INTRODUCTION
Pharmacological intervention, also known as pharmacotherapy, is typically considered the primary approach for the treatment of various diseases. Several studies have shown that the primary goal of pharmacotherapy is to enhance patient care by optimizing therapeutic effectiveness and minimizing the potential for drug toxicity. However, drug administration is inevitably associated with the potential for adverse drug reactions (ADRs), which can range from mild responses to life-threatening events [1,2]. For almost half a century, the World Health Organization (WHO) has provided a definition that describes ADRs, as “a response to a drug that is noxious and unintended and which occurs at doses normally used in man for the prophylaxis, diagnosis, or therapy of disease, or for modification of physiological function” [3].
According to previous studies, ADRs can manifest in both ambulatory and hospitalized patients, but variation often exists in terms of incidence rates, types, and intensity of these reactions [4]. A comprehensive study conducted in the late 1990s and early years of the 21st century showed that ADRs significantly contribute to hospital admissions, ranking as the fourth or sixth leading cause of death [5]. Over the past decade, several studies have shown that these reactions account for approximately 0.2%–59.6% of hospital admissions [6–12]. A recent report conducted by Abu et al. [13] also showed an incidence rate of 0.03%–17.11%. Moreover, hospitalized patients have been reported to be more vulnerable to ADRs due to several factors, including the use of more complex treatment regimens, compromised organ functions, and underlying health issues [12].
In line with previous reports, approximately 6.5%–20% of patients who are admitted to the hospital experience ADRs during treatment [2,6,11,14]. The repercussions of these reactions are substantial, contributing to a decline in patients’ quality of life, an increase in hospital length of stay and mortality rate, and an increment in healthcare costs [14]. Given the gravity of these repercussions, vigilant monitoring throughout the medication administration process is imperative to promptly identify ADRs and ensure patients’ safety. The active participation of Healthcare Professionals (HCPs) worldwide in activities relating to their detection, assessment, understanding, and prevention, collectively known as pharmacovigilance (PV), has proven highly beneficial in upholding patient safety [15], but there are still problems of underreporting in most countries [16,17].
Despite the growth in ADRs reporting in recent years, Indonesia’s reporting rates have consistently remained low. Based on the global reporting map of the World Health Organization, the yearly count of reports in Indonesia is below 10,000 [18]. In addition, low reports from HCPs, who are key players, are attributed to various factors, such as lack of awareness and knowledge on what, when, and to whom to report, lack of time, unavailability of reporting forms, uncertainty regarding the suspected drug, workload for taking care of patients, and the lack of incentive or remuneration [19].
The essential aspect of evaluating these reactions lies with establishing a causal relationship between medications and adverse clinical events [20,21]. While ADRs causality evaluation is extensively practiced in high-income countries as part of PV activities, there is a noticeable scarcity of studies addressing this topic in low- and middle-income countries. A precise categorization of causality for ADRs, particularly in institutions providing high-complexity care, holds the potential to facilitate early diagnosis, prevent recurrence, and optimize drug therapy, thereby enhancing the overall quality of patient care [22].
The World Health Organization Collaborating Centre for International Drug Monitoring-Uppsala Monitoring Centre (WHO-UMC) causality evaluation system and the Naranjo probability scale are widely established and frequently used methodologies for determining causation. Most global regulatory bodies recommend using the WHO-UMC scale for standardized case causality assessment, while many clinicians use the Naranjo Probability Scale (NPS) due to its simplicity [23,24]. The NPS is widely employed by the majority of HCPs in Indonesia for conducting ADR causality analysis. The NPS has been introduced as part of the undergraduate education program at the university. It can also be readily accessed on the manual ADR report sheet issued by the PV Center. HCPs are more acquainted with NPS due to this reason. Despite the availability of necessary tools, the study of ADRs and their causality, drawing upon a substantial volume of primary data collected over the years, remains relatively unexplored in Indonesia. Therefore, this study aims to gather a comprehensive overview of spontaneous ADR reports recorded by the ADR monitoring unit at a national cancer hospital in Indonesia. This includes assessments of the drug-reaction relationship, as well as the severity of the reaction carried out by HCPs.
METHODS
Study design
This hospital-based retrospective cross-sectional study was conducted to evaluate ADRs spontaneously reported by HCPs at Dharmais Cancer Hospital (National Cancer Center Indonesia) from January 2021 to December 2022. Furthermore, the study was initiated after obtaining clearance from the Institute’s Ethical Committee, under the reference number 049/KEPK/I/2023, on February 2, 2023.
Data collection
Data were obtained from all ADRs reports submitted to ADRs monitoring unit during the study period. All spontaneous reports from physicians, pharmacists, or nurses that complied with the validation criteria (data completeness; identifiable patient, identifiable reporter, at least one ADRs, at least one suspected drug, and level of causality derived from reporter’s assessment using NPS) were also collected.
Data classification
Spontaneous reports were characterized based on the following criteria:
Age
Regarding age, reports were classified into 4 groups, namely 1–17 years old (child), 18–24 years old (adolescence), 25–64 years old (adult), and ³65 years old (elderly). This classification was already used by the PV Center of Indonesia.
Clinical manifestations
Clinical manifestations of ADRs were defined based on the Medical Dictionary for Regulatory Activities (MedDRA) term using MedDRA Version 25.1 at the System Organ Class (SOC) level [25].
Suspected medications
The suspected drug was classified according to active ingredients using the anatomical therapeutic chemical (ATC) classification up to the second level (therapeutic subgroup).
