INTRODUCTION
Nowadays, the utilization of plants as traditional medicine has been used as an alternative to medicinal compounds. Medicinal plants, with the bioactive content, can be developed into various formulations that potentially give therapeutic effects. Medicines with natural ingredients are confirmed to be safer and have lower toxicity compared to chemical medicines [1,2]. Indonesia, as a country with numerous biological diversities, can be a rich storehouse of different medicinal plants. Medicinal plants used in various kinds of research are found in the family Basellaceae with diverse genera, which have been analyzed in terms of pharmacological activity and developed into traditional medicines [3,4].
Family Basellaceae with the genus Anredera becomes the genus that has the most widespread, with up to 12 species. One of these species, Anredera cordifolia (Ten.) Steenis is growing in tropical and sub-tropical areas [2]. Anredera cordifolia (Ten.) Steenis, or known as Binahong, originated in the United States of America [5]. The spread of Binahong in various countries has different names, such as “luo kui shu” in China, “Madeira ranker” in South Africa, and “heartleaves Madeira vine” in USA [6]. Anredera cordifolia (Ten.) Steenis has been analyzed for its pharmacological activity, especially in the leaf and has been standardized by Farmakope Herbal Indonesia, which makes it easier to be developed into standardized herbal medicine and phytopharmaca [4].
Anredera cordifolia (Ten.) Steenis leaves are empirically believed to have efficacy in healing many diseases in Indonesia’s society, such as helping the post-surgical recovery process become faster, stimulating blood circulation, overcoming indigestion disease, boosting the immune system, and facilitating micturition. Anredera cordifolia (Ten.) Steenis leaves can also cure several diseases such as hypertension, diabetes mellitus, gout, kidney disease, heart inflammation, and preventing stroke [6]. However, some activities have scientifically been researched, such as anti-bacterial, antioxidant, wound-healing ability, and anti-inflammatory activity [6–9].
Several studies describe the activity of A. cordifolia (Ten.) Steenis, with a focus on its anti-inflammatory and wound-healing properties, has been reported in scientific journals. These studies were conducted in vitro, in vivo, in silico, and in clinical study settings [10]. Determining the Anredera cordifolia (Ten.) Steenis secondary metabolites responsible for these properties are crucial for the development of medicinal products [7,8]. The development of these medicinal plants can therefore be guided by research describing the secondary metabolites responsible for their anti-inflammatory and wound-healing properties, which will facilitate their registration as traditional medicines and their commercial distribution. Scientific information on the secondary metabolites responsible for A. cordifolia (Ten.) Steenis leaves anti-inflammatory and wound-healing effects will be included in this review.
METHODOLOGY
The process of literature discovery is related to the scientific data on bioactive content and anti-inflammatory activity through Google Scholar and the PubMed database. In seeking the most current information, the literature search was based on recent studies from the past decade to support this research. The keywords in database discovery were “A. cordifolia (Ten.) Steenis; phytochemicals content”, “Anti-inflammatory activity of A. cordifolia (Ten.) Steenis”, “Anti-inflammatory mechanism of A. cordifolia (Ten.) STeenis”, “Wound healing of A. cordifolia (Ten.) Steenis”, and “Compound isolation in A. cordifolia (Ten.) Steenis”. The scientific data relevant to the active metabolites associated with anti-inflammatory activity and wound-healing properties are presented in Table 2. This study exclusively includes data from scientific journals that are at least at Sinta 4 level and real research articles. This data did not enclose the data from a thesis or unpublished article.
Botanical aspect
Anredera cordifolia (Ten.) Steenis is included in spreading succulent plants which can be 5 m long. The root is in the form of a rhizome with a soft structure and can be propagated in a vegetative or generative way. This plant has a cylindrical, soft structure, and reddish green stem. The single leaf has a heart shape, light green in color, with short petioles around 1–2 cm in length, the arrangement of the leaves is alternate, with length around 5–10 cm and width around 3–7 cm. The leaf blade is very thin with a pointed tip, split base, flat or wavy edge, and smooth and slippery surface. The flowers have long stems and appear in the leaf axils, the stalk has a length of around 2–3 mm. The petals are also 2–3 mm with a fragrant aroma, whitish beige in color, and consist of 5 strands [6]. The part of the plant primarily used for its anti-inflammatory activity and wound-healing properties is the leaves.
Bioactive content
Binahong plants, A. cordifolia (Ten.) Steenis has secondary metabolite compounds, such as flavonoid, polyphenols, alkaloid, steroid, glycoside, saponin, tannin, triterpenoid, ascorbic acid, and also contains mono polysaccharides including L-arabinose, D-galactose, L-rhamnose, and D-glucose [6,11]. The data on the secondary metabolite content in the extract of A. cordifolia (Ten.) Steenis leaves are listed in Table 1. Also, secondary metabolites that have active anti-inflammatory and wound-healing activity are attached in Table 2.
Biological activity
Anti-inflammatory activity
Inflammation is the body’s response to damage, trauma, or infection, and involves processes in immunology, physiology, and behavior mediated by cytokines [12]. Proinflammatory cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-a are involved in the inflammatory response [13]. Non-steroidal anti-inflammatory medications, or NSAIDs, and steroids are commonly used in treatment because they can both reduce swelling and relieve pain. On the other hand, prolonged use of NSAIDs can result in gastrointestinal disorders, cardiovascular problems, and chronic kidney disease, while steroids can induce osteoporosis, decreased immunity, and other negative effects. According to the World Health Organization, traditional medicine is used as a substitute by 65% of people worldwide. Plants that contain active ingredients have the potential to yield novel anti-inflammatory medicines, as these compounds can be separated and extracted for therapeutic purposes [14].
Based on the information in Table 2 regarding studies on anti-inflammatory activity and wound-healing properties. Additionally, details regarding secondary metabolites will be provided, including Anredera cordifolia (Ten.) Steenis leaves and the extract or isolate that demonstrated anti-inflammatory properties both in vivo and in vitro. A few anti-inflammatory strategies include the suppression of inflammatory mediators or the inflammatory phase that occurs during the healing process of burns, diabetic wounds, hyperglycemia wounds, and wounds from surgery. Figure 1 summarizes many processes of A. cordifolia (Ten.) Steenis leaves’ anti-inflammatory action and wound healing have been studied in vivo, in vitro, in silico, and in clinical studies.
