INTRODUCTION
Bioanalysis is a widely utilized technique for quantifying the levels of pharmaceutical substances and their metabolic byproducts in diverse biological samples, i.e., serum, plasma, cerebrospinal fluid, saliva, and urine [1–3]. Bioanalytical methodologies are employed to determine the suitability of implementing a quantitative analytical technique in the context of a biochemical process [4]. Validation refers to the systematic process of documenting laboratory experiments to ascertain the suitability and reliability of the method being employed for its intended uses [5–6]. This approach is utilized to assess bio availability and conduct bio equivalence studies, quantitatively analyze pharmaceuticals and their metabolites, facilitate drug development, explore clinical pharmacokinetics, engage in scientific research, and monitor therapeutic drug levels [7]. Bio analytical techniques are considered to be at the forefront of technological advancements as a result of their continuous evolution and continual enhancements [8]. Ifenprodil (Fig. 1) is a potential neuromodulatory drug, which has been recently discovered category of N-methyl-D-aspartame (NMDA) receptor antagonist [9–12]. This medication exhibits a specific inhibitory effect on NMDA receptors that incorporate the NR2B subunit [9,13,14]. Ifenprodil is employed in the treatment of cerebrovascular diseases and peripheral artery obliterative illness[15–16]. Furthermore, the safety and efficacy of Ifenprodil are being still assessed in confirmed COVID-19 patients by Novotech (Australia) sponsored by Algernon Pharmaceuticals with clinical trial ID: NCT04382924. The efficacy of this drug is yet to be established [17].
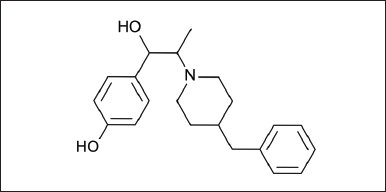 | Figure 1. Chemical structure of Ifenprodil. [Click here to view] |
A limited range of analytical methodologies has been established for the analysis of Ifenprodil. The detection of Ifenprodil in animal plasma has been established in the literature using a liquid chromatographic approach [10]. The approach is distinguished by its extreme sensitivity, simplicity, specificity, rapidity, and cost effectiveness. The main objective of the development of a bioanalytical method was to establish its appropriateness for the intended use and evaluate its possible advantages for researchers.
MATERIALS AND METHODS
Reagents and materials
The injectable Ifenprodil tartrate and the reference standard dextromethorphan were purchased from Supriya Life Science, Mumbai. HPLC-grade FA, ammonium formate, and acetonitrile (ACN) were procured from the Merck chemical division located in Mumbai. During the experiment, we employed the water sourced from the Milli-Q water purifying system [18].
Preparation of buffer solution
Accurately weighed 0.77 g of ammonium formate in a 1,000 ml volumetric flask add about 900 ml of Milli-Q water added and degas to sonicate and finally make up the volume with water then pH adjusted to 2.5 with a FA solution.
Preparation of mobile phase
Mixture of ammonium formate (pH 2.5) and CAN in the ratio of 60:40% W/V.
Ifenprodil standard solution
In the developmental phase of the bioanalytical reverse phase high-performance liquid chromatography (RP-HPLC) process, the preparation of the Ifenprodil stock solution involved dissolving the precisely weighed standard drug in ACN. Ifenprodil standard solution had a concentration of 2,000 ng/ml.
Preparation
A working standard of Ifenprodil, measuring 8 mg, was diluted in a 10 ml solution of diluent. Subsequently, a volume of 0.1 ml from the aforementioned solution underwent additional dilution, resulting in a final volume of 10 ml. Then, mix 500 µl with plasma to achieve a final volume of 2 ml. To create working standard solutions for method development, calibration curves, and quality control (QC) samples, suitable dilutions were made using the mobile phase from the stock solution. The resulting solution and standard solutions were transferred to polypropylene vials and stored in a freezer maintained at −20°C [19].
Extraction procedure
By using a micropipette, transfer 200 µl of plasma into an Eppendorf microtube. Following this, mix the contents by adding 300 µl of ACN and vortexing the tubes. In the same tube, introduce 500 µl of Ifenprodil and IS, vortexing again to ensure thorough mixing. Subsequently, add 500 µl of diluent and vortex for 5 minutes. Centrifuge the tubes at 5,000 rpm for 20 minutes, separate the supernatant liquid, and immediately inject 10 µl of the eluate into the HPLC system [20].
