INTRODUCTION
Fisetin is a dietary flavonoid which abundant in several fruits and vegetables including onions, strawberries, tomatoes, apples, kiwis, persimmons, cucumbers, nuts, wins, and grapes [1]. It has been documented to display many physiological and pharmacological effects, including antioxidant, anti-inflammatory, and neuroprotective properties, cardioprotective, anti-diabetic and anticancer effects [2–4]. Due to its strong anticancer properties, fisetin has been employed to impede viability and trigger apoptosis [5]. Interestingly, it has been demonstrated to exhibit significant anticancer properties in several forms of cancer such as prostate, breast, pancreatic, colon, liver, kidney, oral, stomach, lung, bladder, ovarian, cervical, and skin cancer [6]. Nonetheless, the impact of fisetin on cholangiocarcinoma (CCA) remains uncertain, and the available data are limited.
The anticancer potential of fisetin has been validated through various actions, including inhibition of cell proliferation, suppression of cell invasion and migration, prevention of angiogenesis, and promotion of apoptosis [5,6]. Through mechanisms including the suppression of cyclin-dependent kinases, the inhibition of PI3K/Akt/mTOR signaling pathway, the modulation of MAPK, and the regulation of NF-B, therefore, fisetin causes cell death and inhibits cell proliferation in various types of cancer cells [7,8]. In addition, fisetin prompts apoptosis through diverse mechanisms, including the inhibition of cyclooxygenase-2, the suppression of the EGFR pathway, the stimulation of the caspase cascade, and the modulation of mitochondrial function [5]. Furthermore, fisetin triggers autophagy in cancer cells by suppressing the mTORC1 and 2 signaling [9]. Fisetin has also been documented to diminish the migratory and invasive capabilities of lung cancer cells through alteration of the ERK signaling pathway [10]. Fisetin is highly effective against a variety of cancer cells and is involved in a wide range of mechanisms, according to the data collected, but CCA cells have limited knowledge.
CCA or bile duct epithelial carcinoma which one of the most leading causes of cancer-correlated mortality in Thailand [11]. Due to its resistance to treatment, propensity for local and distant invasion, rapid growth, fast metastasis, and poor prognosis, CCA is one of the most aggressive kinds of cancer [12]. Alternative therapeutic approaches are urgently needed. Fisetin may be the greatest option to investigate in CCA as there is now no effective treatment for the disease. Studies have demonstrated that fisetin provides beneficial effects in combating gastrointestinal tract cancer, including stomach, colon, and liver cancer, and it may also be helpful against CCA [6]. According to studies, fisetin inhibits liver cancer cells from proliferating and migrating, which results in decreased tumor cell colonies and stops cell cycle distribution in the S phase [13]. Similar to our earlier research, fisetin inhibited the proliferation of breast and cervical cancer cells by decreasing the level of the cyclin D1 and cyclin E mRNA [14]. For more information, fisetin was confirmed to reduce phospho-p65 and myelocytomatosis, which decreased CCA cell survival and increased the amount of cellular death when combined with gemcitabine [15].
Despite the considerable number of in vitro studies dedicated to exploring the anticancer potential and mechanisms of fisetin, its effects on CCA are constrained and lack comprehensive information. Therefore, the aim of this investigation was to compare the two different CCA phenotypes of KKU-100, which exhibit slow growth and migration, with those of KKU-M52, which exhibit rapid growth and migration, and to determine the inhibitory effects of fisetin on these two CCA cell types.
MATERIALS AND METHODS
Cells and culture
Two CCA cell lines, KKU-100 (Slow proliferation and migration) and KKU-M452 (Rapid proliferation and migration), were graciously supplied by the Faculty of Medicine at Khon Kaen University. Two cells were grown in DMEM media with 10% fetal bovine serum, 1% antibiotics, and a humidified 5% CO2 environment.
