INTRODUCTION
Cancer is a complex group of diseases characterized by abnormal cellular proliferation that progress through various stages, transforming normal cells into neoplastic cells. The fundamental traits necessary for tumor development include sustained proliferative signaling, evasion of growth suppressors, acquisition of replicative immortality, potential for invasion and metastasis, induction of angiogenesis, and resistance to cell death. These traits are commonly referred to as the “hallmarks” of cancer. They are associated with specific deregulated genes and molecular mechanisms. As our understanding of the biological processes underlying cancer development and progression deepens, new cancer biomarkers are being identified, providing insights into the disease’s evolution. For instance, changes in DNA methylation, genetic mutations, alterations in microRNA levels, and the presence of circulating proteins or nucleic acids in the blood or tissue can be significant indicators of cancer onset and progression [1,2].
The detection and analysis of neoplastic biomolecular alterations enable early identification of cancer, monitoring its progression, and predicting treatment responses, thereby facilitating more personalized and effective patient care. Consequently, exploring new biomarkers that offer detailed insights is essential for advancing the fight against cancer [1,2].
Squamous cell carcinoma (SCC) is a common type of skin cancer that occurs in both humans and dogs. While this carcinoma often presents benign clinical behavior, it can also exhibit local invasion and metastatic potential characteristics. In most cases, the classical treatment involves the surgical removal of the tumor. However, over time, a growing number of mutations, biomolecules, and pathways associated with SCC have been identified, driving the development of new and more effective therapies [3,4].
Syndecan-1, a heparan sulfate proteoglycan, maintains normal cell morphology. Its interactions with various extracellular and intracellular proteins and its mediation of cell signaling in response to environmental stimuli make it a significant factor in cancer biology. Syndecan-1 is involved in several key processes essential for tumorigenesis, including cell growth and migration, apoptosis, angiogenesis, invasion, and metastasis. In cases of skin cancer, including SCC, the compromised function of Syndecan-1 may significantly contribute to tumor progression [5–7].
This review will discuss the expression Syndecan-1 in SCC and the potential for future therapies based on modulating this marker’s expression.
METHODS
A comprehensive literature search was conducted using specific keywords and multiple databases to investigate the role of Syndecan in SCC and explore potential therapeutic strategies. The primary keywords used in this search included “Syndecan,” “ SCC,” “Syndecan-1,” “Syndecan-4,” “Syndecan inhibition,” “Syndecan gene therapy,” “Syndecan-targeted therapy,” “SCC treatment,” “cancer cell proliferation and migration,” and “extracellular matrix interactions in SCC.” These keywords were strategically combined using Boolean operators (AND, OR) to refine the search and target specific aspects of the research topic. For instance, combinations such as “Syndecan AND SCC,” “Syndecan-1 OR Syndecan-4 AND SCC,” “Syndecan inhibition AND SCC treatment,” and “extracellular matrix AND Syndecan AND SCC” were employed to ensure comprehensive coverage of relevant studies.
The literature search was conducted across multiple databases, including PubMed, Scopus, Web of Science, and Google Scholar, to ensure an extensive collection of relevant studies. This multi-database approach utilized both broad and specific search queries to capture a wide range of articles related to the role of Syndecan in SCC and its therapeutic implications.
After the search, the selected articles were thoroughly reviewed, and relevant data were extracted. The extracted data were then synthesized to comprehensively understand Syndecan’s multifaceted role in SCC and identify promising therapeutic strategies.
CUTANEOUS SQUAMOUS CELL CARCINOMA (CSCC)
SCC is one of the most common malignant neoplasms, frequently occurring in the head and neck, cervix, esophagus, lungs (non-small cell tumors), and skin. This malignancy arises from the uncontrolled growth of epithelial cells and typically occurs in organs lined with squamous epithelium. Studies have shown that, regardless of the tissue of origin, patients with SCC exhibit common molecular characteristics, such as those associated with exposure to ultraviolet (UV) radiation. SCC has a significant tendency to metastasize to nearby lymph nodes and distant organs, posing substantial challenges for both treatment and management of the disease [3].
CSCC, a type of keratinocyte carcinoma found in the epidermis or associated structures, is the most common form of non-melanoma skin cancer in both humans and dogs [4].