Causality assessment
The causality of ADRs was assessed using the NPS, and the scale consisted of a questionnaire, which contained 10 questions with the options yes, no, and do not know. In addition, the probability was determined based on a rating system consisting of 4 categories, including definite (score >9), probable (5–8), possible (1–4), or doubtful (0) [26].
Seriousness of reactions
Serious reactions were classified as fatal, life-threatening, requiring hospitalization, or prolongation of existing hospitalization, leading to persistent or significant disability or incapacity, a congenital anomaly or birth defect, and other medically important conditions [27].
Severity assessment
A severity assessment was performed using the NCI CTCAE V.5.0 scale [28]. The NCI CTCAE V.5.0 scale categorized adverse events (AEs) into different severity levels, ranging from grade 1 to grade 5 (grade 1, mild; grade 2, moderate; grade 3, severe; grade 4, life-threatening; grade 5, death-related to AE).
Data analysis
Data obtained were inputted into Microsoft Excel 2019 and analyzed using the Statistical Package for the Social Sciences (SPSS) version 21. A descriptive analysis was then conducted to evaluate the frequencies and percentages.
RESULTS
A total of 564 spontaneous ADR reports were received by ADRs monitoring unit of Dharmais Cancer Hospital during the period from January 1st, 2021, to December 31st, 2022. There were a total of 553 ADRs reported in 280 patients.
Patient characteristics
Demographic trends in the incidence of ADRs showed that most of the reactions were found in the female group (65%) and among the adult age category, namely between 25 and 64 years old (74%). In addition, 15% of ADRs had been reported in the elderly group, while 6%–5% were reported in the child and adolescent groups, respectively. The patients were categorized into 3 diagnostic groups based on their underlying primary disease, including malignant solid tumor, hematologic malignancy, and non-cancer, as shown in Table 1. The highest number of ADRs was observed in patients with malignant solid tumors, where breast cancer had the highest incidence (32.1%), followed by head and neck cancer (11.1%) and lung cancer (10.4%) (Fig. 1). Among the subcategories of hematological malignancy, acute myeloid leukemia was the most common primary disease observed (4.3%), as shown in Figure 1. Table 1 displays the number of medications taken by patients at the time adverse reactions occurred. Approximately 67% of patients utilized between 6 and 10 medications, while 24% used less than 5%–9% consumed more than 10.
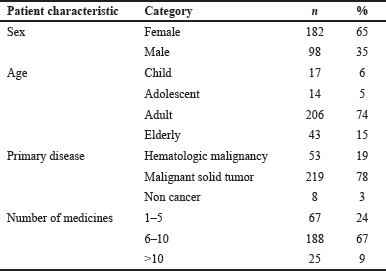 | Table 1. Descriptive data of patients identified with ADR. [Click here to view] |
ADRs characteristic
The classification of ADRs based on SOC showed that gastrointestinal (GI) system disorders were the most prevalent, accounting for 42.7% of cases, followed by skin and subcutaneous tissue (13.4%) and nervous system disorders (12.8%). The types and occurrence of ADRs in various organ systems are summarized in Table 2. Nausea remained the most common (11.6%), followed by vomiting (9.9%), and constipation (7.2%) in this current study. Furthermore, apart from the top 3, rash (5.6%), diarrhea (4.9%), dyspnea (n = 3.8%), peripheral neuropathy (3.4%), abdominal pain (2.7%), pruritus (2.5%), and headache (2.4%) were also documented as top contributors. Figure 2 shows the 10 most prominent ADRs identified in this study.
Suspected drug characteristics
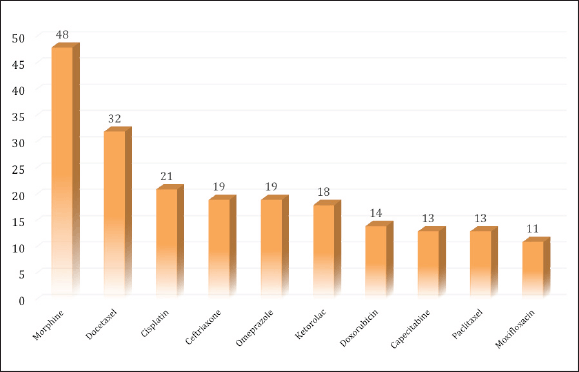
The most common therapeutic category of medications causing ADRs according to the ATC classification included antineoplastic and immunomodulating therapies, contributing 43.2% of the overall drug count. This group comprised antineoplastic drugs, accounting for 41.2%, and endocrine therapy was 2.0%. In addition, the second most suspected drugs were drugs classified in the therapeutic category affecting the nervous system (18.3%) which were dominated by analgesics (15.9%). Meanwhile, the third most suspected drug was systemic anti-infective drugs (14.1%), with antibacterials being the main contributor (8.3%) (Table 3). Morphine, Docetaxel, and Cisplatin were the most frequently reported causing ADRs. The top 10 most frequent drug suspects is shown in Figure 3. Table 4 shows the top ten suspected drugs along with the corresponding reactions.