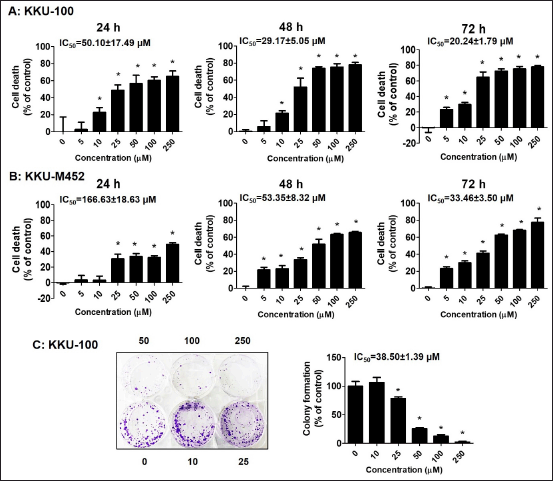 | Figure 1. Growth inhibitory effect of fisetin on CCA cells. (A-B) KKU-100 and KKU-M452 cells (1 × 104 cells/well) were treated with fisetin (0–250 µM) for 24–72 hours. The cancer cells death was determined by SRB assay and expressed as a percentage of control cells. (C) KKU-100 cells (500 cells/well) were exposed to fisetin (0–250 µM) for 24 hours and cultured further for 15 days. After incubation period, the colonies were fixed, stained, and counted. *Statistical difference from control cells (p < 0.05). [Click here to view] |
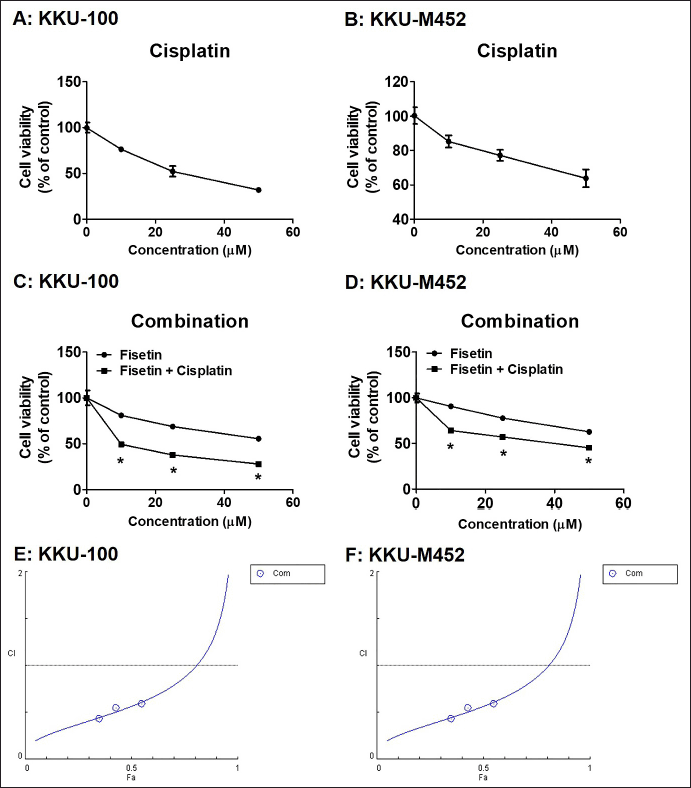 | Figure 2. Effects of the fisetin, cisplatin and in combination with cisplatin, on proliferation in KKU-100 and KKU-M452 cells. (A-B) KKU-100 and KKU-M452 cells (1 × 104 cells/well) were treated with cisplatin (0–50 µM) for 24 hours. The cancer cells viability was determined by SRB assay and expressed as a percentage of control cells. (C-D) Two CCA cells were exposed to fisetin (0–50 µM) with or without 10 µM cisplatin for 24 hours and measured cell viability with SRB assay. (E-F) Chou-Talalay method Fa-CI plot of fisetin plus cisplatin. CI was plotted on the y-axis as a function of effect level (Fa) on the x-axis to evaluate drug synergism. CI <1, CI = 1 and CI >1 refers to synergism, additivity and antagonism, respectively. Data are represented as mean ± SE (n = 3), *p < 0.05 versus fisetin treatment alone. [Click here to view] |
Cytotoxic assay
1 × 104 CCA cells were plated into 96-well culture plates and cultivated for overnight. Fisetin (0–250 µM) was applied to cells in increasing concentrations for the three specified times (24, 48, and 72 hours), and the cytotoxic effects were measured by the sulforhodamine B (SRB) method. In brief, the media was discarded, the cells were treated with 10% trichloroacetic acid for fixation and then exposed to a 0.4% SRB solution for staining for 1 hour at room temperature. The cells were then solubilized in 10 mM Tris base buffer, and an optical density was read by spectrophotometer at 540 nm.
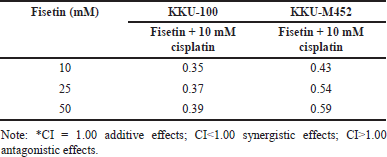 | Table 1. CI analysis of fisetin and 10 mM cisplatin. [Click here to view] |
In combination assay between fisetin with anticancer drug, cisplatin, was explored by SRB assay. Briefly, the CCA cells were incubated with 0–50 µM fisetin, 0–50 µM cisplatin, and fisetin (0–50 µM) in combination with anticancer drugs cisplatin (10 µM) for 24 hours. Next, the cells were exposed to SRB, solubilized, and read the absorbance at 540 nm.
Colony formation assay
6-well plates containing 500 cells per well of KKU-100 cells were plated and grown overnight. The cells were incubated with fisetin (0–250 µM), after which they were switched to fresh full DMEM media and continued to be cultured for an additional 15 days. The colonies underwent a process of washing, fixation, and staining with a 0.3% crystal violet buffer. The colonies’ numbers were captured, counted, and calculated.