In vitro studies on anti-inflammatory activity
Inhibition of inflammatory cytokines
The ethanol extract of A. cordifolia (Ten.) Steenis leaves also showed anti-inflammatory activity in human blood sample tests. Human red blood cell samples are mixed with ethanol extract of A. cordifolia (Ten.) Steenis leaves with concentrations of 0.01%; 0.02%; 0.04%; and 0.08% w/v, respectively, under isotonic conditions. It is proven that ethanol extract concentration in A. cordifolia (Ten.) Steenis leaves of 0.01% w/v show its ability to inhibit the hemolysis of red blood cells. Hemolysis of red blood cell membrane that causes inflammation. As the concentration of the extract increases, the extract will be able to stabilize the red blood cell membrane against hemolysis. However, in the ethanol extract of A. cordifolia (Ten.) Steenis, even at the lowest concentration of 0.01% w/v, it was already able to inhibit hemolysis. Oleic acid compound from ethanol extract of A. cordifolia (Ten.) Steenis leaves is known to take a part in inhibiting inflammation [34,46].
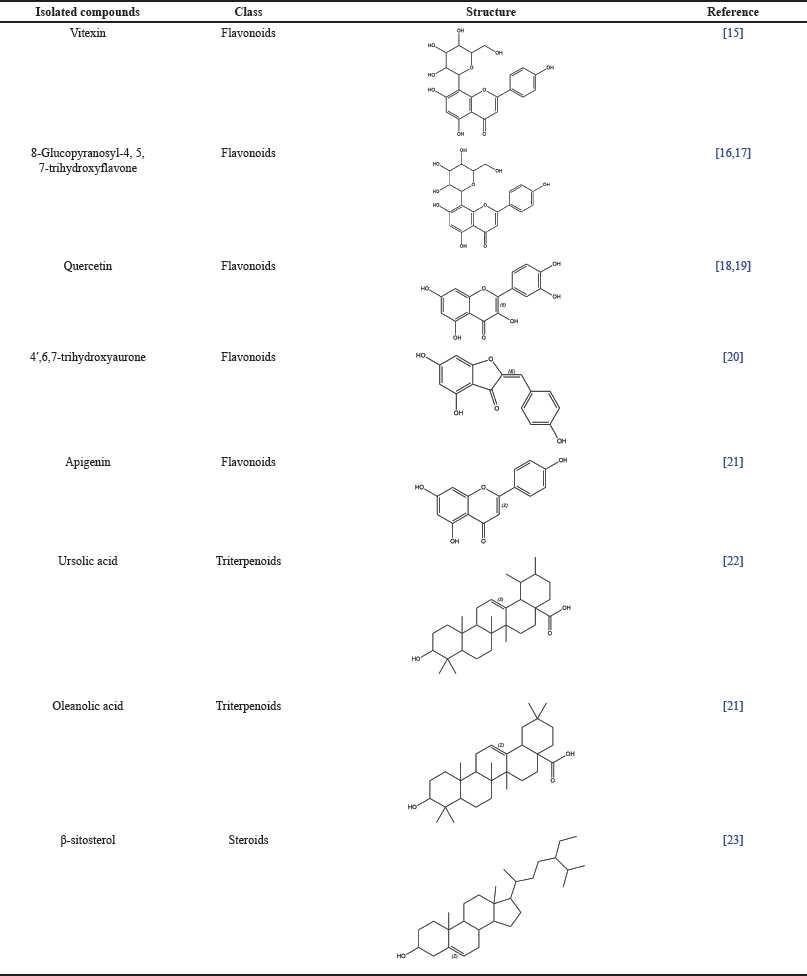 | Table 1. Anti-inflammatory and wound healing compounds reported in A. cordifolia (Ten.) Steenis leaves. [Click here to view] |
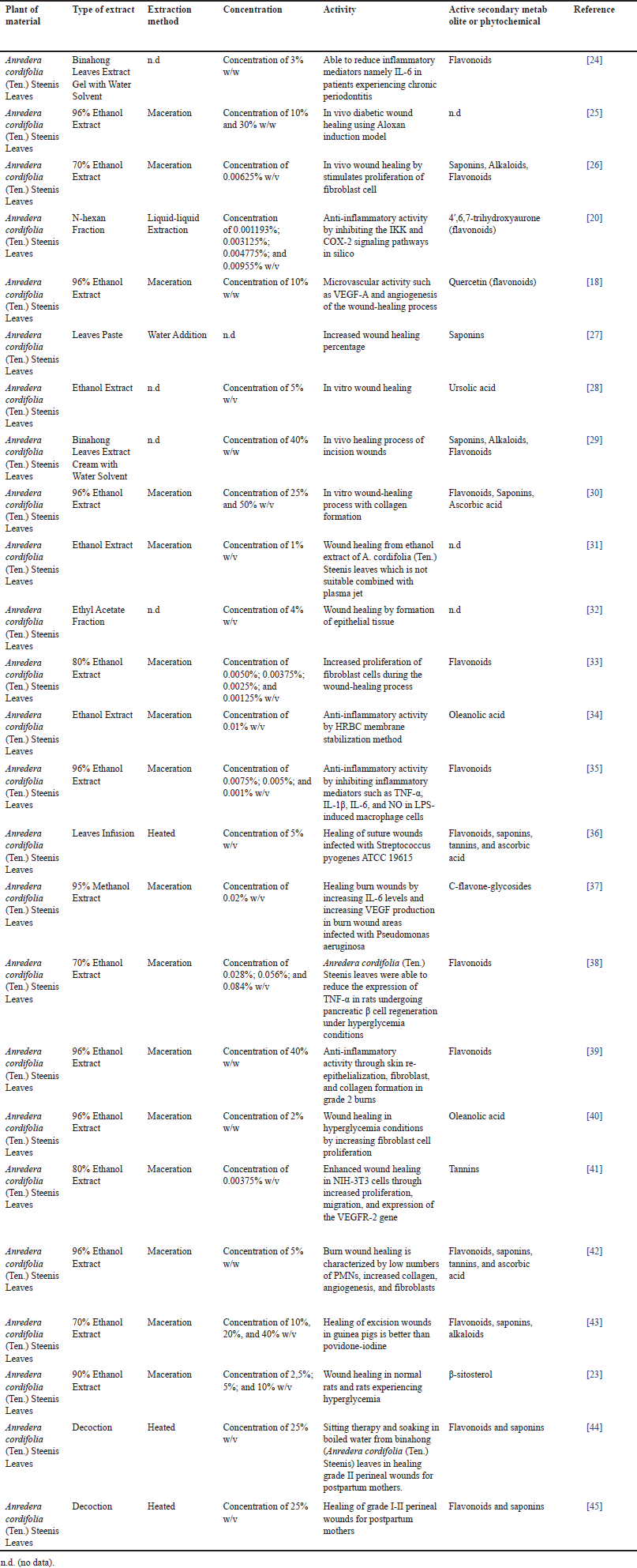 | Table 2. Summarized data of anti-inflammatory and wound healing activity from A. cordifolia (Ten.) Steenis leaves. [Click here to view |
 | Figure 1. (a) Several mechanisms of anti-inflammatory activity and (b) Wound healing. [Click here to view] |
Other research also investigates the mechanism of anti-inflammatory activity by examining the inhibition of inflammatory cytokines in vitro using a 96% ethanol extract of A. cordifolia (Ten.) Steenis leaves. The 96% ethanol extract of A. cordifolia (Ten.) Steenis leaves at concentrations of 0.0075%, 0.005%, and 0.001% w/v was tested on macrophage cell lines (RAW 264.7) induced by Lipopolysaccharide (LPS) to observe its potential in inhibiting the mediators TNF-α, IL-1β, IL-6, and nitric oxide (NO). LPS increases cytokine production as inflammatory mediators. The most significant decrease in TNF-α and IL-1β levels from the 96% ethanol extract of A. cordifolia leaves was observed at a concentration of 0.005% w/v, compared to the negative control as normal cells, with values of 250.3 ± 22.7 and 909.2 ± 19.6 pg/ml, respectively. The reduction in NO levels from the 96% ethanol extract of A. cordifolia (Ten.) Steenis was most notable at a concentration of 0.005% w/v, measuring 22.8 ± 2.1 pg/ml, although this result was still significantly higher than that of normal cells but lower than the positive control, which consisted of LPS-stimulated cells without the extract. Meanwhile, the most significant decrease in IL-6 levels was observed at a concentration of 0.001% w/v, with a value of 217.8 ± 14.7 pg/ml. The data on the active compounds from the 96% ethanol extract of A. cordifolia leaves that act as anti-inflammatory agents has not yet been documented in the journal. However, it has been explained that flavonoid compounds play a role in inhibiting inflammatory mediators [35].