METHOD VALIDATION
The validation procedure was confirmed to meet the specifications set by the US FDA.
System suitability
The purpose of this indicator is to find out the operational status of the instrument and provide authorization to proceed with the analysis of the subsequent set of samples. System appropriateness tests are commonly employed in chromatographic procedures to verify that the system possesses enough sensitivity, specificity, and reproducibility for the ongoing analytical run and its application in a specific study. To identify the appropriateness of the system, samples that were representative of the batch were incorporated at the commencement, and conclusion of each batch. The concentration of Ifenprodil in the system suitability samples was adjusted to 2,000 ng/ml. The concentration of 2,000 ng/ml was present in the mobile phase. The %CV values for both peak area and retention time were computed for Ifenprodil and the IS over six consecutive injections. The objective was to determine if these %CV values were within the range.
Specificity and selectivity
The study entailed the examination of plasma samples acquired from six samples to detect any potential chromatographic interference that may occur at the retention periods of Ifenprodil and IS.
Calibration curve
A calibration curve was constructed using eight data points by introducing accurate volumes of a working solution into a blank plasma sample. The aforementioned procedure yielded ultimate concentrations of Ifenprodil of 200, 500, 1,000, 1,500, 2,000, 2,500, 3,000, and 4,000 ng/ml. The calibration curve was created by plotting the ratio of peak areas for the transition pair of Ifenprodil and the IS against the nominal concentration of the calibration standards. Linear regression analysis was utilized for data interpretation. Standards were considered acceptable if their back-calculated concentrations fell within a deviation range of ±15% (SD) from the nominal value. However, a slightly wider range of ±20% was set for the Lower limit of quantification (LLOQ).
Precision and accuracy
The assessment of intraday precision encompassed the examination of six duplicate samples with varying concentrations, encompassing the LLOQ, Low-quality control (LQC), Medium quality control (MQC), and high-quality control (HQC) samples. The assessment of the method’s reproducibility involved the validation of its day-to-day variance, with a specific focus on inter-day precision. The analysis involved the examination of six sets of samples with varying quantities, encompassing the LLOQ, LQC, MQC, and HQC samples. The analysis was conducted on three distinct occasions.
The precision of intra and inter-day assays was assessed by computing the coefficient of variation (%CV), a measure obtained by dividing the SD by the mean and presenting the result as a percentage.
To assess both intra-assay and inter-assay precision, six replicates were analyzed at four levels of samples, namely LQC, MQC, HQC, and LLOQ. Intra-assay accuracy was evaluated by analyzing on the same day, while inter-assay accuracy was assessed over three distinct days. The accuracy of measurements was assessed by computing the percentage ratio between the determined concentration and the known concentration.
Recovery
The determination of analyte recovery was conducted by comparing the analytical outcomes of extracted samples at three distinct concentrations LQC, MQC, and HQC in six repetitions with the outcomes of extracted standards (without any processing), which serve as a reference for 100% recovery. An experimental study was undertaken to assess the recovery of IS at a specific dose of 2,000 ng/ml.
Dilution integrity
The process of evaluating dilution integrity entails the procedure of introducing a concentration of analyte within the upper limit of quantification control through the addition of analyte to the matrix. It was subsequently followed by diluting the resulting mixture using a blank matrix.
Ruggedness
The recovery percentages and %CV for Ifenprodil were determined by two separate analysts using two different columns (Sonoma C18, Luna silica). The results obtained for the LQC, MQC, and HQC samples were found to fall within acceptable ranges. The results showed that the approach utilized demonstrated a significant level of resilience.
Matrix effect
The matrix effect refers to changes in the analyte response caused by interference from unidentified components in the sample matrix. This effect is assessed at LQC and HQC concentration levels. The peak area response is examined in the presence and absence of matrix ions, and the % matrix factor is determined. When the IS is introduced with the analyte, a normalized factor is calculated. The acceptable variability in the matrix factor should be below 15%.
Stability
The assessment of the stability research was carried out as an essential element of the process of validating the approach. A stability test was done to assess the possible disintegration of Ifenprodil under different conditions.