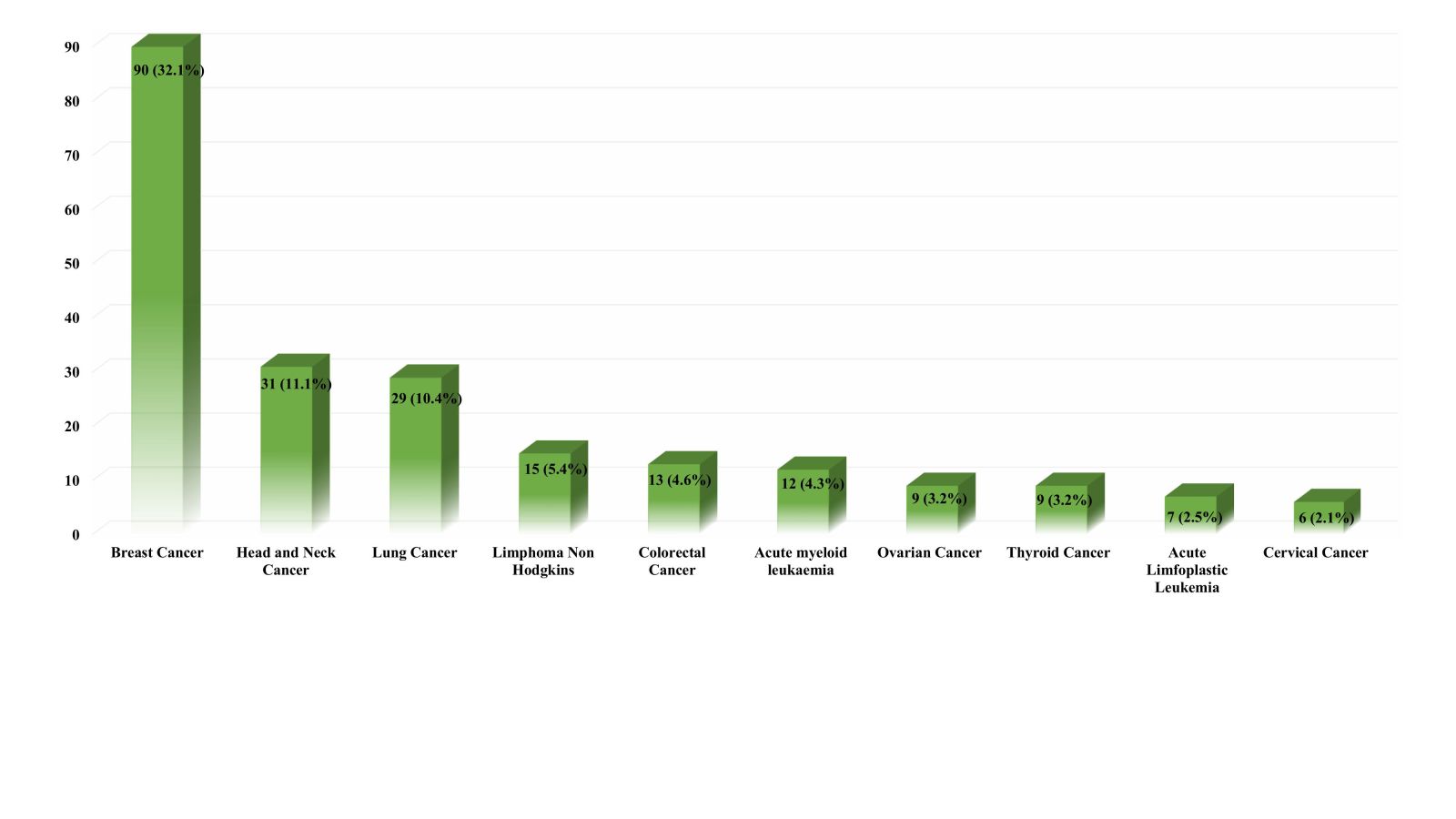 | Figure 1. Top 10 primary diagnoses among patients experiencing ADRs at Dharmais Cancer Hospital. [Click here to view] |
Assessment of ADRs
Causality assessment by the NPS showed that among 553 ADRs, 62.4%, 27.8%, 8.9%, and 0.9% were probable, definite, possible, and doubtful, respectively (Fig. 4).
In terms of seriousness, 21.7% of reports were classified as serious, comprising 5 life-threatening cases, 114 cases requiring hospitalization or prolonged hospitalization, and 1 case related to another medically important condition (Figs. 5a and b). The medications identified as the causative agents for the life-threatening cases were asparaginase (L01XX02), docetaxel (L01CD02), paclitaxel (L01CD01), and morphine (N02AA01). These drugs caused dyspnea and severe allergic responses, specifically anaphylaxis. In addition, one of the medically important conditions was related to oxaliplatin-induced seizures.
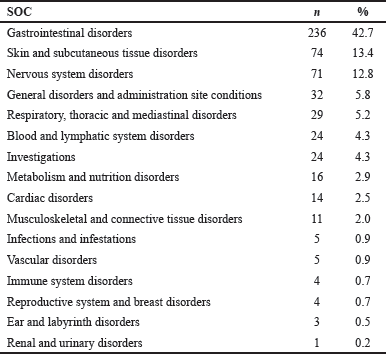 | Table 2. Type of ADRs according to the SOC. [Click here to view] |
Severity assessment using the NCI CTCAE V.5 scale showed that among 553 ADRs, the highest was grade 2, moderate (48.8%), 29% was grade 1 (mild), 20.6% was severe (grade 3), and 1.1% as life-threatening (grade 4). These reactions were either transient or could be treated with additional therapy. In addition, no deaths resulting from ADRs were observed in the current study, as shown in Figure 5c. Intervention through hospitalization or prolonged hospitalization was required for ADRs with a severity level of 3 (severe), while those at a severity level of 4 required urgent and rigorous medical intervention. Certain ADRs were rated severity 3 (severe) due to limiting patient self-care ADLs (Activities of Daily Living), including some cases of abdominal pain, insomnia, constipation, and fatigue.