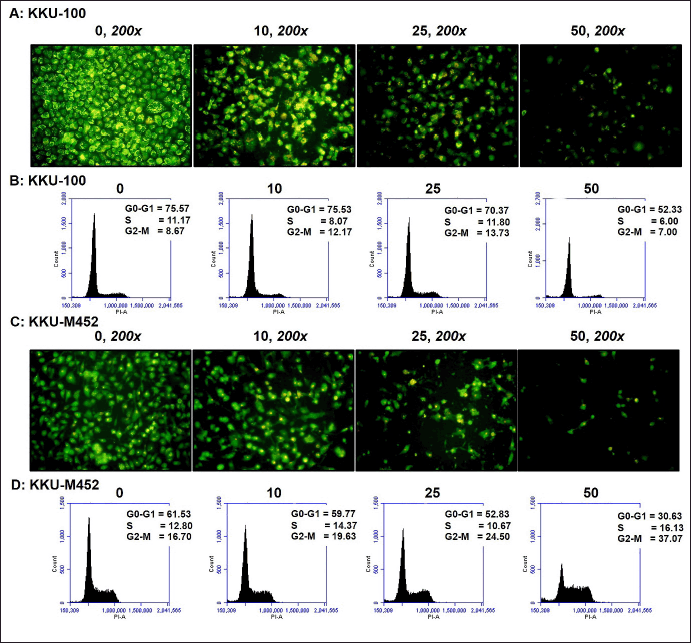 | Figure 3. Apoptotic induction and cell cycle arrest effect of fisetin on CCA cells. (A and C) KKU-100 and KKU-M452 cells (1 × 104 cells/well) were treated with fisetin (0–50 µM) for 24 hours. The apoptotic induction was determined by AO/EB double staining assay. (B and D) CCA cells (2.5 × 105 cells/well) were exposed to fisetin (0–50 µM) for 24 hours, stained with PI solution, and then measured the cell cycle by flow cytometry. [Click here to view] |
Acridine orange/Ethidium bromide (AO/EB) double staining assay
Two CCA cells were placed in 96-well cultured plates (1 × 104 cells/well) for overnight. Fisetin (0–50 µM) was incubated in the cells in increasing concentrations for 24 hours, and the AO/EB test was used to measure the apoptotic effects. After discarding the DEME medium, the CCA cells were washed with PBS buffer and stained for 15 minutes at room temperature with AO and EB (1 µg/ml). Fluorescence inverted microscopy was used to capture the apoptotic cells (200 × magnification, CKX53, Olympus).
Cell cycle distribution assay
Two CCA cells were placed in 6-well culture plates (2.5 × 105 cells/well) for overnight. Fisetin (0–50 µM) was added to cells in increasing concentrations for 24 hours, after which the distribution of the cell cycle was assessed using a flow cytometric method. In brief, the cells were exposed to PBS buffer for washing and then incubated with 70% ethanol for fixation. Next, the cells were washed many times in distilled water before staining with propidium iodide (PI) for 30 minutes. The flow cytometric method was used to determine the cell cycle distribution using BD Accuri C6 Plus software (BD Biosciences, CA, USA).
Transwell migration assay
CCA cells were seeded into the upper chamber (2 × 104 cells/well) in a serum-free medium with fisetin (0–50 µM) for 24 hours, and the lower chamber was added with a complete medium. Following a 24-hour incubation period, migrating cells at the membrane’s bottom surface were preserved with 100% methanol, stained with 2.5% crystal violet, and photographed under a light microscope (200x magnification, CKX53 Olympus, USA).
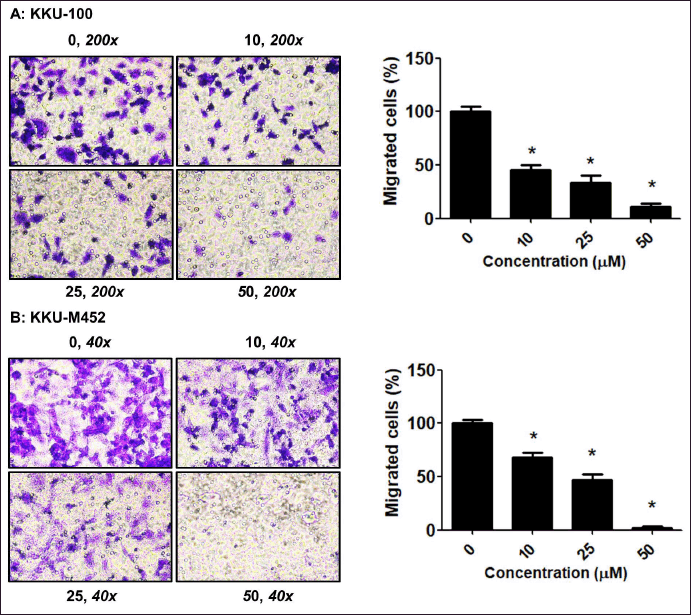 | Figure 4. Migratory inhibition of fisetin on CCA cells. (A-B) CCA cells (2 × 104 cells/well) were treated with fisetin (0–50 µM) for 24 hours. The migration was determined by Transwell migration assay and expressed as a percentage of control cells. *Statistical difference from control cells (p < 0.05). [Click here to view] |
Wound healing assay
CCA cells were plated in 24-well culture plates (2.5 × 105 cells/well) for overnight. The cells were subjected to wound creation using 200 l pipette tips while being exposed to fisetin (0–50 µM) for 24 hours. Next, the cells were exposed to PBS buffer for washing and the closer of the wound was determined under a light microscope (40x magnification, CKX53 Olympus, USA).