Freeze-thaw stability
A temperature of −20°C was maintained for the storage of six replicates each for the LQC, MQC, and HQC samples. The samples were subsequently thawed completely at ambient temperature and promptly refrozen back to a temperature of −20°C. The aforementioned procedure was repeated twice, and thereafter, the samples were directly injected into RP-HPLC.
Bench-top stability
The primary aim of the bench-top stability experiment was to evaluate the stability of Ifenprodil in rat plasma during 8 hours of exposure on the laboratory bench. The assessment was carried out using three distinct concentrations, specifically the LQC, MQC, and HQC Each concentration was reproduced six times.
Auto sampler stability
Determination of Ifenprodil concentrations in plasma was performed through the analysis of samples representing LQC, MQC, and HQC. The specimens were administered at regular intervals of 1 hour for 24 hours, as a component of stability investigation. The samples were compared to the freshly generated samples at the initial time point (0 hours) in six repetitions to assess various methods. The evaluation of sample stability was based on assessing the assay data against predefined criteria for accuracy, specifically within a standard deviation range of ±15%, as well as precision.
Short-term stability
The temporal or spectral stability of a time or frequency signal within a short measurement period is usually not exceeding 100 seconds.
Long-term stability
The examination of stability within a certain setting. This study entails the execution of studies aimed at evaluating the many physical, chemical, biological, bio pharmaceutical, and microbiological characteristics of a pharmaceutical compound. The investigations are conducted both during and after the projected shelf-life and storage duration of the samples. The storage conditions employed in these studies are indicative of the conditions anticipated in the target market [21].
RESULTS AND DISCUSSION
Optimized method
Several studies were undertaken to examine the effects of different ACN compositions in the mobile phase on the resolution of Ifenprodil. The most favorable peak shape was seen while employing a mobile phase used as a mixture of ammonium formate pH−2.5/ FA and ACN (60:40%). This specific composition led to minor deviations in the retention time of Ifenprodil while keeping its peak response unaltered. The optimized conditions and chromatograms for blank, ifenprodil, and dextromethorphan (IS) are given in Table 1 and Figures 2 and 3 respectively.
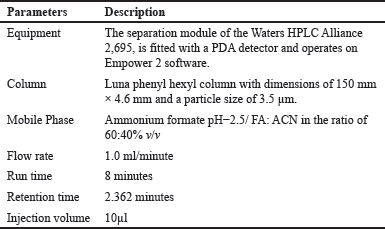 | Table 1. Summary of optimized chromatographic conditions. [Click here to view] |
 | Figure 2. Blank (rat plasma) chromatogram. [Click here to view] |
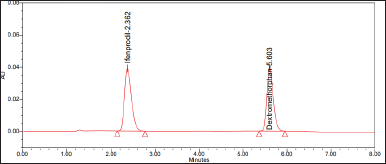 | Figure 3. Chromatogram of Ifenprodil and dextromethorphan IS. [Click here to view] |
Specificity
The assessment of specificity entailed the usage of six separate rat plasma samples. Figures 2 and 3 depict the chromatograms obtained from plasma samples collected from rats. No co-eluting peaks originating from naturally occurring substances were seen at the specific retention time associated with Ifenprodil. The analyte Ifenprodil was eluted with a retention time of 2.362 minutes. However, the execution duration of the sample was optimized to 8 minutes.
Sensitivity
The percentage coefficient of variation values for the area ratio of Ifenprodil and the IS were found to be 0.10% and 99.43%, respectively. Hence, it effectively exhibited a capacity for sensitivity.
Linearity
The standard curves exhibited linearity in the concentration range of 200–4,000 ng/ml for Ifenprodil, with an average correlation coefficient (r2) of 0.9998. Quantification of samples involved determining the ratio of the analyte peak area to the peak area of the IS. The plotted data comprised ratios of peak areas to plasma concentrations Table 2 provides the linearity data of ifenprodil and Figure 4 shows the linearity curve of ifenprodil.