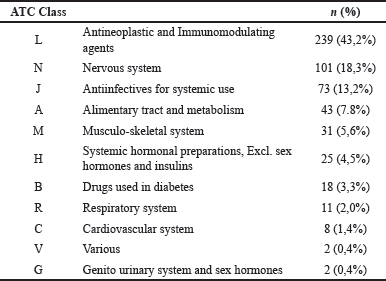 | Table 3. Descriptive data on suspected drugs based on ATC code. [Click here to view] |
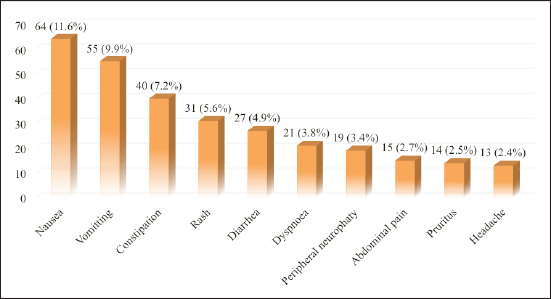 | Figure 2. Top 10 ADRs reported by HCPs. [Click here to view] |
 | Figure 4. Distribution of causality assessment by HCPs using NPS. [Click here to view] |
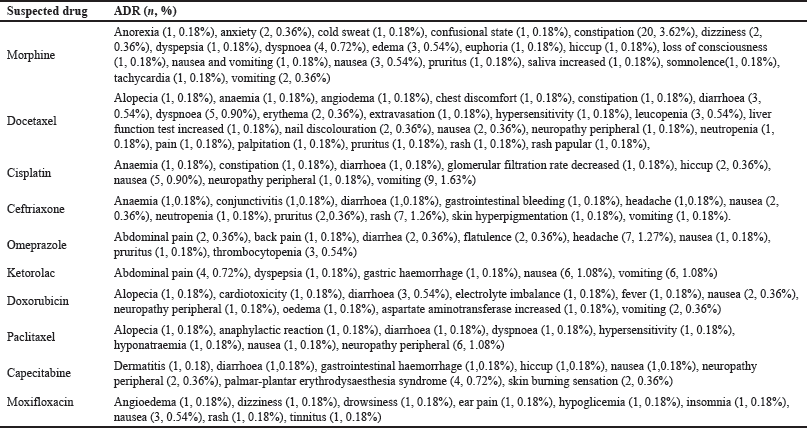 | Table 4. A list of top ten suspected drugs with corresponding reactions. [Click here to view] |
Regarding reporter qualification, pharmacists were the most frequent reporters (91%) of ADRs in this study, followed by doctors (6%) and nurses (13%) (Fig. 6).
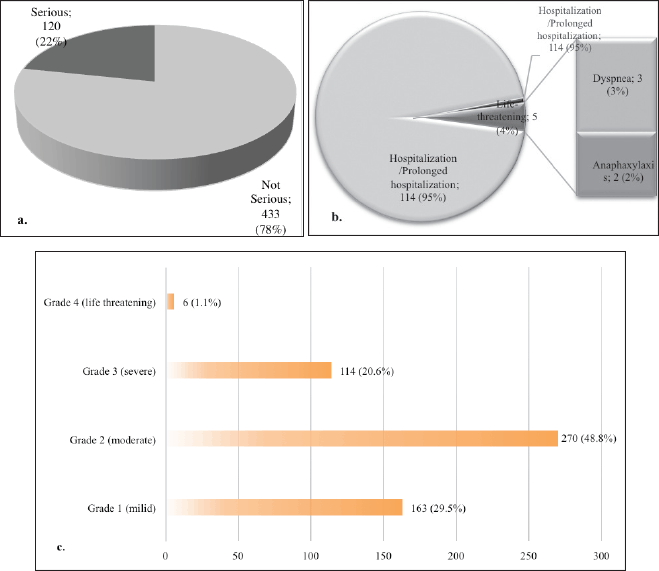 | Figure 5. Descriptive details of the seriousness and severity level of ADRs; a. Seriousness of ADRs; b. The classification of the seriousness of ADRs; c. severity level of ADRs. [Click here to view] |
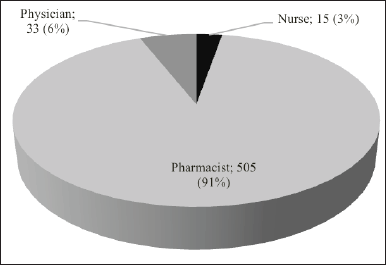 | Figure 6. Distribution of ADRs reporter HCPs based on reporter qualification. [Click here to view] |
DISCUSSION
ADRs remain a great concern in clinical practice, despite extensive studies dedicated to their occurrence. Implementing a continuous monitoring and reporting program in hospitals provides some beneficial purposes. These include evaluating the safety of pharmacological therapies, measuring the incidence of ADRs over a certain period, and educating HCPs about the effects of drugs, thereby enhancing their knowledge and awareness [29]. Periodic assessment of ADRs frequency and trends is crucial since disseminating this information to HCPs contributes to enhancing drug safety within the institution and on a national and global scale.
Upon collecting and analyzing ADRs data, it was observed that most of the ADRs occurred among adult patients in the age group of 25–64 years old (74%). In addition, the average age of the patients was 50.01 ± 14.08 years, indicating a more susceptible age group to developing ADRs during treatment in the hospital. This result was similar to the previous studies [30–34]. The majority of the reactions found could be attributed to the fact that individuals in this age group usually have multiple comorbidities, leading to the consumption of more medications. The drug regimen which involve a combination of five or more medications is called polypharmacy [35], which might increase the potential risk of ADR-related events [36,37]. In this study, the incidence of ADRs was higher among females (65%) compared to males (35%). Among the female population, breast cancer patients showed the highest potential to experience ADRs (32.1%). This is probably due to the high prevalence of breast cancer in Indonesia, where it continues to remain in the top position [38]. High rates of ADRs in the female population with breast cancer have also been reported by several studies [31,34,39].