In vivo studies on anti-inflammatory activity
Inhibition of inflammatory cytokines
The condition of diabetes mellitus is closely related to the increase of cytokine levels, such as TNF-α and IL-1β. The increase of TNF-α and IL-β levels occurred in diabetic rats induced by Streptozotocin (STZ) [47]. 70% ethanol extract of dried and powdered A. cordifolia (Ten.) Steenis leaves, given to rats that have been induced by STZ, so can improve the blood glucose level. On this condition, three concentrations of ethanol extract 70% of A. cordifolia (Ten.) Steenis leaves that are boiled with water each as much as 0.028% w/v (P1); 0.056% w/v (P2); and 0.084% w/v (P3) are given. After STZ induction on extract untreated rats, the blood glucose is measured and found to be 278.33 mg/dl (P1); 249.33 mg/dl (P2); and 219 mg/dl (P3), respectively. The improvement of TNF-α expression occurred after the STZ induction and the damage to the pancreas β cell. However, after the allocation of 70% ethanol extract powder of A. cordifolia (Ten.) Steenis leaves, show that the three concentrations obtained the highest concentration of 0.084% w/v, which resulted in the increase of β pancreas cell number by 32.76. On the other hand, a decreased level in P1 group to 115.00 mg/dl; P2 into 116.00 mg/dl; P3 into 102.67 mg/dl also happened. It can be concluded that the therapy of boiled powder ethanol extract water 70% of A. cordifolia (Ten.) Steenis leaves influence in the form of the decrease of TNF-α expression and β cell pancreas regeneration, which can stimulate insulin production [38]. It is comparable to the flavonoid content contained in 70% ethanol extract of A. cordifolia (Ten.) Steenis leaves that actively play a role as the inhibitor, which can prevent TNF-α production and increase insulin secretion by β cell pancreas [48].
In silico study on anti-inflammatory activity
Cyclooxygenase-2 (COX-2) inhibition
Research about the anti-inflammatory activity of A. cordifolia (Ten.) Steenis leaves were not only conducted on the extract, but isolate of A. cordifolia (Ten.) Steenis leaves as well. The n-hexane fraction of A. cordifolia (Ten.) Steenis leaves, tested using in silico methods, showed concentrations of 0.001193%; 0.003125%; 0.004775%; and 0.00955% w/v. In the molecular docking results, it is known that the N-Hexane fraction of A. cordifolia (Ten.) Steenis leaves contain 4′,6,7-trihydroxyaurone, which is the derivative of flavonoids. The possible interactions between compound 4′,6,7-trihydroxyaurone and two specific proteins, COX-2 and inhibitor κB kinase (IKK), have been investigated. Strong binding affinity of 4′,6,7-trihydroxyaurone for IKK protein is shown by the compound’s docking score of −9.0 kcal/mol. In the meantime, a substantial binding affinity for COX-2 protein is shown by the binding score of −8.1 kcal/mol for COX-2 protein. IKK is stimulated by the nuclear factor-kappa B (NF-κB) as a signal that arranges the inflammation. The signaling pathway of the NF-κB is activated by cytokines such as IL-1 and tumor necrosis factors (TNF). COX-2 protein is also a mediator of pro-inflammatory. Hence, the potential of 4′,6,7-trihydroxyaurone compound is significant in blocking IKK and COX-2 signal [20].
Clinical studies on anti-inflammatory activity
Inhibition of inflammatory cytokines
The inflammatory mediator will be stimulated when the inflammation occurs because of the reaction or system changes [49]. The leaf of A. cordifolia (Ten.) Steenis, mainly the extracted one, has proven to inhibit the pro-inflammatory in macrophages induced by LPS. Mediators of pro-inflammatory referred are TNF-α, IL-6, IL-1β, and NO [35]. The extract of A. cordifolia (Ten.) Steenis leaves packaged in a gel preparation with a concentration of 3% w/w have been tested on humans with chronic periodontitis [24]. This chronic periodontitis is a condition where the teeth get infected, which causes tooth inflammation, unadhered teeth, and leading to tooth decay [50]. The investigated values are the decrease of Il-6 level in gingival sulcus fluid, both before and after Scaling and Root Planing (SRP) treatment. The teeth with 3% w/w of A. cordifolia (Ten.) Steenis gel extract proven to experience the reduction of IL-6 level, from 265.79 to 81.19 pg/ml. SRP treatment also helps the reduce process of IL-6 level [24]. The active compound content of A. cordifolia (Ten.) Steenis extract was not specifically known in the research. However, flavonoid is suspected to play a role in inhibiting pro-inflammatory mediators, such as TNF-α, IL-1β, IL-6, COX-2, and NO [51].
