna HB, et al. Bacillus velezensis CE 100 inhibits root rot diseases (Phytophthora spp.) and promotes growth of Japanese Cypress (Chamaecyparis obtusa Endlicher) Seedlings. Microorganisms. 2021;9:821.
39. Sandhu JS, Sidhu MK, Yadav IS. Control of fungal diseases in agricultural crops by chitinase and glucanase transgenes BT—sustainable agriculture reviews. In: Lichtfouse E, editor. Cham, Switzerland: Springer International Publishing; 2017. p. 163–212. CrossRef
40. Stintzi A, Heitz T, Prasad V, Wiedemann-Merdinoglu S, Kauffmann S, Geoffroy P, et al. Plant “pathogenesis-related” proteins and their role in defense against pathogens. Biochimie. 1993;75(8):687–706.
41. Yu J, Liu Q, Liu Q, Liu X, Sun Q, Yan J, et al. Effect of liquid culture requirements on antifungal antibiotic production by Streptomyces rimosus MY02. Bioresource Technol. 2008 Apr;99(6):2087–91.
42. Yasir M, Aslam Z, Kim SW, Lee SW, Jeon CO, Chung YR. Bacterial community composition and chitinase gene diversity of vermicompost with antifungal activity. Bioresource Technol. 2009 Oct;100(19):4396–403.
43. Kawase T, Yokokawa S, Saito A, Fujii T, Nikaidou N, Miyashita K, et al. Comparison of enzymatic and antifungal properties between family 18 and 19 chitinases from S. coelicolor A3(2). Biosci Biotechnol Biochem. 2006 Apr;70(4):988–98.
44. Verburg JG, Huynh QK. Purification and characterization of an antifungal chitinase from arabidopsis thaliana. Plant Physiol. 1991 Feb;95(2):450–5.
45. Leah R, Tommerup H, Svendsen I, Mundy J. Biochemical and molecular characterization of three barley seed proteins with antifungal properties. J Biol Chem. 1991 Jan;266(3):1564–73.
46. Wang S, Wu J, Rao P, Ng TB, Ye X. A chitinase with antifungal activity from the mung bean. Protein Expr Purif. 2005 Apr;40(2):230–6.
47. Fleuri LF, Kawaguti HY, Sato HH. Production, purification and application of extracellular chitinase from Cellulosimicrobium cellulans 191. Brazil J Microbiol?: [publication of the Brazilian Society for Microbiology]. 2009 Jul;40(3):623–30.
48. Zarei M, Aminzadeh S, Zolgharnein H, Safahieh A, Daliri M, Noghabi KA, et al. Characterization of a chitinase with antifungal activity from a native Serratia marcescens B4A. Brazil J Microbiol?: [publication of the Brazilian Society for Microbiology]. 2011 Jul;42(3):1017–29.
49. Chang WT, Chen CS, Wang SL. An antifungal chitinase produced by Bacillus cereus with shrimp and crab shell powder as a carbon source. Curr Microbiol. 2003 Aug;47(2):102–8.
50. Mohammadi M, Karr AL. β-1,3-Glucanase and chitinase activities in soybean root nodules. J Plant Physiol [Internet]. 2002;159(3):245–56. Available from: https://www.sciencedirect.com/science/article/pii/S0176161704702278