Several studies have shown that ADRs often impact various physiological systems or organs. In addition, the GI system emerged as the most frequently affected system, since 2 of the 3 main classes of drugs manifest symptoms related to the GI system. For instance, morphine dominantly causes constipation, while cisplatin-based chemotherapy induces nausea and vomiting (CINV) [30,31,40]. The next most common systems associated with the ADRs were skin and subcutaneous tissue, which is commonly found in cancer treatment and easy to observe [41]. According to previous studies, rash, and pruritus were the two most common ADRs in this SOC, and similar results were obtained by Garg et al. [40] and Sharma et al. [33]. The nervous system was the other most common system affected in the ADRs. The prevalence of neuroactive drug utilization in cancer patients was indisputably substantial, comprising premedication as well as adjuvant or supportive therapy.
In the current study, the most common ADRs reported were nausea, followed by vomiting and constipation. This finding is in accordance with the investigations conducted by Poddar et al. [34], which identified nausea and vomiting as the most prevalent ADRs, while Tamang et al. [31] found constipation to be the most frequent. CINV is a debilitating adverse reaction of cancer therapy, impacting at least 40% of patients [42] and becoming the most prevalent and fearful adverse reaction experienced by patients. As much as 72.2% incidence of ³ grade 2 CNIV was observed in this study. This value is higher than the findings reported by Dranitsaris et al. [42], where about 42.2% of patients encountered ³ grade 2 CNIV. This population is highly prone to experiencing a significant deterioration in their quality of life [43–45]. In a study conducted by Surendiran et al. [46], reactions, such as nausea and vomiting belonged to the category of “definitely preventable.” Previous studies have shown that the administration of appropriate prophylactic medications, with careful consideration of the timing and duration of administration, could potentially prevent additional episodes of CINV [47,48]. Following nausea and vomiting, constipation emerged as the second most prevalent ADRs in our study, with a prevalence rate of approximately 7.2%. This percentage was lower than the values reported by other studies [30,49,50]. It is important to note that the constipation cases identified in the present study were classified as grade 1 and 2 and could be effectively treated with laxatives and appropriate dietary modification.
Morphine is the drug with the highest contribution to the incidence of ADRs in this study since it is the first-line therapy for cancer-related pain experienced by most patients [51–53]. Based on the WHO step ladder, morphine as a strong opioid could be given to patients, starting from mild pain with or without a combination of NSAIDs and adjuvants [54]. According to previous reports, there was no maximum dose limit required, indicating that the term “ceiling dose” often had an adverse impact when patients were not properly monitored, both in terms of efficacy and drug safety [54,55].
Based on this study, most of ADRs occurred due to the medications from the antineoplastic group (ATC code L01), specifically docetaxel and cisplatin, as the second and third suspected substances, respectively. Docetaxel, a member of the taxane class, was extensively employed as an antineoplastic agent in various chemotherapy protocols. TP (docetaxel and cisplatin), TCH (docetaxel, carboplatin, and trastuzumab), and TPF (docetaxel, cisplatin, and 5-fluorouracil) regimens were the preferred treatment options for breast cancer in current hospital. Currently, taxane-based regimens are the predominant option for chemotherapy in the treatment of breast cancer in Indonesia [56,57]. Cisplatin is a commonly used chemotherapeutic drug for the treatment of various types of cancer. However, the prevalence of its adverse effects, particularly nausea and vomiting, restricted its extensive application [33,39,58,59].
Causality assessment of ADRs using NPS showed that the majority of the reactions belonged to the “probable” category, followed by the “definite” and “possible” categories with the lowest percentage of doubtful cases. This finding is similar to the studies conducted by Tamang et al. [31], Garg et al. [40], and Surendiran et al. [46] where the causality of most ADRs was probable. Wahlang et al. [30] and Chopra et al. [58] found that most ADRs were in the “possible” category, followed by the “probable” category. Most of the reactions in the current study were moderate, followed by mild categories. According to studies conducted on severity assessment using the Hartwig and Siegel scale, most reactions were moderate [31,40], and some were mild [30,33,46]. Withdrawal and alteration of pharmacological therapy are typically unnecessary for both mild and moderate reactions.
To obtain an accurate ADRs diagnosis, comprehensive data used in causality analysis is essential. Therefore, the evaluation carried out by HCPs who initially detected ADRs is likely to be more reliable and trustworthy than that conducted by an external entity, such as the PV center. This is because the HCPs have the privilege of repeatedly observing the patient’s condition, accessing essential data such as laboratory results and diagnostic tests, and consulting with the doctor to gain insights into their experience with the incident and the potential for re-challenge. The instruments utilized in causality assessment often include queries that indirectly prompt HCPs to get the information required for addressing the presented inquiries.
The current study documented that pharmacists reported a larger number of ADRs compared to other HCPs. A consistent finding was also reported in previous studies [60–64]. This might be attributed to the better awareness, knowledge, attitude, and practice of ADRs and PV concepts among pharmacists which is due to the pharmacists’ education on drug safety [65,66]. In addition, monitoring ADRs is a clinical pharmacy activity mentioned in the Minister of Health’s Decree on Pharmaceutical Service Standards in hospitals in Indonesia.