Wound-healing activity
The process of wound-healing involves cells that experience four phases, such as hemostasis, inflammation, proliferation, and remodeling. The first phase is the hemostasis phase which involves platelets. The inflammatory phase will secrete the cytokine (TNF, Interferon, and Interleukin) and activate the growth factors [epidermal growth factor, transformation growth factor β (TGF-β), fibroblast growth factor, and platelets derivative growth factor]. The next phase is the proliferation phase which is indicated by the gradual replacement of damaged cells that are dominated by platelets and macrophages. The remodeling phase will maximize the power of structural integrity of new tissue, epithelial growth, and scar tissue formation [33,52].
In vitro studies on wound-healing activity
The research about wound healing from A. cordifolia (Ten.) Steenis leaves, were also examined in vitro, besides through in vivo. It has been proven that 70% A. cordifolia (Ten.) Steenis leaves ethanol extract can heal wounds in NIH-3T3 cells. NIH-3T3 cells are the line of isolated fibroblast cell line NIH/Swiss mice embryos. The increase of proliferation in 3T3 cells was observed in the experimental group with concentrations of 0.00625%; 0.0125%; 0.025%; 0.05%; and 0.1% w/v. The highest proliferation level is at the concentration of 0.00625% w/v with a value of 127.89 ± 16.12 compared to the positive control, Aloclair® gel. The increase in proliferation in fibroblast cells will support wound closure faster. Its important role in the wound-healing process is because fibroblast proliferation can contribute to the formation of granulation tissue and collagen deposition, which are essential for wound repairActive compounds in the 70% ethanol extract A. cordifolia (Ten.) Steenis leaves that play a significant role in enhancing the proliferation of NIH-3T3 fibroblast cells include saponins, alkaloids, and flavonoids [26,53].
It is also supported by research on the flavonoid content of 80% A. cordifolia (Ten.) Steenis leaves ethanol extract which when combined with 80% ethanol extract of Artocarpus lacucha Buch. -Ham. will result in proliferation-increasing activity in NIH-3T3 cells. The combination of extracts at the concentration of 0.00375% w/v becomes the most effective compared to the combination of concentrations of 0.0050%; 0.0025%; and 0.00125% w/v in stimulating proliferation increase. The percentage increase in the proliferation phase is around 125.44% ± 0.38% [33]. Deeper in vitro wound-healing research on NIH-3T3 cells is also conducted on 80% A. cordfolia leaves ethanol extract at a concentration of 0.00375% w/v combined with A. lacucha Buch. -Ham. The observation is on the secretion of growth factors, namely excretion of VEGFR-2, the improvement of cell proliferation and migration. The combination of the two extracts contributes to proliferation increase values after cell incubation for 24, 48, and 72 hours, which are 124.33% ± 0.32%; 128.52% ± 0.41%; and 118.35% ± 0.22%, respectively. Meanwhile, the effect on VEGFR-2 expression is around 1.58 ± 0.02. The tannin content in the extract has the potential to migrate fibroblast tissue, so it can increase VEGFR-2 [41].
In vivo studies on wound-healing activity
Incision/excision wound and infection healing
Wound does not only occur in soft tissue only, but it can also injure hard tissue. In post-tooth extraction, pain and swelling will probably be felt, even if the wound is long-lasting. The initial phase when experiencing wound is signed by the inflammation as the preparation of the wound-healing process. Granulation tissue and fibroblast are also constructed and result in the growth factor, VEGF-A. VEGF-A is an important mediator in angiogenesis formation and is called an important factor in wound healing. The distribution of 96% ethanol extract of A. cordifolia (Ten.) Steenis leaves with a concentration of 10% w/w has proven to be able to improve VEGF-A expression on the third and seventh day by 17.30 ± 2.75 and 14.50 ± 2.99, respectively, compared to the controlling group which is only 8.10 ± 3.34 in third day and 8.60 ± 2.36 in seventh day. The mean expression of VEGF-A on the third and seventh days was twice as high as that of the control group. If the amount of VEGF-A increases on the third day, it indicates that a response has already occurred in the wound area and the formation of new blood vessels has begun by the seventh day. Similarly, angiogenesis will occur more rapidly. Quercetin, which is a flavonoid derivative, has been studied for its role in enhancing the expression of VEGF-A. Quercetin, which is a flavonoid derivation, is investigated to take a part in increasing VEGF-A expression [18].
Incisional wounds or cut wounds are cases that frequently occur and almost carried out by veterinarians every day. Incisional wounds can be caused by an accident experienced by the animal itself, medical treatment as in surgery. Every surgery that involves the skin uses an incision as the method of opening the skin. Research on incision wounds has been proven effective with the leaf paste of A. cordifolia (Ten.) Steenis, the hydrogel of ethanol extract from A. cordifolia (Ten.) Steenis leaves, as well as the cream made from the extract of A. cordifolia (Ten.) Steenis leaves. The saponin content A. cordifolia (Ten.) Steenis leaf paste can increase TGF-β1 activity, which is one of the factors of growth. The giving of A. cordifolia (Ten.) Steenis leaves paste twice a day in 12 seems to give the biggest effect. It can be seen from the wound healing percentage of 76% compared to the positive controlling group, povidone-iodine, which is only 48%. It also applies to ursolic acid content in A. cordifolia (Ten.) Steenis leaves ethanol extract hydrogel that gives incision wound-healing effect with a diameter of 7.854 ± 0.010 seconds after the treatment. In wound healing, ursolic acid will stimulate the peroxisome proliferator-activated receptor-α expression. The 40% w/w concentration of the A. cordifolia (Ten.) Steenis leaves cream extract also gives effect, in the form of decreasing the diameter of wound incision by 1.1954 ± 1.222928. Flavonoid compounds might contribute to the wound-healing process [27–29].
The treatments commonly used for wound healing are still chemical substances. However, the utilization of chemical medicine topically can give adverse effects, including irritation on the skin surface, allergic reactions, and mild pain. Plasma jet is known to have activity in expediting mice’s wound healing, especially in inflammatory, re-epithelization, and wound contraction phases. The combination of the extract with the plasma jet should be able to enhance the potential to improve the plasma jet’s performance in expediting wound healing through re-epithelization improvement percentage. The concentration of A. cordifolia (Ten.) Steenis leaves ethanol extract that was used is 1% w/v with 14 days of testing on excision-wounded mice. On the 14th day, the combination of A. cordifolia (Ten.) Steenis leaves extract and plasma jet is able to reach 100% in re-epithelization. On the other hand, the decrease of Malondialdehyde level, which is a biomarker of oxidative stress, occurred and hindered the wound-healing process. Macroscopic observation also verifies that the combination of A. cordifolia (Ten.) Steenis leaves ethanol extract and plasma jet provide less wound diameter closure than only A. cordifolia (Ten.) Steenis leaves ethanol extract. This is due to the antioxidant content of A. cordifolia (Ten.) Steenis leaves and the oxidative properties in jet plasma, which when combined, will reduce the wound-healing effects [31].