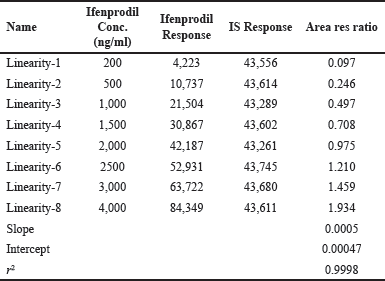 | Table 2. Linearity data of Ifenprodil in rat plasma. [Click here to view] |
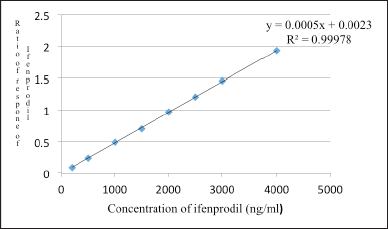 | Figure 4. Linearity curve of Ifenprodil in rat plasma. [Click here to view] |
LOD and LOQ
The determination of LOD and LOQ was conducted by independent investigations utilizing the calibration curve approach. The bioanalytical RP-HPLC method established the determination of the LOD and LOQ for the compound by incrementally injecting decreasing amounts of standard solutions. For Ifenprodil, the LOD is 60 ng/ml, with a corresponding signal-to-noise (S/N) value of 3. The LOQ, representing the reliable quantification threshold, is 200 ng/ml, and the observed S/N ratio at this level is 10 Table 3 provides LOD and LOQ data for ifenprodil.
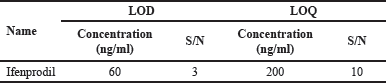 | Table 3. LOD and LOQ data for Ifenprodil. [Click here to view] |
Precision and accuracy
The evaluation of precision and accuracy within a single assay was carried out by analyzing six replicates, each consisting of Ifenprodil at six different QC levels. The concept of accuracy is commonly understood as the condition in which data is confined inside a certain range of 85%–115% of the true values. In contrast, precision was assessed through the calculation of the %CV, with an acceptable range of ±15%, except for the minimum quantifiable level. In the context of LLQC, it is anticipated that the level of accuracy will fall between the range of 80%–120%, while the RSD is required to be below 20%. The data for precision and accuracy were provided in Table 4.
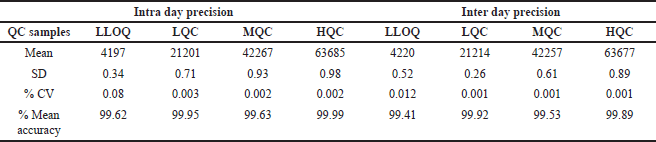 | Table 4. Accuracy and precision data of the Ifenprodil. [Click here to view] |
Recovery of analyte
The evaluation of the recovery of both the drug and IS was conducted at three distinct concentration levels, specifically at low, medium, and HQC levels. The determination of recovery was conducted by comparing the reaction of duplicate samples to that of the undiluted standard solution. The assessment of extraction efficiency, which involves recovering an analyte from a sample matrix, requires comparing the analytical response obtained from a known quantity of added analyte with the response obtained from the sample matrix. ACN was chosen for the extraction process due to the specific properties of Ifenprodil The recovery of analyte data is given in Table 5.
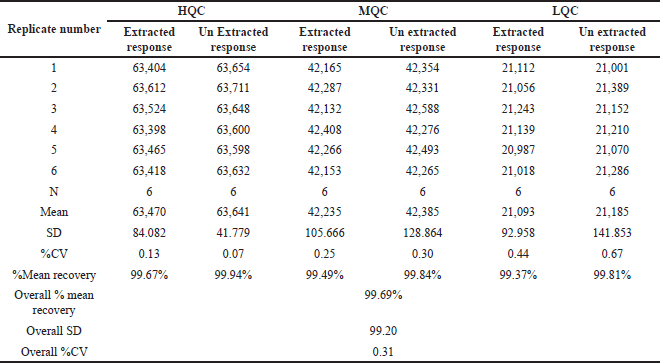 | Table 5. Recovery data of Ifenprodil. [Click here to view] |
Stability
Bench top stability
Percentage mean accuracy values for Ifenprodil HQC, LQC, and MQC were found to be 99.63%, 99.60%, and 99.63%. Benchtop stability findings are presented in Table 6.
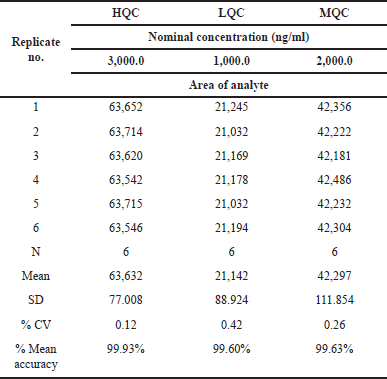 | Table 6. Bench top stability data of Ifenprodil. [Click here to view] |
Freeze-thaw at −20°C
The values for mean accuracy of Ifenprodil were found to be 99.56%, 99.78%, and 99.59% respectively. Freeze-thaw stability findings were presented in Table 7.