However, this study had some inherent limitations. While it was possible to access the original reports, not all missing data could be retrieved. Moreover, since this study was conducted at a single institution, it is important to note that the results might not be able to be generalized and may not fully represent the overall picture. This study did not provide paired drug-ADRs information, thereby restricting the comprehension of medications that could potentially be accountable for the incidence of ADRs. Furthermore, variations in results could inevitably arise due to factors such as study design, demographics, sample size, and the characteristics of specific clinical practices or regions, which may be influenced by local regulations.
CONCLUSION
In conclusion, ADRs were caused by various suspected drugs, and reactions varied from mild to life-threatening. This study emphasized the importance of actively monitoring patients to rapidly identify and manage ADRs. Further prospective studies are needed to explore ADRs reporting from HCPs. The use of online reporting systems and tools for the detection and assessment of ADRs is recommended to save time and facilitate reporting among HCPs.
ACKNOWLEDGMENTS
The authors are grateful to the clinical pharmacists’ team at the Department of Pharmacy, both technical and administrative for their cooperation in this study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This study was supported by the Ministry of Research, Technology, and Higher Education Republic of Indonesia, which provided financial support through Hibah Penelitian Disertasi Doktor [PDD] [Grant No. 018/E5/PG.02.00.PL/2023; 2147/UN1/DITLIT/DitLit/PT.01.03/2023).
ETHICAL APPROVALS
Ethical approval details are given in 'Methods section'.
CONFLICT OF INTEREST STATEMENT
The authors report no financial or any other conflicts of interest in this work.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Kiguba R, Karamagi C, Bird SM. Incidence, risk factors and risk prediction of hospital-acquired suspected adverse drug reactions: a prospective cohort of Ugandan inpatients. BMJ Open. 2017;7(1):1–11.
2. Montastruc JL, Lafaurie M, de Canecaude C, Durrieu G, Sommet A, Montastruc F, et al. Fatal adverse drug reactions: a worldwide perspective in the World Health Organization pharmacovigilance database. Br J Clin Pharmacol. 2021 Nov;87(11):4334–40.
3. WHO. International drug monitoring: the role of national centres. Report of a WHO meeting. Geneva, Switzerland: World Health Organization—Technical Report Series; 1972;Vol. 498, p. 1–25.
4. Kane Gill SL, Den Bos J Van, Handler SM. Adverse drug reactions in hospital and ambulatory care settings identified using a large administrative database. Ann Pharmacother [Internet]. 2010 May 4;44(6):983–93. Available from: https://doi.org/10.1345/aph.1M726
5. Le Louët H, Pitts PJ. Twenty-First Century Global ADR Management: a need for clarification, redesign, and coordinated action. Ther Innov Regul Sci. 2023 Jan;57(1):100–3.
6. Angamo MT, Chalmers L, Curtain CM, Bereznicki LRE. Adverse-drug-reaction-related hospitalisations in developed and developing countries: a review of prevalence and contributing factors. Drug Saf. 2016;39(9):847–57.
7. Sonal Sekhar M, Adheena Mary C, Anju PG, Hamsa NA. Study on drug related hospital admissions in a tertiary care hospital in South India. Saudi Pharm J SPJ Off Publ Saudi Pharm Soc. 2011 Oct;19(4):273–8.
8. Yadesa TM, Kitutu FE, Deyno S, Ogwang PE, Tamukong R, Alele PE. Prevalence, characteristics and predicting risk factors of adverse drug reactions among hospitalized older adults: a systematic review and meta-analysis. SAGE Open Med. 2021;9:20503121211039100.
9. Klopotowska JE, Wierenga PC, Stuijt CCM, Arisz L, Dijkgraaf MGW, Kuks PFM, et al. Adverse drug events in older hospitalized patients: results and reliability of a comprehensive and structured identification strategy. PLoS One. 2013;8(8):e71045.
10. Schurig AM, Böhme M, Just KS, Scholl C, Dormann H, Plank-Kiegele B, et al. Adverse drug reactions (ADR) and emergencies. Dtsch Arztebl Int. 2018 Apr;115(15):251–8.
11. Alhawassi TM, Krass I, Bajorek B, Pont LG. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging. 2014;9:2079–86.
12. Kauppila M, Backman JT, Niemi M, Lapatto-Reiniluoto O. Incidence, preventability, and causality of adverse drug reactions at a university hospital emergency department. Eur J Clin Pharmacol. 2021 Apr;77(4):643–50.
13. Abu SF, Shafie AA, Chandriah H. Cost estimations of managing adverse drug reactions in hospitalized patients: a systematic review of study methods and their influences. Pharmacoepidemiology. 2023;Vol. 2:. 120–39.
14. Bond CA, Raehl CL. Adverse drug reactions in United States hospitals. Pharmacotherapy. 2006 May;26(5):601–8.
15. Pal SN, Duncombe C, Falzon D, Olsson S. WHO strategy for collecting safety data in public health programmes: complementing spontaneous reporting systems. Drug Saf. 2013;36(2):75–81.
16. Olsson S, Pal SNSN, Dodoo A. Pharmacovigilance in resource-limited countries. Expert Rev Clin Pharmacol. 2015;8(4):449–60.
17. Vallano A, Cereza G, Pedròs C, Agustí A, Danés I, Aguilera C, et al. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol. 2005;60(6):653–8.
18. Badan Pengawas Obat dan Makanan Republik Indonesia. Buletin berita MESO Badan POM. 2021;39(1):23–9.
19. Putri RA, Ikawati Z, Rahmawati F, Yasin NM. An awareness of pharmacovigilance among healthcare professionals due to an underreporting of adverse drug reactions issue: a systematic review of the current State, Obstacles, and Strategy. Curr Drug Saf. 2023;19:317–31.