The ethyl acetate fraction of A. cordifolia (Ten.) Steenis leaves at a concentration of 4% w/v have been proven to heal incision wounds by enhancing epithelialization during the proliferation phase. Increased proliferation can be seen on the fourth day after the treatment of the incision wound, with an average of 2.500 ± 0.548. The proliferation phase in the wound-healing process, happens from the third until the fourteenth day and the epithelization process of the incision wound occurs in the first 48 hours [32]. Another study found that 96% ethanol extract of A. cordifolia (Ten.) Steenis leaves at concentrations of 25% and 50% w/v stimulates the collagen formation in the wound-healing process experienced by a mouse that infected with Staphylococcus aureus. The thickness of collagen formation after the administration of 96% ethanol extract of A. cordifolia (Ten.) Steenis leaves reached 50% w/v, indicated by a blue color in the wound area [30]. The study explains that the active flavonoid compounds contained in the extract of A. cordifolia (Ten.) Steenis leaves may play a role in wound healing, particularly during the proliferation and remodeling phases, as well as in collagen formation.
The leaves of A. cordifolia (Ten.) Steenis was prepared in the form of an infusion with concentrations of 5% and 20% w/v for treatment on rats infected with Streptococcus pyogenes in stitched wounds, and then the wound-healing process was observed after treatment. The observation is conducted over 8 days, but on the fifth day, there is a change in the suture wound part after the infusion of A. cordifolia (Ten.) Steenis leaves are given. The infusion supply group of 5% w/v A. cordifolia (Ten.) Steenis leaves serves the most significant effect or can be said as the best compared to the infusion supply group of 20% w/v A. cordifolia (Ten.) Steenis leaves, as seen from the best wound closure. The flavonoid, saponin, tannin, and ascorbic acid content play an important part in the wound-healing process. Flavonoids are important in increasing fibroblast cells and new tissue formation. Saponin takes part in increasing the proliferation and releasing the growth factors. Then, tannin plays a role in stopping the bleeding. Besides, ascorbic acid is necessary for collagen formation [36,54].
Excision wounds are related to the process of broader tissue loss that is caused by sharp things. The excision wound healing has been analyzed on 70% of A. cordifolia (Ten.) Steenis leaves ethanol extract with concentrations of 10%, 20%, and 40% w/v. The examination was conducted on a guinea pig that was given excision wound treatment around 2 cm long in its back area. The observation of the wound-healing process is organized through wound length measurement after the treatment that is compared to the positive control, povidone-iodine, in 15 days. After the observation in 15 days, the best wound healing is in the 40% w/v concentration reach 100% healed, 20% w/v concentrate reaches 86.67%, and 10% w/v concentrate reaches 81.67%; meanwhile, the positive control only reaches 68.33%. It might be caused by the flavonoid, saponin, and alkaloid content in A. cordifolia (Ten.) Steenis leaves extract that takes a part in wound healing, but the compound with the highest potential has not been found yet [43].
Wound healing for diabetic conditions
Diabetes mellitus becomes one of the causes of chronic diseases if someone gets complications. One of the complications caused by Diabetes Mellitus is diabetic wounds. It is defined as the necrosis of tissue or dead tissue that is induced by the embolism of a large blood vessel in a particular part of the body that stops blood supply. It can be happened as the result of prolonged inflammation so it requires more time to heal the wound [55]. Anredera cordifolia (Ten.) Steenis is utilized as one of the traditional medicine alternatives in diabetic wound conditions. The application of 96% A. cordifolia (Ten.) Steenis leaves ethanol extract has been proven to heal diabetic wounds. The concentrates are around 10% and 30% w/w packed in gel supply, then tested on the mice that have been given diabetic condition treatment using Alloxan induction, then subsequently given wound treatment. The decrease in the diameter of diabetic wounds is observed on 1st, 6th, 12th, and 19th day. The treatment groups of both 10% and 30% w/w concentrations significantly experience wound diameter decrease. On the 19th day, the 30% w/w concentration extract makes the diameter of the wound become 0.2 cm, this is not much different from the 10% w/w concentration which is only 0.3 cm. Hence, it can be concluded that 10% w/w concentration is able to provide a good and efficient effect on the diabetic wound-healing stage compared to the positive control, chloramphenicol [25].
The diabetic wound healing also possessed 96% A. cordifolia (Ten.) Steenis leaves ethanol extract with 2% w/w concentration that is packed in the cream supply. The mechanism of the diabetic wound-healing process is conducted through the improvement of fibroblast tissue proliferation. Rabbits are used as the tested animal giving alloxan as the hyperglycemia model. The observation of wound diameter in the experimental group compared to a positive control group, Sanoskin® cream, showed that the average of the experimental group is 99.61 ± 84.99 which is similar to the positive group, 96.33 ± 83.13 on average [40].
Acute diabetic wounds are not as bad as chronic diabetic wounds which are extremely difficult to heal. Acute diabetic wounds usually take 14 days to heal. The giving of 90% A. cordifolia (Ten.) Steenis leaves ethanol extract with concentrations of 2.5%, 5%, and 10% w/v have been evidenced to make the acute diabetic wound-healing process faster. The treatment on mice is done through the STZ application to treat hyperglycemia conditions, with 14 days of observation after the extract application. The result shows that 10% concentrate is much better in making the wound heal quicker compared to 2.5% and 5% w/v concentrate. The higher the extract’s concentration, the higher the active compound that is attracted to the extract. Active compounds that have potential in wound healing are B-sitosterol, part of steroid and phenolic, saponin, and oleic acid which are involved in triterpenoid [23].
Burns healing
Burn is the shape of tissue damaged or lost which is caused by the contact with heat sources, such as fire, hot water, chemical materials, electricity, and radiation. Burn is a wound that occurs due to direct and indirect contact of the body surface with objects that produce heat. Burn is a type of trauma that has high morbidity and mortality, so it needs specialized care from the initial to the advanced phase. The wound will cause inflammation and infection, problems in wound closure on extensive bare or non-epithelialized wounds and/or in structures or functional organs, and hypermetabolism [56].