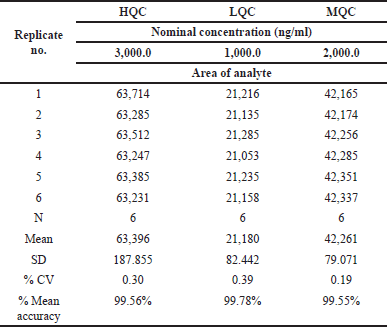 | Table 7. Freeze thaw stability data of Ifenprodil. [Click here to view] |
Short-term stability
Percentage mean accuracy values for Ifenprodil HQC, LQC, and MQC were found to be 99.63%, 99.60%, and 99.63%. Short-term stability results were found in Table 8.
 | Table 8. Short term stability results for Ifenprodil. [Click here to view] |
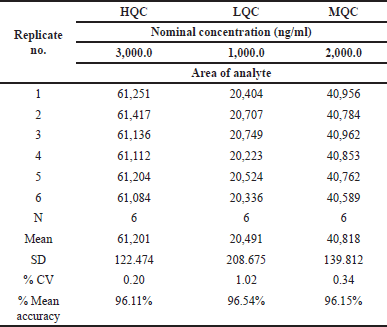 | Table 9. Long-term stability results for Ifenprodil. [Click here to view] |
Long-term stability (28 days)
The % CV and mean accuracy for Ifenprodil were found to be within the acceptable limit, which passed the long-term stability Long-term stability results were found in Table 8.
DISCUSSION
The developed RP-HPLC method was optimized for estimating Ifenprodil in rat plasma, employing dextromethorphan was used as an IS. Analytes were separated using ammonium formate pH 2.5/ FA and ACN (60:40% v/v) at 1 ml/minute flow rate on a Luna phenyl hexyl Column (150 mm × 4.6 nm: particle size 3.5 µm). The method was also validated following United states food and drug administration (USFDA) guidelines. The retention time of drug and IS was discovered at 2.362 and 5.603 minutes, respectively. In the rat plasma matrix, liquid–liquid extraction yielded clean samples with a consistent mean recovery of 99.69%. The method was linear over the ranges of 200–4,000 ng/ml, with r2 of 0.9998. Therefore, the proposed method could be considered valid for routine drug analysis in biological matrices.
CONCLUSION
The suggested RP-HPLC method for the determination of Ifenprodil in rat plasma is rapid, sensitive, and repeatable, with linear dynamic ranges of 200–4,000 ng/ml, respectively. It has been validated and adheres to the USFDA standards, demonstrating a high level of accuracy and precision following the guidelines. The selected method was suitable for pharmacokinetics and bioequivalence studies of Ifenprodil samples.
ACKNOWLEDGMNT
The authors express gratitude to the principal and administration of KL College of Pharmacy, KL Deemed University, for their support. Appreciation is extended to Clintech Solution, Hyderabad, Telangana, India, for providing essential facilities for conducting this research. In addition, thanks are offered to the principal and administration of Vasavi Institute of Pharmaceutical Sciences, Kadapa.