Risks associated with the development of CSCC
Several risk factors are associated with CSCC, which can be environmental, genetic, or epigenetic. UV radiation is one of the primary contributors to this type of cancer [8]. Other risk factors include male gender, advanced age, fair skin, immunosuppression, chronic skin ulcers, HPV infection, smoking, certain medical conditions (such as chronic lymphocytic leukemia and non-Hodgkin’s lymphoma), use of BRAF inhibitors, chronic skin inflammation, and genetic skin disorders like recessive dystrophic epidermolysis bullosa [9,10]. The complex molecular mechanisms associated with CSCC and its high mutational burden can lead to various clinical manifestations, including Bowen’s disease, erythroplasia of Queyrat, and Bowenoid papulosis [9,11].
Carcinogenesis and molecular changes in CSCC
Understanding the progression of CSCC requires identifying the genes involved and investigating new therapeutic strategies. CSCC is characterized by a high mutational load, often resulting from exposure to UV radiation, as discussed earlier [12]. Endogenous factors, including errors in DNA repair, genome editing, mitotic errors, and reactive oxygen species, can cause mutations. For CSCC to develop, mutations must occur in long-lived cells within the epidermal compartment, particularly in the basal layer [12,13].
One of the earliest genetic alterations in CSCC is the TP53 tumor suppressor gene mutation, frequently induced by UV radiation [7]. Mutations in other genes, such as p63 and p73, are also common. Additional genes that are often deregulated include the histone acetyltransferase p300 (EP300), Polybromo-1 (PBR1), ubiquitin-specific peptidase 28 (USP28), and the conserved ubiquitinated helix-loop-helix kinase (CHUK). Mutations in NOTCH1 and NOTCH2, which result in truncated Notch receptors and impaired signaling, are prevalent in sun-exposed skin and play a crucial role in the early stages of CSCC progression [7,13,14].
Moreover, CSCC frequently exhibits mutations that activate the alpha catalytic subunit of phosphatidylinositol-4,5-bisphosphate 3-kinase (PIK3CA) and the p21 transforming protein (HRAS), as well as mutations in transforming growth factor beta receptors (TGFBR1 and TGFBR2). The transforming growth factor beta signaling pathway has a dual role in CSCC progression, initially functioning as a tumor suppressor but potentially becoming oncogenic at later stages [7,13,14].
SCCs often exhibit mutations or amplifications in receptor tyrosine kinase genes, such as epidermal growth factor receptor, fibroblast growth factor receptor 3, tyrosine-protein kinase, and receptor tyrosine-protein kinase (ERBB4), leading to uncontrolled cell proliferation [15,16].
The expression of molecules associated with epithelial-mesenchymal transition, such as E-Cadherin and Syndecan-1, is also relevant. Figure 1 depicts various genes and molecules. As described, these genes are not deregulated initially; their deregulation occurs with increasing tumor aggressiveness [15,16].
Several studies have highlighted the wide range of mutations involved in the pathogenesis of CSCC, reflecting the complexity of its molecular landscape. Beyond these mutations, CSCC also exhibits other genetic and epigenetic alterations that add to the disease’s intricate nature. Consequently, research has increasingly concentrated on understanding the mechanisms underlying this type of cancer to identify factors that can clarify its progression and inform the development of new therapeutic approaches [15–17].
The tumor microenvironment is equally important in the carcinogenesis of CSCC due to the presence of cancer-associated fibroblasts and tumor-associated macrophages, which significantly influence tumor progression, invasion, metastasis, and response to chemotherapy. The extracellular matrix also plays a vital role by providing structural support to the skin and interacting with SCC cells, affecting the cellular processes involved in tumor progression [7,13,14].
In summary, the development of CSCC is a complex process driven by the accumulation of genetic and epigenetic abnormalities. UV radiation is a key factor contributing to the high mutation rates observed in these tumors. Ongoing research efforts are focused on enhancing our understanding of CSCC biology, aiming to develop more effective therapeutic strategies and interventions [7,14].
SYNDECAN-1 (SDC1 OR CD138)
Structure and function
Syndecans are type I transmembrane proteoglycans composed of four isoforms: Syndecan-1 (SDC1 or CD138), Syndecan-2 (SDC2 or fibroglycan), Syndecan-3 (SDC3 or N-Syndecan), and Syndecan-4 (SDC4, amphoglycan or ryudocan) [18–22]. Each isoform has a central protein structure that varies in the number of attached glycosaminoglycan (GAG) side chains and is expressed in different cells, levels, and locations depending on the developmental stage [18,23,24].
Syndecan-1 (SDC1), encoded by the CD138 gene, has been extensively studied. During development, SDC1 is located on the basolateral surface of epithelial cells and in plasma cells, fibroblasts, and mesenchymal cells. Syndecan-2 (SDC2) is primarily found in endothelial and mesenchymal cells, Syndecan-3 (SDC3) in cells derived from the neural crest, and Syndecan-4 (SDC4) is ubiquitously expressed at lower levels across nearly all cell types [5,20].