In this inflammatory phase, IL-6 will be secreted by macrophage cells as a sign of inflammation after injury. Macrophage cells will stimulate the secretion of growth factor, VEGF, which takes a core role in enhancing the proliferation phase and the formation of new tissue. The research found that about 95% of A. cordifolia (Ten.) Steenis leaves methanol extract on mice that are treated to have burned and infected by Pseudomonas aeruginosa. The 0.02% w/v concentration 95% A. cordifolia (Ten.) Steenis leaves methanol extract is able to increase IL-6 and VEGF secretion compared to the controlling group. The increase of IL-6 secretion in the experimental group is around 1.40 ± 0.22 compared to the control group which is only 0.42 ± 0.12. In VEGF secretion, the experimental group is 27.99 ± 2.15, which is higher than the control group that only reached 14.44 ± 0.56. C-flavone-glycosides compounds from A. cordifolia (Ten.) Steenis leaves actively play a role in burn healing by optimizing the IL-6 secretion in the inflammatory phase and acceleration in the proliferation phase [37].
Burns have 4 degrees, namely degree 1 for burns that damage the epidermis, degree 2 for reaching the shallow of the epidermis or one-third of the superficial dermis, then degree 2 also for wounds that cause deep damage to two-thirds of the superficial dermis and underlying tissue. Furthermore, in degree 3, the burn causes damage to all layers of the skin (dermis and epidermis) and deeper layers. Grade 4, the highest degree, which means damage to the entire skin layer and surrounding structures such as subcutaneous fat, fascia, muscle, and bone [56]. Burn wound-healing research has been proven to be effective on 96% of A. cordifolia (Ten.) Steenis leaves ethanol extract with a concentration of 40% w/w that was packed both in cream or oral suspension on mice that were treated degree 2 burn model. 96% An. cordifolia (Ten.) Steenis leaves ethanol extract in cream supply has better effectivity compared to oral suspension with an average of re-epithelization thickness around 43.45 µm. On the contrary, oral suspension only reaches an average of re-epithelization thickness at 28.46 µm. Microscopically also observed the formation of fibroblast and collagen tissue. The flavonoid content, especially quercetin, saponins, and ascorbic acid are considered to play a vital role in burn wound healing [39].
The giving of 96% A. cordifolial (Ten.) Steenis leaves ethanol extract in the form of cream supply with a concentration of 5% w/w, which scientifically also has potential in burn healing. The burn healing model is applied to white mouse to easier wound healing observation through histolatologic tissue collection. Observation variables are collagen deposition score, PMN score, angiogenesis degree, and fibrosis. Collagen deposition score and PMN score are 2.0 ± 0.00 and 2.0 ± 0.00, respectively. It is related to the secondary metabolite of A. cordifolia (Ten.) Steenis leaves, namely flavonoid, saponin, tannin, and ascorbic acid [42,57,58].
Clinical studies on wound healing activity
Post-surgery wound healing
After giving birth, a mother will experience the postpartum period, which tends to experience perineal injury due to spontaneous injury or episiotomy after childbirth. In perineal injuries, there will be wounds, which can cause infection and inhibit the wound-healing process if not properly treated. The healing process of postpartum wounds must be treated immediately, and the utilization of traditional medicine can be an option for healing postpartum wounds. Indonesian medicinal plants such as binahong leaves or known as A. cordifolia (Ten.) Steenis are believed to help the wound-healing process quicker. Based on research that has been conducted, the use of A. cordifolia (Ten.) Steenis leaves processed into a decoction supply with a concentration of 25% w/v are proven to be able to heal postpartum wounds. This decoction is a plant extraction process carried out by boiling the plant parts used at a certain temperature. The results prove that A. cordifolia (Ten.) Steenis leaves decoction can heal degrees 1 and 2 perineal wounds in postpartum maternal care. Flavonoid and saponin as secondary metabolites contained in A. cordifolia (Ten.) Steenis leaves are believed to play a role in healing postpartum wounds [44,45].
CONCLUSION
The literature review indicates that A. cordifolia (Ten.) Steenis leaves play a significant role in both anti-inflammatory and wound-healing activities. The secondary metabolites found in these leaves include flavonoids, alkaloids, tannins, steroids, triterpenoids, and saponins. Several chemical structures of the secondary metabolites, whether extracted or isolated, contribute actively to anti-inflammatory and wound-healing effects. Anti-inflammatory activity has been studied through in vitro, in vivo, in silico, and clinical studies, focusing on mechanisms such as inhibiting inflammatory cytokines. Wound-healing activity also has been explored in vitro, in vitro, and clinical study, both in incision/excision wound and infection healing, diabetic wound healing, burn healing, and post-surgery wound healing. However, the lack of studies directly linking secondary metabolites to pharmacological activity poses a challenge in presenting comprehensive scientific data. Further research is required to be conducted to discover more about secondary metabolite content in A. cordifolia (Ten.) Steenis leaves that role in anti-inflammatory and wound-healing activity, as well as in other pharmacological effects.
ACKNOWLEDGMENT
The authors show their gratitude to the Faculty of Pharmacy Sanata Dharma University for the great support in writing this article.
AUTHOR CONTRIBUTIONS
All of the authors gave great contributions toward the conception and design, data collection, and data analysis and interpretation; took part in writing the article and revised it critically; agreed to submit the article to this journal; gave final agreement upon the published version of the article; and agreed to be responsible on all of the aspects. All authors have filled the requirement to be the author based on guidance from the International Committee Medical Journal Editor (ICMJE).
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors reported that there was no conflict of financial importance or other conflict of importance in writing this article.
ETHICAL APPROVALS
This research did not involve experiments on animals or humans as subjects.
DATA AVAILABILITY
All of the collected and analyzed data are attached to this article.
PUBLISHER NOTE
This journal remains neutral with respect to jurisdictional claims in the affiliation of published institutions.
REFERENCES
1. Salim A, Kristanto DF, Subianto F, Sundah JE, Jamaica PA, Angelika T, et al. Phytochemical screening and therapeutic effects of Binahong (Anredera cordifolia (Ten.) Steenis) Leaves. Indones J Life Sci. 2021;3(2):43–55.
2. Alba TM, de Pelegrin CMG, Sobottka AM. Ethnobotany, ecology, pharmacology, and chemistry of Anredera cordifolia (Basellaceae): a review. Rodriguesia. 2020;71:1–11.
3. Dewi NKSM, Fakhrudin N, Wahyuono S. A comprehensive review on the phytoconstituents and biological activities of Nyctanthes arbor-tristis L. J Appl Pharm Sci. 2022;12(8):9–17.
4. Indonesia KKR. Farmakope herbal Indonesia. Edisi II. Jakarta, Indonesia: Direktorat Jenderal Kefarmasian dan Alat Kesehatan; 2017.
5. Weng Z, Shen S. Complete chloroplast genome characterization and phylogenetic analysis of Anredera cordifolia (Tenore) Steenis (Basellaceae). Mitochondrial DNA Part B Resour. 2021;6(7):1867–8.