ROS formation assay
6-well plates containing 2.5 × 105 cells/well of CCA cells per well were seeded for overnight. The ROS formation was assessed using a flow cytometry test after cells were exposed to fisetin at increasing doses (0–50 µM) for 24 hours. The cells were washed multiple times in distilled water before being stained for 30 minutes at 37°C with DCF-DA (25 µM). The ROS generation was measured by flow cytometric method using BD Accuri C6 Plus software (BD Biosciences, CA, USA).
Statistical analysis
The aforementioned all of these data were examined by using GraphPad Prism 5.0 statistical software (GraphPad Software, CA, USA). The mean ± SE were used to represent measurement data. A statistically significant difference, indicating p < 0.05, was observed when comparing mean values across multiple groups using the Student t-test. To evaluate the combination index (CI) between fisetin and cisplatin, the CI was determined by Chou and Talalay method (Chou, 2010) using the CompuSyn software (ComboSyn, Inc., New York, NY, USA).
RESULTS
Fisetin inhibited cell growth and colony formation
Two CCA cells, KKU-100 and KKU-M452, were incubated with fisetin (0–250 µM) for 24–72 hours, respectively, to study the fisetin effects on the cell growth of CCA cells. Fisetin suppressed cell growth in a concentration- and time-dependent manner (Fig. 1A and B) with low concentration. The IC50 values were 50.10 ± 17.49, 29.17 ± 5.05, and 20.24 ± 1.79 µM for KKU-100 cells at 24, 48, and 72 hours, respectively, along with 166.63 ± 18.63, 53.35 ± 8.32, and 33.46 ± 3.50 µM for KKU-M452 cells.
Next experiment, the KKU-100 cell was employed as the model, while KKU-M452 cells were unable to form a colony, and this allowed researchers to assess whether fisetin controlled the colonies formation of CCA cells. Cells were incubated with fisetin (0–250 µM), and another was further cultured for 15 days. These findings showed that fisetin inhibited KKU-100 cell colony formation with IC50 values of 38.50 ± 1.39 µM (Fig. 1C). Fisetin showed a potent anticancer impact on CCA cells, and for the benefit of the current investigation, we will be using doses of fisetin ranging from 10 to 50 µM in all studies. Figure 2A–C demonstrated that cisplatin is one of the anticancer drugs to treat CCA and caused a reduction of both CCA cells viability in a concentration-dependent manner with IC50 values were 27.27 ± 2.37 µM and 88.97 ± 21.04 µM for KKU-100 and KKU-M452 cells, respectively. In combination results of fisetin and cisplatin indicated that fisetin significantly potentiated the anticancer effects of cisplatin in each dose of fisetin treatment. The IC50 values of fisetin treatment alone were 56.68 ± 0.76 µM and 86.72 ± 1.98 µM for KKU-100 and KKU-M452 cells, along with fisetin plus cisplatin were 13.34 ± 0.92 µM and 31.02 ± 2.63 µM.
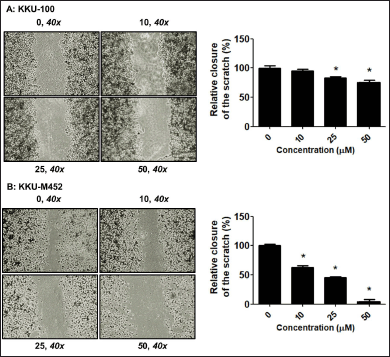 | Figure 5. Migratory inhibition of fisetin on CCA cells. (A-B) CCA cells (2.5 × 105 cells/well) were scratched and treated with fisetin (0–50 µM) for 24 hours. The migration was determined by Wound healing assay and expressed as a percentage of control cells. *Statistical difference from control cells (p < 0.05). [Click here to view] |
The CI was used to examine whether the combination treatment of fisetin and cisplatin had synergistic (CI<1), additive (CI = 1) or antagonistic (CI>1) actions on CCA cells. Fisetin (0–50 μM) plus cisplatin (10 μM) exhibited the strongest activity (CI<1) in KKU-100 cells than KKU-M452 cells (Table 1). Collectively, fisetin enhanced the anticancer drug effects, cisplatin, on KKU-100 than KKU-M452 cells.