20. Marante KB. The challenges of adverse drug reaction evaluation. J Pharmacovigil. 2018;06(03):1–4.
21. Hammad TA, Afsar S, McAvoy LB, Le Louet H. Aspects to consider in causality assessment of safety signals: broadening the thought process. Front Drug Saf Regul. 2023;3(May):1–9.
22. Rezende de Menezes R, Graciano Silva M das D, Pinho Ribeiro AL, Martins Pinto Filho M, Martinho GH, Carvalho Ferreira LE, et al. Causality assessment of adverse drug reactions by applying a global introspection method in a high complexity hospital. Explor Res Clin Soc Pharm. 2021;3:100064.
23. WHO-UMC. The use of the WHO-UMC system for standardised case causality assessment: Available from: http://who-umc.org/Graphics/24734.pdf.2015
24. Sharma S, Gupta AK, Reddy GJ. Inter-rater and intra-rater agreement in causality assessment of adverse drug reactions: a comparative study of WHO-UMC versus Naranjo scale. Int J Res Med Sci. 2017;5(10):4389.
25. MEdDRA. Introductory guide MedDRA version 25.1. analysis. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH); 2022.
26. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.
27. Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician’s guide to terminology, documentation, and reporting. Ann Intern Med. 2004;140(10):795–801.
28. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v.5.0. Cancer Ther Eval Progr [Internet]. 2017;155. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
29. American Society of Hospital Pharmacy. ASHP guidelines on adverse drug reaction monitoring and reporting. American Society of Hospital Pharmacy. Am J Health Syst Pharm. 1995 Feb;52(4):417–9.
30. Wahlang JB, Laishram PD, Brahma DK, Sarkar C, Lahon J, Nongkynrih BS. Adverse drug reactions due to cancer chemotherapy in a tertiary care teaching hospital. Ther Adv drug Saf. 2017 Feb;8(2):61–6.
31. Tamang R, Bharati L, Khatiwada AP, Ozaki A, Shrestha S. Pattern of adverse drug reactions associated with the use of anticancer drugs in an oncology-based hospital of Nepal. JMA J. 2022 Oct;5(4):416–26.
32. Shrestha S, Shakya R, Shrestha S, Shakya S. Adverse drug reaction due to cancer chemotherapy and its financial burden in different hospitals of Nepal. Int J Pharmacovigil. 2017;2:1–7.
33. Sharma PK, Misra AK, Gupta A, Singh S, Dhamija P, Pareek P. A retrospective analysis of reporting of adverse drug reactions to oncology drugs: an experience from a national center of clinical excellence. Indian J Pharmacol. 2018;50(5):273–8.
34. Poddar S, Sultana R, Sultana R, Akbor MM, Azad MAK, Hasnat A. Pattern of adverse drug reactions due to cancer chemotherapy in tertiary care teaching hospital in Bangladesh. Dhaka Univ J Pharm Sci. 2009;8(1):11–6.
35. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? a systematic review of definitions. BMC Geriatr. 2017 Oct;17(1):230.
36. Osanlou R, Walker L, Hughes DA, Burnside G, Pirmohamed M. Adverse drug reactions, multimorbidity and polypharmacy: a prospective analysis of 1 month of medical admissions. BMJ Open. 2022 Jul;12(7):e055551.
37. Rodrigues MCS, De Oliveira C. Drug-drug interactions and adverse drug reactions in polypharmacy among older adults: an integrative review. Rev Lat Am Enfermagem. 2016;24:e2800.
38. The Global Cancer Observatory. Cancer Incident in Indonesia. Int Agency Res Cancer [Internet]. 2020;858:1–2. Available from: https://gco.iarc.fr/today/data/factsheets/populations/360-indonesia-fact-sheets.pdf
39. Kirthi C, Afzal A, Reddy M, Ali S, Yerramilli A, Sharma S. A study on the adverse effects of anticancer drugs in an oncology center of a tertiary care hospital. Int J Pharm Pharm Sci. 2014;6:580–3.
40. Garg K, Ashish T, Kataria SP, Aarushi M, Perumal R. To study the pattern, causality, severity and predictability of adverse drug reactions in patients on cancer chemotherapy: descriptive research-qualitative study. Turkiye Klin J Med Sci. 2022;42(2):130–7.
41. Cury-Martins J, Eris APM, Abdalla CMZ, Silva G de B, Moura VPT de, Sanches JA. Management of dermatologic adverse events from cancer therapies: recommendations of an expert panel. An Bras Dermatol. 2020;95(2):221–37.
42. Dranitsaris G, Molassiotis A, Clemons M, Roeland E, Schwartzberg L, Dielenseger P, et al. The development of a prediction tool to identify cancer patients at high risk for chemotherapyinduced nausea and vomiting. Ann Oncol [Internet]. 2017;28(6):1260–7. Available from: https://doi.org/10.1093/annonc/mdx100
43. Gupta K, Walton R, Kataria SP. Chemotherapy-induced nausea and vomiting: pathogenesis, recommendations, and new trends. Cancer Treat Res Commun [Internet]. 2021;26:100278. Available from: https://www.sciencedirect.com/science/article/pii/S2468294220301131
44. Hoskins P. Guidelines for prevention and treatment of chemotherapy-induced nausea and vomiting in adults. BC Cancer Agency [Internet]. 2012;2021(May 1999):1–6. Available from: http://www.bccancer.bc.ca/NR/rdonlyres/2EB89118-69EA-4AF6-910A-952FFE05F5CB/10717/SCNAUSEA1Nov06.pdf
45. Clark-Snow R, Affronti M Lou, Rittenberg CN. Chemotherapy-induced nausea and vomiting (CINV) and adherence to antiemetic guidelines: results of a survey of oncology nurses. Support Care Cancer. 2018;26(2):557–64.