6. Tedjakusuma F, Lo D. Functional properties of Anredera cordifolia (Ten.) Steenis: a review. IOP Conf Ser Earth Environ Sci. 2022;998(1):1–10.
7. Nawawi RA, Kamaluddin MT, Theodorus. In vivo efficacy of topical Binahong (Anredera cordifolia (Ten.) Steenis) leaf ethanolic extract on transforming growth factor-beta 1 in infected wounds. Asian J Pharm Clin Res. 2021;14(3):99–02.
8. Kurniawan B, Carolia N, Pheilia A. The effectiveness of Binahong leaf extract (Anredera cordifolia (Ten.) Steenis) and mefenamic acid as anti inflamation to white male rat induced by karagenin. Juke. 2014;4(8):151–7.
9. Rodríguez ET. In vitro antibacterial activity of dried extract from Anredera vesicaria rhizomes. Adv Plants Agric Res. 2018;8(3):237–9.
10. Silalahi M. Utilization and bioactivity of Anredera cordifolia (Ten.) Steenis. GSC Biol Pharm Sci. 2024;26(2):128–34.
11. Nury DF, Widjaja T, Kurniawan F. The effect of Binahong (Anredera cordifolia (Ten.) Steenis) medicinal plant extract addition on glucose detection. J Sains dan Seni ITS. 2019;8(2):15–20.
12. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–18.
13. Ahmed AU. An overview of inflammation: mechanism and consequences. Front Biol China. 2011;6(4):274–81.
14. Reis C, Barreto M, Menezes de Faria Pereira S, Leandro da Cruz L, De Souza Passos M, Pereira de Moraes L, et al. Plants as sources of anti-inflammatory agents. Molecules. 2020;25(3726):1–22.
15. Mulia K, Muhammad F, Krisanti E. Extraction of vitexin from binahong (Anredera cordifolia (Ten.) Steenis) leaves using betaine - 1,4 butanediol natural deep eutectic solvent (NADES). AIP Conf Proc. 2017;1823:1–4.
16. Musyaropah R, Supriyatna A. Efektivitas Daun Binahong (Anredera scandens (L.)Moq) Sebagai Obat Penyembuhan Berbagai Luka: review literature. Idea Health J. 2023;3(02):49–54.
17. Djamil R, Winarti W, Zaidan S, Abdillah S. Antidiabetic activity of flavonoid from Binahong leaves (Anredera cordifolia) extract in alloxan induced mice. J Pharmacogn Nat Prod. 2017;3(2):1–4.
18. Khoswanto C. Microvascular activity from the wound healing process in Wistar Rats due to administration of Anredera cordifolia (Ten.) Steenis. J Int Dent Med Res. 2021;14(4):1351–6.
19. Dias MC, Pinto DCGA, Silva AMS. Plant flavonoids: chemical characteristics and biological activity. Molecules. 2021;26(17):1–16.
20. Febriansah R, Lakshita HA. Co-chemotherapeutic effect of n-hexane fraction of binahong (Anredera cordifolia [Tenore] steen.) on wider colon cancer cell line. Open Access Maced J Med Sci. 2021;9(T4):77–82.
21. Sukandar EY, Ridwan A, Sukmawan YP. Vasodilation effect of oleanolic acid and apigenin as a metabolite compound of Anredera cordifolia (Ten) V. Steenis on isolated rabbit aortic and frog heart. Int J Res Ayurveda Pharm. 2016;7(5):82–4.
22. Hanafiah OA, Hanafiah DS, Dohude GA, Satria D, Livita L, Moudy NS, et al. Effects of 3% binahong (Anredera cordifolia) leaf extract gel on alveolar bone healing in post-extraction tooth socket wound in Wistar rats (Rattus norvegicus). F1000Research. 2022;10:1–23.
23. Situmorang GA, Yamamoto Z, Ichwan M, Prayugo B. Anredera cordifolia leaves extract accelerates the wound healing of normal and hyperglycemic rats. Pharmaciana. 2022;12(1):39–48.
24. Syahfery I, Nasution AH, Satria D, Ervina I, Hanafiah OA. Evaluation of il-6 levels after subgingival application Binahong leaf extract gel 3% on patients of chronic periodontitis. Int J Appl Dent Sci. 2020;6(3):615–8.
25. Kintoko K, Desmayanti A. The effectivity of ethanolic extract of binahong leaves (Anredera cordifolia (Tenore) steen) gel in the management of diabetic wound healing in aloxan-induced rat models. J Kedokt dan Kesehat Indones. 2016;7(5):227–36.
26. Hanafiah OA, Abidin T, Ilyas S, Nainggolan M, Syamsudin E. Wound healing activity of binahong (Anredera cordifolia (Ten.) Steenis) leaves extract towards NIH-3T3 fibroblast cells. J Int Dent Med Res. 2019;12(3):854–8.
27. Gurcharan Singh GK a/p, Utami NV, Usman HA. Effect of topical application of Binahong [Anredera cordifolia (Ten.) Steenis] leaf paste in the wound healing process in mice. Althea Med J. 2014;1(1):6–11.
28. Istyastono EP, Yuliani SH. Scarless wound healing gel with Binahong (Anredera cordifolia (Ten.) Steenis) leaves extract and celecoxib as the active ingredients. AIP Conf Proc. 2016;1755:1–5.
29. Winarjo G, Arifin F, Oenarta D. Comparison of the effectiveness of giving Binahong leaf (Anredera cordifolia (Ten.) Steenis) and Papaya leaf (Carica papaya) on skin wound healing in White Rat (Rattus novergicus). J Widya Med Jr. 2021;3(2):78–85.
30. Nuroini F, Ratnaningrum K, Soejoto MRA, Dewi SS, Putri GSA. Binahong leaf extract activity in the 8th day of wound healing infected with Staphylococcus aureus towards collagen tissue. J Teknol Lab. 2021;10(2):124–30.
31. Darmawati S, Nasruddin N, Kurniasiwi P, Mukaromah AH, Iswara A, Putri GSA, et al. Plasma jet effectiveness alteration in acute wound healing by binahong (Anredera cordifolia) extract. Plasma Med. 2020;10(4):259–71.
32. Meriyanti PS, Kamaluddin MT, Theodorus. The topical effect of Binahong fraction leaves [Anredera cordifolia (Ten.) Steenis] on increased epithelization and hydroxyproline level at incision wound in rats. Biomed J Indones. 2020;6(1):1–9.