SDC1 regulates cell adhesion, extracellular matrix composition, cell migration, and growth. It is detected in various tissues, including the skin, oral cavity, tonsils, lips, salivary glands, gastrointestinal tract (esophagus, stomach, and intestine), gallbladder, pancreatic duct cells, hepatocytes, urogenital system, respiratory system, and female reproductive organs [5,6,23,25,26]. SDC1 has a unique structure consisting of extracellular, transmembrane, and cytoplasmic domains. The extracellular domain contains covalently linked GAG chains, primarily heparan sulfate (HS) and chondroitin sulfate [18]. The cytoplasmic domain is divided into C1, C2, and a variable (V) region. The functions of SDC1 can vary significantly depending on the tissue type and the stage of cell development, as illustrated in Figure 2 [27–30].
The GAG chains of SDC1 facilitate interactions with various molecules crucial for intercellular signaling and the maintenance of cell morphology. As a reservoir for growth factors or as a co-receptor for signal transduction, SDC1 is vital for the function of epithelial cells. Despite being the most extensively studied isoform, much remains to be understood about its full range of functions [27,31].
The synthesis of SDC1 begins in membrane-bound ribosomes and is followed by its translocation to the endoplasmic reticulum. In the Golgi apparatus, GAG chains are covalently attached to the core protein, modifying the structure of SDC1. The modified SDC1 is then transported to the cell surface via exocytosis [29,32].
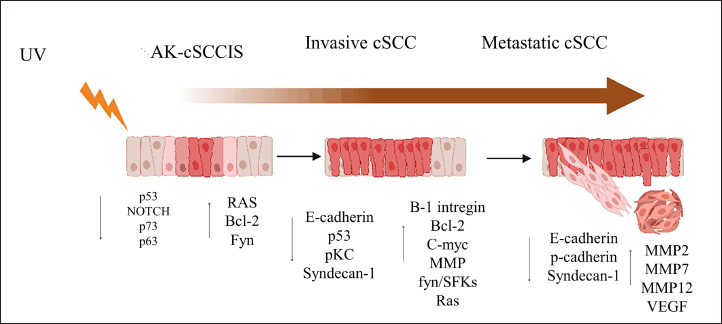 | Figure 1. Molecular changes associated with the progression from AK (a precancerous lesion) to invasive CSCC, [15] . [Click here to view] |
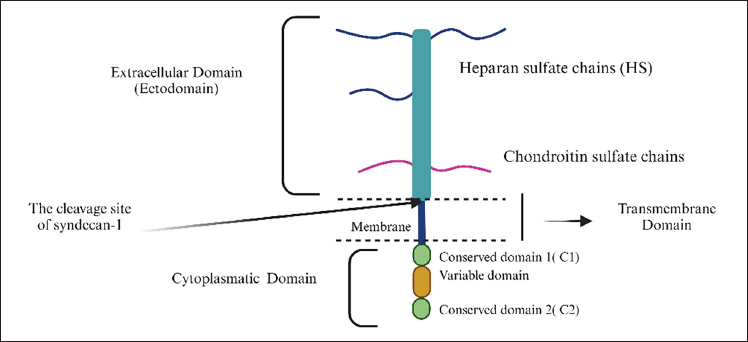 | Figure 2. Structure of Syndecan-1 with three domains: the extracellular domain, the transmembrane domain and the cytoplasmic domain. The arrow indicates where the cleavage of the ectodomain occurs, resulting in its elimination, [33] . [Click here to view] |
SDC1 acts as a co-receptor on the cell surface, facilitating interactions between ligands and their signaling receptors, such as VEGF, WNT, and HGF [29,33–35]. Additionally, proteolytic cleavage of the extracellular domain near the plasma membrane releases a soluble form of the proteoglycan that retains the heparan sulfate chains. This soluble form of Syndecan-1 can either remain in solution or bind to and accumulate in the extracellular matrix [33,35].
Several mechanisms regulate the elimination of Syndecan-1. The phosphorylation of tyrosine residues in the cytoplasmic domain and the interaction between the cytoplasmic domain and Rab5 control this process. Moreover, bacterial virulence factors, insulin, and oxidative stress can also influence the elimination of Syndecan-1 [36,37]. Heparanases can weaken the binding of the core protein, facilitating cleavage, while matrix metalloproteinases (MMPs), when activated, can shed the SDC1 ectodomain, as shown in Figure 3 [28,36–40].