Fisetin-induced cells apoptosis and cell cycle arrest
First, AO/EB double labeling was modified to assess cell apoptosis by morphological analysis in order to determine whether fisetin encouraged CCA cells to undergo apoptosis.
These data demonstrated that, in the untreated group, the nuclei of both KKU-100 and KKU-M452 cells displayed uniform staining, signifying the presence of intact cell membrane morphology in these cells. However, fisetin clearly induced the formation of substantial apoptotic bodies in both CCA cells when administered at concentrations of 10–50 µM after a 24-hour exposure. The highest levels of apoptotic bodies were observed at the 25 µM fisetin dose. Fisetin substantially caused the CCA cells to die at high doses (50 µM); as a result, there were less apoptotic bodies than at 10 and 25 µM. (Figs. 3A and 2C). These results revealed that fisetin significantly decreased the rate of CCA cell proliferation in a concentration-dependent manner, which may be related to an increase in apoptosis.
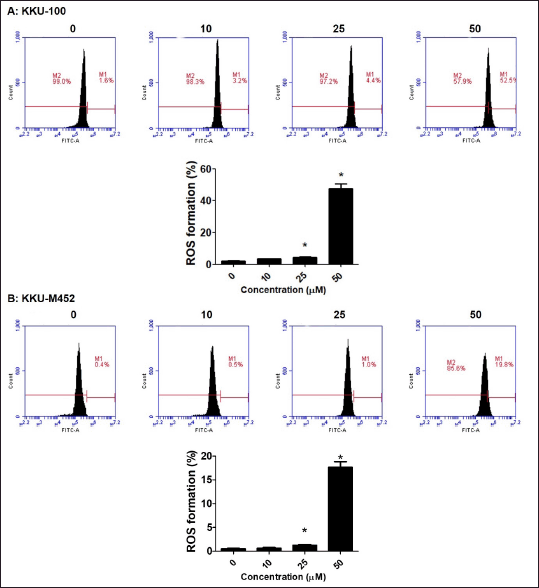 | Figure 6. ROS generation of fisetin on CCA cells. (A-B) CCA cells (2.5 × 105 cells/well) were treated with fisetin (0–50 µM) for 24 hours. The cells were stained with DCF-DA, ROS generation was determined by flow cytometry and expressed as a percentage of control cells. *Statistical difference from control cells (p < 0.05). [Click here to view] |
To validate that fisetin triggered CCA cell death and apoptosis, an analysis of cell cycle distribution was conducted. Fisetin exhibited substantial anticancer effects on both CCA cell types; hence, the cell cycle histograms remained unchanged at the 10 and 25 µM doses when compared with untreated control groups. The dose of 50 µM fisetin robustly triggered cell death in CCA cells and resulted in an alteration of the cell cycle histogram (Fig. 3B and 3D).
Fisetin decreased cells migration
To further validate the impact of fisetin on the motility of CCA cells, wound healing and Trans-well migration methods were performed. The outcomes from both the Trans-well migration (Fig. 4A–B) and wound healing (Fig. 5A–B) assays indicated that fisetin effectively suppressed the migration of KKU-M452 and KKU-100 cells when compared with the untreated group. Two experiments confirmed that fisetin exerted a more pronounced effect on KKU-M452 cells characterized by rapid migration, as opposed to KKU-100 cells which exhibited slower migration. According to this information, fisetin dramatically reduced the ability of CCA cells to migrate.
Fisetin increased ROS generation
To determine if fisetin influences ROS generation in CCA cells and whether the initiation of fisetin-induced cell death is mediated by ROS, the intracellular ROS production in control and treatment cells was observed using a fluorescent probe (DCF-DA). The results indicated that fisetin led to a substantial increase in ROS production in KKU-100 cells, reaching approximately 50% at a dose of 50 µM, in contrast to around 20% in KKU-M452 cells (Fig. 6A–B). These findings imply that the cell death and apoptosis activated by fisetin in two CCA cells might be attributed to the increased production of ROS.
DISCUSSION
CCA is marked by a notable resistance to conventional chemotherapy [16] and a new alternative treatment is urgently needed. Presently, we tested the effectiveness of fisetin, a nontoxic flavonoid molecule, against two different types of CCA cells, KKU-100 with slow growth and migration and KKU-M452 with rapid growth and migration, in order to overcome the limitations of existing chemotherapy regimens. This study showed that fisetin greatly promoted cell death, increased apoptosis, and decreased migration through the generation of ROS levels. KKU-100 cells, which are characterized as having slow proliferation and migration, are more sensitive to the antiproliferative actions of fisetin than are KKU-M452 cells, which are described as having rapid growth and migration. In contrast, KKU-M452 cells were more sensitive to the migratory inhibitory effects of fisetin than KKU-100 cells. These findings showed that fisetin substantially induced apoptosis, migratory inhibition, and cell death in both CCA cells.