33. Nazliniwaty, Hanafiah OA, Pertiwi D, Muhammad M, Satria D. Antioxidant and cell proliferation induction activities combination of hydroalcohol extract of Artocarpus lacucha Buch. Ham. leaves and Anredera cordifolia (Ten) Steenis. Leaves. Rasayan J Chem. 2022;15(2):1563–6.
34. Sutrisno E, Ketut Adnyana I, Sukandar EY, Fidrianny I, Aligita W. Anti-inflammatory study of Anredera cordifolia leaves and Centella asiatica herbs and its combinations using human red blood cell-membrane stabilization method. Asian J Pharm Clin Res. 2016;9(5):78–80.
35. Laksmitawati DR, Widyastuti A, Karami N, Afifah E, Rihibiha DD, Nufus H, et al. Anti-inflammatory effects of Anredera cordifolia and Piper crocatum extracts on lipopolysaccharide-stimulated macrophage cell line. Bangladesh J Pharmacol. 2017;12(1):35–40.
36. Baso SNAS, Azizah N, Rosyidah R, Rinata E. Binahong [Anredera cordifolia (Tenore) Steenis] leaf infusa for suture wound infection convalescence. Indones Midwifery Heal Sci J. 2023;7(4):302–16.
37. Sukrama DM, Wihandani DM, Manuaba AP. Topical Binahong (Anredera cordifolia) leaf extract increases interleukin-6 and VEGF (Vascular endothelial growth factor) during burn wound healing in Wistar Rats infected with Pseudomonas aeruginosa. Biol Med. 2017;9(1):1–6.
38. Latuhihin YG, Watuguly T, Kakisina P, Kustarini Samsuria I. Potential of Binahong (Anredera cordifolia [Tenore] Steen) in reducing TNF-α expression on regeneration of pancreas β cells on White Rats (Rattus norvegicus) diabetes mellitus models. J Biosci Med. 2020;08(06):37–49.
39. Hapsari AF, Setyaningrum DAW, Wardhani RP, Alfina A, Ginting RB, Dzakiy WA. Burn healing with Binahong (Anredera cordifolia (Tenore) Steenis) leaves extract as a topical and systemic treatments. Adv Heal Sci Res. 2017;10:5–9.
40. Sutrisno E, Sukandar EY, Irda Fidrianny IKA. Wound healing in vivo and in vitro study of Binahong leaves (Anredera cordifolia (Ten.) Steenis) and Pegagan (Centella asiatica (L.) Urban) ethanolic extract. Pharmacologyonline. 2018;1:111–6.
41. Nazliniwaty N, Hanafiah OA, Pertiwi D, Muhammad M, Satria D. The activity of combination of ethanol Extract of Artocarpus lacucha Buch.-Ham and Anredera cordifolia Steenis leaves to increase wound healing process on NIH-3T3 cell line. Open Access Maced J Med Sci. 2022;10(A):807–11.
42. Yuniarti WM, Lukiswanto BS. Effects of herbal ointment containing the leaf extracts of Madeira vine (Anredera cordifolia (Ten.) Steenis) for burn wound healing process on albino rats. Vet World. 2017;10(7):808–13.
43. Miladiyah I, Prabowo BR. Ethanolic extract of Anredera cordifolia (Ten.) Steenis leaves improved wound healing in guinea pigs. Universa Med. 2012;31(1):4–11.
44. Zulmi D, Septiani L, Soepardan S. The effect of sitting and soaking therapy with binahong leaf (Anredera cordifolia) decoction on perineal wound healing. Maj Obstet Ginekol. 2019;27(1):28–33.
45. Novelia S, Syamsiah S, Kurnia N. The effect of Pineapple juice on perineal wound healing among post partum women. Nurs Heal Sci J. 2023;3(1):32–6.
46. Serrano JL, Figueiredo J, Almeida P, Silvestre S. From xanthine oxidase inhibition to in vivo hypouricemic effect: an integrated overview of in vitro and in vivo studies with focus on natural molecules and analogues. Evidence-Based Complement Altern Med. 2020;2020:9–11.
47. Randeria SN, Thomson GJA, Nell TA, Roberts T, Pretorius E. Inflammatory cytokines in type 2 diabetes mellitus as facilitators of hypercoagulation and abnormal clot formation. Cardiovasc Diabetol. 2019;18(1):1–15.
48. Ghorbani A, Rashidi R, Shafiee-Nick R. Flavonoids for preserving pancreatic beta cell survival and function: a mechanistic review. Biomed Pharmacother. 2019;111:947–57.
49. Jain P, Pandey R, Shukla SS. Inflammation: natural resources and its applications. India: Springer; 2015. 156 p.
50. Peeran SW, Ramalingam K, Ahmed AM, Abhilash PR, Elhassan A, Ahmed A, et al. Essentials of periodontics & oral implantology. India: Saranraj JPS Publication; 2021. 85 p.
51. Serafini M, Peluso I, Raguzzini A. Session 1: antioxidants and the immune system flavonoids as anti-in?ammatory agents. Proc Nutr Soc. 2010;69:273–8.
52. Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, Conti B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics. 2020;12(8):1–30.
53. Widyarini KD, Sukandar EY, Fidrianny I. Xanthine oxidase inhibitory and antihyperuricemic activities of Anredera cordifolia (Ten) steenis, sonchus arvensis l, and its combination. Int J Pharm Pharm Sci. 2015;7(3):86–90.
54. Kurniawan B, Aryana WF. Binahong (Cassia alata L.) as inhibitor of Escherichia coli growth. Fac Med Lampung Univ. 2015;4(4):100–4.
55. Senneville É, Albalawi Z, van Asten SA, Abbas ZG, Allison G, Aragón-Sánchez J, et al. IWGDF/IDSA guidelines on the diagnosis and treatment of diabetes-related foot infections (IWGDF/IDSA 2023). Clin Infect Dis. 2023;40(3):1–23.
56. Tavares Pereira DDS, Lima-Ribeiro MHM, De Pontes-Filho NT, Carneiro-Leão AMDA, Correia MTDS. Development of animal model for studying deep second-degree thermal burns. J Biomed Biotechnol. 2012;2012:1–7.
57. Alfatinnisa Z, Andriyan MW, Saputra MR, Astuti EP, Sunarno S, Isdadiyanto S, et al. Topical ointment Anredera cordifolia leaves ethanolic extract-loaded nanochitosan promotes wound healing in hyperglycemic rat. 2024;16(1):155–65.
58. Sidhartha E, Yang JJ, Dimara RSN, Rahmawati F, Purba SWD. Phytochemical screening, antioxidant and antifungal activity test of Binahong leaf extract (Anredera cordifolia (Ten.) Steenis). Eur J Adv Chem Res. 2024;5(1):1–8.