Soluble SDC1 and membrane-bound SDC1 exert opposing effects on cancer cells, influencing metastasis, tumor growth, chemokine localization, and leukocyte trafficking. When SDC1 translocates to the nucleus or stroma, it is associated with various pathological effects. Elevated levels of soluble SDC1 have been linked to conditions such as inflammation, sepsis, lung diseases, coagulopathies, pulmonary embolism, and tumor invasion [41,42].
SDC1 expression in CSCC
SDC1 is the most extensively studied among the four Syndecans due to its critical role in tumor progression, particularly in epithelial cancers such as breast carcinoma [43], Oral SCC [44], and head and neck SCC [45]. SDC1 is essential for maintaining cell cohesion; when epithelial cells lose SDC1 expression, they lose their cuboidal shape, adopt a mesenchymal phenotype, and gain migratory and invasive properties. Reduced expression of SDC1 has been observed in several types of carcinomas, including CSCC [46,47].
In CSCC, the role of SDC1 has been relatively understudied in humans and dogs. However, existing research indicates a decrease in SDC1 levels in invasive CSCC, which correlates with tumor differentiation, reinforcing its role as a prognostic marker, as observed in other forms of SCC [48–50]. This reduction may result from transcriptional deregulation or the shedding of the SDC1 ectodomain, driven by constitutive MMPs [51].
In addition to the intensity of SDC1 expression in tumor cells, its nuclear or stromal localization should also be evaluated, as these locations are associated with poor prognosis in some tumors. However, the nuclear localization of soluble SDC1 and its functional significance is not yet fully understood [52–55]. When SDC1 translocates to the nucleus, it can influence gene expression through several mechanisms, such as regulating the transcription machinery by inhibiting DNA topoisomerase, which prevents DNA relaxation and access to transcription factors [56]. SDC1 can also modulate gene expression by altering the acetylation states of histone proteins, thereby impacting tumor behavior [18,34,57]. The HS chains in the SDC1 ectodomain bind to growth factors and other regulatory proteins, suggesting that SDC1 may transport these molecules to the nucleus. The cleavage of CD138 by heparanase can lead to gene activation. This nuclear translocation may also regulate epithelial-mesenchymal plasticity, influencing epithelial-mesenchymal transition and the invasive behavior of tumor cells [18,34,58].
In several human cancers, including breast, gastric, ovarian, and oral carcinomas, stromal SDC1 promotes epithelial cell proliferation. Stromal SDC1 expression has also been observed in SCC [59]. Stromal SDC1 expression facilitates tumor cell invasion and motility during tumor progression and has been correlated with clinicopathological parameters and, consequently, a poor prognosis. This stromal source of proteoglycan may originate from cancer-associated fibroblasts [42].
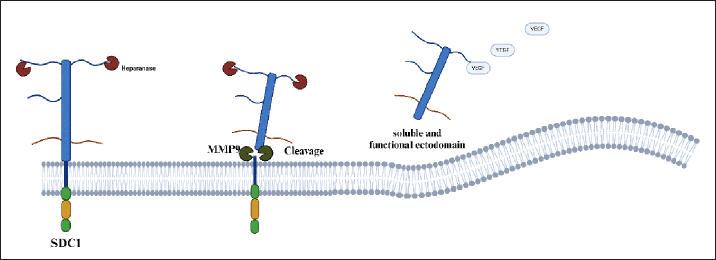 | Figure 3. Illustration of the elimination of SDC1 by MMP9. Once SDC1 becomes soluble, it can bind to various molecules and potentially influence cancer progression. [29] . [Click here to view] |
It is also believed that this variation in SDC1 localization may be related to the occurrence of metastases. Heparanase facilitates the shedding of SDC1, leading to increased levels of MMPs and, consequently, a higher incidence of metastasis [26,60].
Therapies associated with SDC-1
As outlined, SDC1 is expressed in CSCC and is considered a potential prognostic biomarker for the disease. Developing new therapies targeting this marker has been a significant focus of recent research [61,62]. One promising approach involves inactivating molecules that facilitate the elimination of Syndecan-1, as this process plays a crucial role in tumor progression. Heparanase, a molecule associated with poor prognosis and increased metastasis, promotes the elimination of SDC1 and has become a target for new therapeutic strategies [63]. Inhibiting heparanase has successfully reduced invasiveness in human osteosarcomas [64].