46. Surendiran A, Balamurugan N, Gunaseelan K, Akhtar S, Reddy KS, Adithan C. Adverse drug reaction profile of cisplatin-based chemotherapy regimen in a tertiary care hospital in India: an evaluative study. Indian J Pharmacol. 2010 Feb;42(1):40–3.
47. Aapro M, Scotté F, Escobar Y, Celio L, Berman R, Franceschetti A, et al. Practice patterns for prevention of chemotherapy-induced nausea and vomiting and antiemetic guideline adherence based on real-world prescribing data. Oncologist. 2021;26(6):e1073–82.
48. Escobar Álvarez Y, De Castro Carpeño J, Bell D, Drago A, Franceschetti A. Prevention of chemotherapy-induced nausea and vomiting in the real-world setting in Spain. Clin Transl Oncol [Internet]. 2021;23(10):2155–62. Available from: https://doi.org/10.1007/s12094-021-02623-8
49. Saini VK, Sewal RK, Ahmad Y, Medhi B. Prospective observational study of adverse drug reactions of anticancer drugs used in cancer treatment in a tertiary care Hospital. Indian J Pharm Sci. 2015;77(6):687–93.
50. Lau PM, Stewart K, Dooley M. The ten most common adverse drug reactions (ADRs) in oncology patients: do they matter to you? Support Care Cancer. 2004 Sep;12(9):626–33.
51. Swarm RA, Paice JA, Anghelescu DL, Are M, Bruce JY, Buga S, et al. Adult cancer pain, version 3.2019. J Natl Compr Cancer Netw. 2019;17(8):977–1007.
52. Fallon M, Giusti R, Aielli F, Hoskin P, Rolke R, Sharma M, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29(July):166–91.
53. Makhlouf SM, Pini S, Ahmed S, Bennett MI. Managing pain in people with cancer—a systematic review of the attitudes and knowledge of professionals, patients, caregivers and public. J Cancer Educ. 2020;35(2):214–40.
54. World Health Organization. WHO guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents. Geneva, Switzerland: World Health Organization; 2018.
55. Su YJ, Yan YD, Wang WJ, Xu T, Gu ZC, Bai YR, et al. Drug-related problems among hospitalized cancer pain patients: an investigative single-arm intervention trial. Ann Palliat Med. 2021;10(2):2008–17.
56. Haryono SJ, Sutandyo N, Karsono R, Karsono B, Purwanto DJ, Dien A, et al. Treatment pattern and safety results of Docetaxel-(Taxotere®)-based chemotherapy in early breast cancer patients in Indonesia: part of Asia-Pacific Breast Initiative II. Indones J Cancer. 2019;13(1):1.
57. Aldo G, IRsan S, Nur Q, Mulawan U. Neoadjuvan chemotherapy response to local advanced breast cancer patients at Dr Mohammad Hoesin Hospital Palembang. 2022;3:51–8.
58. Chopra D, Rehan HS, Sharma V, Mishra R. Chemotherapy-induced adverse drug reactions in oncology patients: a prospective observational survey. Indian J Med Paediatr Oncol Off J Indian Soc Med Paediatr Oncol. 2016;37(1):42–6.
59. Shin Y, Kim B, Kim W. Cisplatin-induced nausea and vomiting: effect of herbal medicines. Plants. 2022;11(23):1–15.
60. Hussain R, Hassali MA, Hashmi F, Akram T. Exploring healthcare professionals’ knowledge, attitude, and practices towards pharmacovigilance: a cross-sectional survey. J Pharm Policy Pract [Internet]. 2021;14(1). Available from: https://doi.org/10.1186/s40545-020-00287-3
61. Ali I, Ahmad W, Ullah AR, Khan F, Ijaz M, Khan S, et al. Knowledge, attitude, and barriers toward ADRs reporting among health-care professionals at tertiary care health settings in Peshawar, Pakistan: a web based study. Hosp Pharm [Internet]. 2020 Apr 21;56(4):384–91. Available from: https://doi.org/10.1177/0018578720910401
62. Bogolubova S, Padayachee N, Schellack N. Knowledge, attitudes and practices of nurses and pharmacists towards adverse drug reaction reporting in the South African private hospital sector. Heal SA Gesondheid. 2018;23:1–9.
63. Sandberg A, Ehlers P, Torvinen S, Sandberg H, Sivén M. Regulation awareness and experience of additional monitoring among healthcare professionals in finland. Healthcare. 2021;9(11):1–12.
64. SN A, BA D. Pharmacovigilance practices: knowledge and attitudes among the Healthcare Professionals at the Volta Regional Hospital of Ghana. J Pharmacovigil. 2017;05(03):1–9.
65. Opadeyi AO, Fourrier-Réglat A, Isah AO. Educational intervention to improve the knowledge, attitude and practice of healthcare professionals regarding pharmacovigilance in South-South Nigeria. Ther Adv Drug Saf. 2019;10:204209861881627.
66. Alqahtani SS, Ahmad S, Alam N, Kashan Syed N, Syed MH, Khardali A, et al. Healthcare professionals’ awareness, attitudes and practices towards pharmacovigilance and spontaneous adverse drug reaction reporting in Jazan Province, Saudi Arabia: a survey study. Saudi Pharm J [Internet]. 2023;31(6):979–88. Available from: https://doi.org/10.1016/j.jsps.2023.04.021