In numerous investigations and mechanisms, the impact of fisetin on the proliferation of cancer cells has been described. Fisetin exhibits minimal cytotoxicity towards normal cells at doses that are extremely cytotoxic to cancer cells, and it also stimulates cell cycle arrest at several phases [5]. Such as in breast MCF-7 and cervical HeLa cancer cells, fisetin induced both cancer cell death with low IC50 values of 267.10 ± 48.96 and 140.97 ± 22.92 µg/ml, respectively [14]. In our first report about fisetin on CCA cell growth, we introduced two distinct CCA cell lines and used the SRB assay to confirm the cytotoxicity of fisetin at various doses. Fisetin inhibited the growth and proliferation in both CCA cells, achieving this effect at doses corresponding to IC50 values were 50.10 ± 17.49 and 166.63 ± 18.63 µM for KKU-100 and KKU-M452 cells at 24 hours incubation period, respectively. With the correlation of gastric cancer cells, treatment with fisetin (10–20 µM) encouraged the inhibition of growth by stimulating apoptosis and cancer cell cycle arrest at G2/M stages [17]. Numerous findings suggested that fisetin stimulated cell cycle arrest at G1 and G2/M phase [18] and G2/M arrest in prostate and colon cancer cells [19], respectively. The fisetin strongly activated apoptosis was described through the stimulation of morphological alterations in cells that are indicative of apoptosis through the production of caspase 3 and 7 activities [20].
Apoptosis is a form of programmed cell death, typically marked by specific morphological features and energy-dependent biochemical processes. This study found that after both CCA cells were exposed to fisetin for 24 hours, 10–50 M fisetin significantly activated apoptotic bodies than untreated control groups. The transmission and execution of apoptotic signals necessitate the activity of a cascade of caspases [21]. In cervical cancer cells, fisetin stimulated the high levels of pro-apoptotic genes and protein expression, including APAF1, Bad, Bax, Bid and BIK along with suppression of anti-apoptotic genes, including Bcl-2, BIRC8, MCL-1, XIAP/BIRC4, Livin/BIRC7, clap-2/BIRC3. Significantly, the abovementioned variations subsequently resulted in increased levels of caspase 3, 8 and 9 levels, which activated the cancer cells apoptosis [22]. In combination between fisetin and cisplatin (anticancer drug) showed the potentiated effects between two compounds with upregulation of Bax pro-apoptotic and caspases 3 and 9 gene expression [23]. Fisetin modulates the apoptotic and anti-apoptotic protein or gene expression in several cancer cells, which finally leads to the cancer cells apoptosis as well as CCA cells.
For the next effects of fisetin on CCA cells, we focus on migratory suppression. Less is known about the precise association between fisetin and CCA migration. In advanced cancer stages, cancer cells frequently migrate or metastasize to form a secondary tumor. Fisetin dramatically reduced the migration of both CCA cells in studies of wound healing and the Trans-well migration assay, which were similar to fisetin’s suppression of breast cancer cell migration [14]. Matrix metalloproteinases (MMP) 2 and MMP 9 gene expression and enzyme activity are suppressed, according to reports on the mechanism of action [24]. MMPs are crucial participants in the mechanisms underlying tumorigenesis, angiogenesis, invasion, and metastasis [25]. Numerous natural compounds and herbal remedies have been shown to reduce the expression of MMPs, resulting in a significant reduction in migratory capability [26]. For example, Piper nigrum reduced migration in a rat model by reduction of the MMP 2, MMP 9 levels, and vascular endothelial growth factor [27]. It has been confirmed that Cratoxylum formosum, a Thai vegetable, suppressed both gene and protein expression of MMP 2 and MMP 9 [28]. In summary, these results collectively indicated that fisetin attenuates the migration of CCA cells, consequently diminishing tumor advancement and migration. In deep mechanism of fisetin on CCA migration was more interesting in the future in vivo study.