Thus, a promising strategy for CSCC involves inhibiting heparanase using modified heparins and small molecule inhibitors and blocking monoclonal antibodies. Heparanase cleaves the heparan sulfate chains of SDC1 at specific sites, and its inhibition reduces the degradation of SDC1. Although heparin acts as a heparanase inhibitor, its clinical use in cancer therapy is limited due to its anticoagulant effects. However, combination therapies with heparin are currently being developed and are widely utilized [64].
Another promising target related to SDC1 is MMPs. The combined clinical application of MMP inhibitors with chemotherapy may offer an effective strategy to prevent the degradation of SDC1 and the creation of microenvironments conducive to tumor recurrence. As mentioned, chemotherapy can increase the elimination of Syndecan-1 by promoting the production of MMPs, which are zinc-containing endopeptidases secreted by keratinocytes in response to stimuli such as UV radiation [21]. Therefore, the use of MMP inhibitors can reduce Syndecan-1 shedding. An example of such an inhibitor is Batimastat (BB-94), which prevents SDC1 degradation, reduces ascites, and disrupts carcinogenesis in breast, ovarian, and colorectal cancers [28,65].
Therefore, the literature suggests that an ideal therapeutic approach combines chemotherapy with agents that decrease the elimination of SDC1 [28,40]. Additionally, therapies that target growth factors binding to SDC1 and disrupt these interactions to reduce malignancy are of interest. For example, in breast cancer, syntetin—a peptide mimetic of Syndecan docking motifs—interferes with the interactions between integrins, IGFR1, VEGFR, and SDC1, leading to a significant reduction in angiogenesis and tumorigenesis in vivo [28].
Another promising approach involves using the soluble form of SDC1, which functions as a tumor suppressor capable of inhibiting tumor growth. To leverage this property, analogs of SDC1 have been developed by attaching GAG chains to a protein framework, creating synthetic proteoglycans. These synthetic proteoglycans have shown the ability to inhibit the viability of myeloma cells both in vitro and in vivo in a murine model of breast cancer [51].
A proposed therapy involves the overexpression of the C-terminal fragment of SDC1, which may suppress tumor cell migration and invasion. This fragment has been demonstrated to inhibit tumor cell migration and increase basal phosphorylation of Src and FAK. Although these therapies have been primarily investigated in breast cancer and myeloma, no specific therapies targeting CSCC have been described [36].
One study investigated the role of the microRNA miR-10 and found that miR-10 inhibitors decreased the SDC1 expression. This study, therefore, serves as an example of a possible therapy associated with sdc1 expression, thus focusing on solving inhibitors of molecules that cause SDC1 to decrease [66].
CONCLUSION
As mentioned, Syndecan-1 plays a complex role in cancer, particularly in SCC, where its deregulation has been observed, although few studies on the subject exist. Beyond its typical membranous localization, Syndecan-1’s abnormal expression in the nucleus and its presence in the stroma is also significant from a pathological perspective.
Since tumor malignancy is generally linked to reduced expression of syndecan-1, therapies often focus on preventing the loss of Syndecan-1, which can help slow tumor progression. Investigating the mechanisms behind Syndecan-1 loss could be valuable for developing future therapies for SCC.
Therefore, further studies are needed to enhance our understanding of Syndecan-1 expression in this type of cancer. This could provide important insights into tumor progression and pave the way for new therapeutic approaches.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work was supported by the projects UIDB/00772/2020 (Doi:10.54499/UIDB/00772/2020) funded by the Portuguese Foundation for Science and Technology (FCT).
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Jones JL, Poulsom R, Coates PJ. Recent advances in pathology: the 2023 annual review issue of The Journal of Pathology. J Pathol. 2023;260:495–7. CrossRef
2. Vaghari-Tabari M, Ferns GA, Qujeq D, Andevari AN, Sabahi, Z, Moein S. Signaling, metabolism, and cancer: an important relationship for therapeutic intervention. J Cell Physiol. 2021;236:5512–32. CrossRef
3. Desai N, Divatia MK, Jadhav A, Wagh A. Aggressive cutaneous squamous cell carcinoma of the head and neck: a review. Curr Oncol. 2023;30:6634–47. CrossRef
4. Anjos DS, Bueno C, Magalhães LF, Magalhães GM, Mattos E, Pinto MMR, et al. Electrochemotherapy in canine cutaneous squamous cell carcinomas.