LIST OF ABBREVIATIONS
HQC, High-quality control; IS, Internal standard; LQC, Low-quality control; LLOQ, Lower limit of quantification; MQC, Medium quality control; RP-HPLC, Reverse phase high-performance liquid chromatography; S/N, Signal-to-noise; USFDA, United states food and drug administration.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICT OF INTERESTS
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
The study protocol was approved by the Institutional Animal Ethics Committee of KL College of Pharmacy, Andhra Pradesh, India (Approval No: 1250/PO/RcBi/S/11/CPCSEA, Date: 24/06/2023).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Xue YJ, Gao H, Ji QC, Lam Z, Fang X, Lin Z, et al. Bioanalysis of drug in tissue: current status and challenges. Bioanalysis. 2012;4(21):2637–53. CrossRef
2. Tijare LK, Rangari NT, Mahajan UN. A review on bioanalytical method development and validation. Asian J Pharm Clin Res. 2016;9(3):6–10. CrossRef
3. Mu R, Yuan J, Huang Y, Meissen JK, Mou S, Liang M, et al. Bioanalytical methods and strategic perspectives addressing the rising complexity of novel bioconjugates and delivery routes for biotherapeutics. BioDrugs. 2022;36(2):181–96. CrossRef
4. Tiwari G, Tiwari R. Bioanalytical method validation: an updated review. Pharm Methods. 2010;1(1):25–38. CrossRef
5. Peters FT, Drummer OH, Musshoff F. Validation of new methods. Forensic Sci Int. 2007;165(2-3):216–24. CrossRef
6. Refsgaard JC, Henriksen HJ. Modelling guidelines––terminology and guiding principles. Adv Water Resour. 2004;27(1):71–82. CrossRef
7. Penner N, Xu L, Prakash C. Radiolabeled absorption, distribution, metabolism, and excretion studies in drug development: why, when, and how? Chem Res Toxicol. 2012;25(3):51–31.CrossRef
8. Van Peer A. Variability and impact on design of bioequivalence studies. Basic Clin Pharmacol Toxicol. 2010;106(3):146–53. CrossRef
9. Williams K. Ifenprodil, a novel NMDA receptor antagonist: site and mechanism of action. Curr Drug Targets. 2001;2(3):285–98. CrossRef
10. Yang J, Lu C, Song W, Li J, Ding Y, Zhu Y, et al. Determination of ifenprodil by LC–MS/MS and its application to a pharmacokinetic study in healthy Chinese volunteers. Acta Pharm Sin B. 2013;3(3):180–4. CrossRef
11. Chen YW, Chiu CC, Wang JN, Hung CH, Wang JJ. Ifenprodil for prolonged spinal blockades of motor function and nociception in rats. Pharmacol Rep. 2016;68(2):357–62. CrossRef
12. Murakami HK, Takano A, Ogai Y, Tsukamoto S, Murakami M, Funada D, et al. Study of effects of ifenprodil in patients with methamphetamine dependence: protocol for an exploratory, randomized, double-blind, placebo-controlled trial. Neuropsychopharmacol Rep. 2019;39(2):90–9 CrossRef
13. Reynolds IJ, Miller RJ. Ifenprodil is a novel type of N-methyl-D-aspartate receptor antagonist: interaction with polyamines. Mol pharmacol. 1989;36(5):758–65.
14. Kew JN, Trube G, Kemp JA. A novel mechanism of activity-dependent NMDA receptor antagonism describes the effect of ifenprodil in rat cultured cortical neurones. J Physiol. 1996;497(3):761–72. CrossRef
15. Amico-Ruvio SA, Paganelli MA, Myers JM, Popescu GK. Ifenprodil effects on GluN2B-containing glutamate receptors. Mol Pharmacol. 2012;82(6):1074–81. CrossRef
16.Sirrieh RE, MacLean DM, Jayaraman V. A conserved structural mechanism of NMDA receptor inhibition: a comparison of ifenprodil and zinc. J Gen Physiol. 2015;146(2):173–81. CrossRef
17. Novotech (Australia) and Algernon pharmaceuticals, saffety and efficacy of NP-120(Ifenprodil) for the treatment of hospitalised patient with confirmed COVID-19 disease. Clinical Trial ID NCT04382924, Novotech; 2021.
18. Pavani B, Narender M, Prasanth DSNBK, Chakravarthi G. Development and validation of a novel bioanalytical method for the simultaneous determination of glecaprevir and pibrintasvir in human plasma using reversed phase high-performance liquid chromatography. Egypt.Pharm.J. 2022;21(4):424–31. CrossRef
19. Ishaq BM, Reddy LS, Basha GM, Sreenivasulu M, Chetty CM, Ahad HA. Rapid and sensitive bioanalytical method development and validation for quantification of metoprolol using LC-MS/MS in human plasma. J Chem Soc Pak. 2020;42:171–5. CrossRef
20. Pavani B, Narender M, Prasanth DSNBK, Chakravarthi G. A novel bioanalytical method for simultaneous determination of olanzapine and samidorphan in human plasma using RP-HPLC. Ind J Pharm Edu Res. 2023;57(3):873–82.
21. Guidance for Industry, bioanalytical method validation, US Department of Health and Human Services. Food and Drug Administration Center for Drug Evaluation and Research (CDER), Centre for Veterinary Medicine (CVM); 2001.