Elevated intracellular ROS levels lead to the death and apoptosis of cancer cells [29] such as multiple myeloma, liver, oral, skin and oral cancer cells. ROS and mitochondria play an important role in instigating apoptosis in pathological situations, that is, the accumulation of ROS within cancer cells frequently initiates mitochondrial impairment, elevates caspase three levels, and ultimately induces apoptosis in cancer cells [30]. According to this study, fisetin stimulated intracellular ROS production in both types of CCA cells, but had a noticeable effect on KKU-100 cells than KKU-M452 cells. Likewise, fisetin activates apoptosis in lung cancer through the production of ROS levels, which stimulates mitochondrial dysfunction, apoptosis and DNA fragmentation [31] via suppression of ERK1/2 and NF-κB pathways [31]. Moreover, fisetin also caused endoplasmic reticulum (ER) stress-induced production of mitochondrial ROS generation and Ca2+, with the involvement of MAPK signaling [31]. ER stress related to ROS formation is involved in the activation of apoptosis and autophagy [32]. The ROS scavenger molecule N-acetyl cysteine decreased fisetin-activated apoptosis in multiple myeloma and oral cancer cells [33,34]. The association between ROS signaling and fisetin-induced apoptosis in CCA cells will undergo further investigation. According to the findings, large amounts of ROS generation may be able to control how fisetin accelerated CCA cell death, lowered cell viability, and triggered apoptosis, which in turn caused mitochondrial malfunction and the caspase cascade to be induced in cancer cells. ROS may cause CCA cell death and apoptosis.
CONCLUSION
The current research is an initial investigation into the anticancer properties of fisetin and the mechanisms that led to the apoptosis and death of CCA cells. Taken together, according to the available research, fisetin has anticancer effects in human CCA cells by reducing the possibility of cell growth, death, and migration. Moreover, fisetin may exert its function in inhibiting CCA cells through the elevation of intracellular ROS levels. According to our discoveries, fisetin has the potential to be regarded as a promising option for the treatment of CCA.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to the Faculty of Medicine, Mahasarakham University for providing facilities and equipment.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research project was financially supported by Mahasarakham University.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Adhami VM, Syed DN, Khan N, Mukhtar H. Dietary flavonoid fisetin: a novel dual inhibitor of PI3K/Akt and mTOR for prostate cancer management. Biochem Pharmacol. 2012;84(10):1277–81. CrossRef
2. Chuang JY, Chang PC, Shen YC, Lin C, Tsai CF, Chen JH, et al. Regulatory effects of fisetin on microglial activation. Molecules. 2014;19(7):8820–39. CrossRef
3. Liu Y, Cao H, Zhao Y, Shan L, Lan S. Fisetin-induced cell death in human ovarian cancer cell lines via zbp1-mediated necroptosis. J Ovarian Res. 2022;15(1):1–9. CrossRef
4. Prakash D, Gopinath K, Sudhandiran G. Fisetin enhances behavioral performances and attenuates reactive gliosis and inflammation during aluminum chloride-induced neurotoxicity. Neuromolecular Med. 2013;15(1):192–208. CrossRef
5. Kubina R, Krzykawski K, Kabala-Dzik A, Wojtyczka RD, Chodurek E, Dziedzic A. Fisetin, a potent anticancer flavonol exhibiting cytotoxic activity against neoplastic malignant cells and cancerous conditions: a scoping, comprehensive review. Nutrients. 2022;14(13):2604. CrossRef
6. Imran M, Saeed F, Gilani SA, Shariati MA, Imran A, Afzaal M, et al. Fisetin: an anticancer perspective. Food Sci Nutr. 2021;9(1):3–16. CrossRef
7. Mukhtar E, Adhami VM, Sechi M, Mukhtar H. Dietary flavonoid fisetin binds to beta-tubulin and disrupts microtubule dynamics in prostate cancer cells. Cancer Lett. 2015;367(2):173–83. CrossRef
8. Ren Q, Guo F, Tao S, Huang R, Ma L, Fu P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-kappaB p65 and MAPK signaling pathways in septic AKI mice. Biomed Pharmacother. 2020;122:109772. CrossRef
9. Chou RH, Hsieh SC, Yu YL, Huang MH, Huang YC, Hsieh YH. Fisetin inhibits migration and invasion of human cervical cancer cells by down-regulating urokinase plasminogen activator expression through suppressing the p38 MAPK-dependent NF-kappaB signaling pathway. PLoS One. 2013;8(8):e71983. CrossRef
10. Liao YC, Shih YW, Chao CH, Lee XY, Chiang TA. Involvement of the ERK signaling pathway in fisetin reduces invasion and migration in the human lung cancer cell line A549. J Agric Food Chem. 2009;57(19):8933–41. CrossRef
11. Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24(3):349–56. https://doi.org/10.1097/MOG.0b013e3282fbf9b3
12. Braconi C, Patel T. Cholangiocarcinoma: new insights into disease pathogenesis and biology. Infect Dis Clin North Am. 2010;24(4):871–84. CrossRef
13. Sundarraj K, Raghunath A, Panneerselvam L, Perumal E. Fisetin, a phytopolyphenol, targets apoptotic and necroptotic cell death in HepG2 cells. Biofactors. 2020;46(1):118–35. CrossRef
14. Buranrat B, Junking M. Effect of fisetin on the proliferation and migration of human breast and cervical cancer cells. Trop J Pharm Res. 2022;21(1):79–85.