5. Yang Z, Chen S, Ying H, Yao W. Targeting syndecan-1: new opportunities in cancer therapy. Am J Physiol Cell Physiol. 2022;323:C29–45. CrossRef
6. Altemeier WA, Schlesinger SY, Buell CA, Brauer R, Rapraeger AC, Parks WC, et al. Transmembrane and extracellular domains of syndecan-1 have distinct functions in regulating lung epithelial migration and adhesion. J Biol Chem. 2012;287:34927–35. CrossRef
7. Chang MS, Azin M, Demehri S. Cutaneous squamous cell carcinoma: the frontier of cancer immunoprevention. Annu Rev Pathol Mech Dis. 2022;17:101–19. CrossRef
8. Zhang H, Zhong A, Chen J. Immune checkpoint inhibitors in advanced cutaneous squamous cell carcinoma: a systemic review and meta-analysis. Skin Res Technol. 2023;29:e13229. CrossRef
9. Guscetti F, Nassiri S, Beebe E, Rito Brandao I, Graf R, Markkanen E. Molecular homology between canine spontaneous oral squamous cell carcinomas and human head-and-neck squamous cell carcinomas reveals disease drivers and therapeutic vulnerabilities. Neoplasia. 2020;22:778–88. CrossRef
10. Brougham NDL, Tan ST. The incidence and risk factors of metastasis for cutaneous squamous cell carcinoma-implications on the T-classification system: skin squamous cell carcinoma metastasis. J Surg Oncol. 2014;110:876–82. CrossRef
11. Pickering CR, Zhou JH, Lee JJ, Drummond JA, Peng SA, Saade RE, et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res. 2014;20:6582–92. CrossRef
12. Inman GJ, Wang J, Nagano A, Alexandrov LB, Purdie KJ, Taylor RG, et al. The genomic landscape of cutaneous SCC reveals drivers and a novel azathioprine associated mutational signature. Nat Commun. 2018;9:3667. CrossRef
13. Brown VL, Harwood CA, Crook T, Cronin JG, Kelsell DP, Proby CM. p16INK4a and p14ARF tumor suppressor genes are commonly inactivated in cutaneous squamous cell carcinoma. J Invest Dermatol. 2004;122:1284–92. CrossRef
14. Hassan S, Purdie KJ, Wang J, Harwood CA, Proby CM, Pourreyron C, et al. A unique panel of patient-derived cutaneous squamous cell carcinoma cell lines provides a preclinical pathway for therapeutic testing. Int J Mol Sci. 2019;20:3428. CrossRef
15. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:237–47. CrossRef
16. South AP, Purdie KJ, Watt SA, Haldenby S, Den Breems NY, Dimon M, et al. NOTCH1 mutations occur early during cutaneous squamous cell carcinogenesis. J Invest Dermatol. 2014;134:2630–8. CrossRef
17. Patel GK, Yee CL, Terunuma A, Telford WG, Voong N, Yuspa SH, et al. Identification and characterization of tumor-initiating cells in human primary cutaneous squamous cell carcinoma. J Invest Dermatol. 2012;132:401–9. CrossRef
18. Couchman JR. Syndecan-1 (CD138), carcinomas and EMT. Int J Mol Sci. 2021;22:4227. CrossRef
19. Ibrahim SA, Gadalla R, El-Ghonaimy EA, Samir O, Mohamed HT, Hassan H, et al. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, notch and EGFR signaling pathways. Mol Cancer. 2017;16:57. CrossRef
20. Karászi K, Vigh R, Máthé M, Fullár A, Oláh L, Füle T, et al. Aberrant expression of syndecan-1 in cervical cancers. Pathol Oncol Res. 2020;26:2255–64. CrossRef
21. Teng YHF, Aquino RS, Park PW. Molecular functions of syndecan-1 in disease. Matrix Biol. 2012;31:3–16. CrossRef
22. Couchman JR. Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol. 2010;26:89–114. CrossRef
23. Czarnowski D. Syndecans in cancer: a review of function, expression, prognostic value, and therapeutic significance. Cancer Treat Res Commun. 2021;27:100312. CrossRef
24. Saunders S, Jalkanen M, O’Farrell S, Bernfield M. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547–56. CrossRef
25. Frontiers Production Office. Erratum: the role and therapeutic value of syndecan-1 in cancer metastasis and drug resistance. Front Cell Dev Biol. 2022;10:1074502. CrossRef
26. Annaval T, Wild R, Crétinon Y, Sadir R, Vivès RR, Lortat-Jacob H. Heparan sulfate proteoglycans biosynthesis and post synthesis mechanisms combine few enzymes and few core proteins to generate extensive structural and functional diversity. Molecules. 2020;25:4215. CrossRef
27. Szatmári T, Ötvös R, Hjerpe A, Dobra K. Syndecan-1 in cancer: implications for cell signaling, differentiation, and prognostication. Dis Markers. 2015;2015:1–13. CrossRef
28. Barbouri D, Afratis N, Gialeli C, Vynios DH, Theocharis AD, Karamanos NK. Syndecans as modulators and potential pharmacological targets in cancer progression. Front Oncol. 2014;4:4. CrossRef
29. Guo S, Wu X, Lei T, Zhong R, Wang Y, Zhang L, et al. The role and therapeutic value of syndecan-1 in cancer metastasis and drug resistance. Front Cell Dev Biol. 2022;9:784983. CrossRef
30. Liao S, Liu C, Zhu G, Wang K, Yang Y, Wang C. Relationship between SDC1 and Cadherin signalling activation in cancer. Pathol Res Pract. 2020;216:152756. CrossRef
31. Reg?s E, Karászi K, Reszegi A, Kiss A, Schaff Z, Baghy K, et al. Syndecan-1 in liver diseases. Pathol Oncol Res. 2020;26:813–9. CrossRef
32. Akl MR, Nagpal P, Ayoub NM, Prabhu SA, Gliksman M, Tai B, et al. Molecular and clinical profiles of syndecan-1 in solid and hematological cancer for prognosis and precision medicine. Oncotarget. 2015;6:28693–715. CrossRef
33. Alexopoulou AN, Multhaupt HAB, Couchman JR. Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol. 2007;39:505–28. CrossRef
34. Kumar-Singh A, Parniewska MM, Giotopoulou N, Javadi J, Sun W, Szatmári T, et al. Nuclear syndecan-1 regulates epithelial-mesenchymal plasticity in tumor cells. Biology. 2021;10:521. CrossRef
35. Rangarajan S, Richter JR, Richter RP, Bandari SK, Tripathi K, Vlodavsky I, et al. Heparanase-enhanced shedding of syndecan-1 and its role in driving disease pathogenesis and progression. J Histochem Cytochem. 2020;68:823–40. CrossRef
36. Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a Timp-3–sensitive metalloproteinase. J Cell Biol. 2000;148:811–24. CrossRef
37. Park PW, Foster TJ, Nishi E, Duncan SJ, Klagsbrun M, Chen Y. Activation of syndecan-1 ectodomain shedding by Staphylococcus aureus α-toxin and β-toxin. J Biol Chem. 2004;279:251–8. CrossRef
38. Curran S, Dundas SR, Buxton J, Leeman MF, Ramsay R, Murray GI. Matrix metalloproteinase/tissue inhibitors of matrix metalloproteinase phenotype identifies poor prognosis colorectal cancers. Clin Cancer Res. 2004;10:8229–34. CrossRef
39. Chen Y, Hayashida A, Bennett AE, Hollingshead SK, Park PW. Streptococcus pneumoniae sheds syndecan-1 ectodomains through ZmpC, a metalloproteinase virulence factor. J Biol Chem. 2007;282:159–67. CrossRef
40. Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, et al. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Biol Chem. 2003;278:40764–70. CrossRef
41. Ramani VC, Purushothaman A, Stewart MD, Thompson CA, Vlodavsky I, Au JL, et al. The Heparanase/Syndecan-1 axis in cancer: mechanisms and therapies. FEBS J. 2013;280:2294–306. CrossRef
42. Maeda T, Alexander CM, Friedl A. Induction of syndecan-1 expression in stromal fibroblasts promotes proliferation of human breast cancer cells. Cancer Res. 2004;64:612–21. CrossRef
43. Pham SH, Pratt K, Okolicsanyi RK, Oikari LE, Yu C, Peall IW, et al. Syndecan-1 and -4 influence Wnt signaling and cell migration in human breast cancers. Biochimie. 2022;198:60–75. CrossRef
44. Shetty P, Gonsalves N, Desai D, Pandit S, Aradhya C, Shahid M, et al. Expression of Syndecan-1 in different grades of oral squamous cell carcinoma: an immunohistochemical study. J Cancer Res Ther. 2022;18:191. CrossRef
45. Chen CL, Ou DL. Expression of Syndecan-1 (CD138) in nasopharyngeal carcinoma is correlated with advanced stage and poor prognosis. Hum Pathol. 2006;37:1279–85. CrossRef