15. Kim N, Lee SH, Son JH, Lee JM, Kang MJ, Kim BH, et al. Fisetin reduces cell viability through up-regulation of phosphorylation of ERK1/2 in cholangiocarcinoma cells. Anticancer Res. 2016;36(11):6109–16. CrossRef
16. Zheng Q, Zhang B, Li C, Zhang X. Overcome drug resistance in Cholangiocarcinoma: new insight into mechanisms and refining the preclinical experiment models. Front Oncol. 2022;12:850732. CrossRef
17. Yan W, Chen S, Zhao Y, Ye X. Fisetin inhibits the proliferation of gastric cancer cells and induces apoptosis through suppression of ERK 1/2 activation. Oncol Lett. 2018;15(6):8442–6. CrossRef
18. Haddad AQ, Fleshner N, Nelson C, Saour B, Musquera M, Venkateswaran V, et al. Antiproliferative mechanisms of the flavonoids 2,2’-dihydroxychalcone and fisetin in human prostate cancer cells. Nutr Cancer. 2010;62(5):668–81. CrossRef
19. Khan N, Jajeh F, Eberhardt EL, Miller DD, Albrecht DM, Van Doorn R, et al. Fisetin and 5-fluorouracil: effective combination for PIK3CA-mutant colorectal cancer. Int J Cancer. 2019;145(11):3022–32. CrossRef
20. Guo G, Zhang W, Dang M, Yan M, Chen Z. Fisetin induces apoptosis in breast cancer MDA-MB-453 cells through degradation of HER2/neu and via the PI3K/Akt pathway. J Biochem Mol Toxicol. 2019;33(4):e22268. CrossRef
21. Ashe PC, Berry MD. Apoptotic signaling cascades. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):199–214. CrossRef
22. Afroze N, Pramodh S, Shafarin J, Bajbouj K, Hamad M, Sundaram MK, et al. Fisetin deters cell proliferation, induces apoptosis, alleviates oxidative stress and inflammation in human cancer cells, HeLa. Int J Mol Sci. 2022;23(3):1707. CrossRef
23. Jafarzadeh S, Baharara J, Tehranipour M. Apoptosis induction with combined use of cisplatin and fisetin in cisplatin-resistant ovarian cancer cells (A2780). Avicenna J Med Biotechnol. 2021;13(4):176–82.
24. Tsai CF, Chen JH, Chang CN, Lu DY, Chang PC, Wang SL, et al. Fisetin inhibits cell migration via inducing HO-1 and reducing MMPs expression in breast cancer cell lines. Food Chem Toxicol. 2018;120:528–35. CrossRef
25. Toth M, Sohail A, Fridman R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol Biol. 2012;878:121–35. CrossRef
26. Kumar GB, Nair BG, Perry JJP, Martin DBC. Recent insights into natural product inhibitors of matrix metalloproteinases. Medchemcomm. 2019;10(12):2024–37. CrossRef
27. Deng Y, Sriwiriyajan S, Tedasen A, Hiransai P, Graidist P. Anti-cancer effects of Piper nigrum via inducing multiple molecular signaling in vivo and in vitro. J Ethnopharmacol. 2016;188:87–95. CrossRef
28. Buranrat B, Mairuae N, Kanchanarach W. Cytotoxic and antimigratory effects of Cratoxy formosum extract against HepG2 liver cancer cells. Biomed Rep. 2017;6(4):441–8. CrossRef
29. Nakamura H, Takada K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021;112(10):3945–52. CrossRef
30. Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5(5):415–8. CrossRef
31. Kang KA, Piao MJ, Hyun JW. Fisetin induces apoptosis in human nonsmall lung cancer cells via a mitochondria-mediated pathway. In Vitro Cell Dev Biol Anim. 2015;51(3):300–9. CrossRef
32. Rengarajan T, Yaacob NS. The flavonoid fisetin as an anticancer agent targeting the growth signaling pathways. Eur J Pharmacol. 2016;789:8–16. CrossRef
33. Jang KY, Jeong SJ, Kim SH, Jung JH, Kim JH, Koh W, et al. Activation of reactive oxygen species/AMP activated protein kinase signaling mediates fisetin-induced apoptosis in multiple myeloma U266 cells. Cancer Lett. 2012;319(2):197–202. CrossRef
34. Su CH, Kuo CL, Lu KW, Yu FS, Ma YS, Yang JL, et al. Fisetin-induced apoptosis of human oral cancer SCC-4 cells through reactive oxygen species production, endoplasmic reticulum stress, caspase-, and mitochondria-dependent signaling pathways. Environ Toxicol. 2017;32(6):1725–41. CrossRef