46. Balasubramanian P, Lang JC, Jatana KR, Miller B, Ozer E, Old M, et al. Multiparameter analysis, including EMT markers, on negatively enriched blood samples from patients with squamous cell carcinoma of the head and neck. PLoS One. 2012;7:e42048. CrossRef
47. Anttonen A, Kajanti M, Heikkilä P, Jalkanen M, Joensuu H. Syndecan-1 expression has prognostic significance in head and neck carcinoma. Br J Cancer. 1999;79:558–64. CrossRef
48. Ahmed Haji Omar A, Haglund C, Virolainen S, Häyry V, Atula T, Kontio R, et al. Epithelial and stromal syndecan-1 and -2 are distinctly expressed in oral- and cutaneous squamous cell carcinomas. J Oral Pathol Med. 2013;42:389–95. CrossRef
49. Mukunyadzi P, Sanderson RD, Fan CY, Smoller BR. The level of syndecan-1 expression is a distinguishing feature in behavior between keratoacanthoma and invasive cutaneous squamous cell carcinoma. Mod Pathol. 2002;15:45–9. CrossRef
50. Bayer-Garner IB, Smoller BR. The expression of syndecan-1 is preferentially reduced compared with that of E-cadherin in acantholytic squamous cell carcinoma. J Cutan Pathol. 2001;28:83–9. CrossRef
51. Stepp MA, Pal-Ghosh S, Tadvalkar G, Pajoohesh-Ganji A. Syndecan-1 and its expanding list of contacts. Adv Wound Care. 2015;4:235–49. CrossRef
52. Chen L. Sanderson RD. Heparanase regulates levels of syndecan-1 in the nucleus. PLoS One. 2009;4:e4947. CrossRef
53. Hsia E. Richardson TP, Nugent MA. Nuclear localization of basic fibroblast growth factor is mediated by heparan sulfate proteoglycans through protein kinase C signaling. J Cell Biochem. 2003;88:1214–25. CrossRef
54. Brockstedt U. Immunoreactivity to cell surface syndecans in cytoplasm and nucleus: tubulin-dependent rearrangements. Exp Cell Res. 2002;274:235–45. CrossRef
55. Fedarko NS, Ishihara M, Conrad HE. Control of cell division in hepatoma cells by exogenous heparan sulfate proteoglycan. J Cell Physiol. 1989;139:287–94., CrossRef
56. Szatmári T, Mundt F, Kumar-Singh A, Möbus L, Ötvös R, Hjerpe A, et al. Molecular targets and signaling pathways regulated by nuclear translocation of syndecan-1. BMC Cell Biol. 2017;18:34. CrossRef
57. Jinesh GG, Brohl AS. Classical epithelial-mesenchymal transition (EMT) and alternative cell death process-driven blebbishield metastatic-witch (BMW) pathways to cancer metastasis. Signal Transduct Target Ther. 2022;7:296. CrossRef
58. Ribatti D, Tamma R, Annese T. Epithelial-mesenchymal transition in cancer: a historical overview. Transl Oncol. 2020;13:100773. CrossRef
59. Hadler-Olsen E, Wetting HL, Rikardsen O, Steigen SE, Kanapathippillai P, Grénman R, et al. Stromal impact on tumor growth and lymphangiogenesis in human carcinoma Xenografts. Virchows Arch. 2010;457:677–92. CrossRef
60. Yang N, Friedl A. Syndecan-1-induced ECM fiber alignment requires integrin Αvβ3 and syndecan-1 ectodomain and heparan sulfate chains. PLoS One. 2016;11:e0150132. CrossRef
61. Hadler-Olsen E, Winberg JO, Uhlin-Hansen L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumor Biol. 2013;34:2041–51. CrossRef
62. Aragão AZB, Belloni M, Simabuco FM, Zanetti MR, Yokoo S, Domingues RR, et al. Novel processed form of syndecan-1 shed from SCC-9 cells plays a role in cell migration. PLoS One. 2012;7:e43521. CrossRef
63. Bertrand J, Bollmann M. Soluble syndecans: biomarkers for diseases and therapeutic options. Br J Pharmacol. 2019;176:67–81. CrossRef
64. Ritchie JP, Ramani VC, Ren Y, Naggi A, Torri G, Casu B, et al. SST0001, a chemically modified heparin, inhibits myeloma growth and angiogenesis via disruption of the heparanase/Syndecan-1 axis. Clin Cancer Res. 2011;17:1382–93. CrossRef
65. Yu W, Yang L, Li T, Zhang Y. Cadherin signaling in cancer: its functions and role as a therapeutic target. Front Oncol. 2019;9:989. CrossRef
66. Xiong Z, Jiang B, Li G. Downregulation of miR-10a inhibits cutaneous squamous cell carcinoma cell proliferation, migration, and invasion by targeting Syndecan-1. Int J Clin Exp Pathol. 2020;13(10):2502–12.