INTRODUCTION
Interstitial lung diseases (ILDs) encompass a diverse range of lung conditions, with pulmonary fibrosis (PF) representing the ultimate common pathway of these disorders. ILDs differ in many ways, but they are grouped together because they have similarities clinically, radiographically, and physiologically. Among ILDs, idiopathic pulmonary fibrosis (IPF) is the most common and severe type [1].
IPF is a chronic, progressive, and predominantly fatal lung ailment of uncertain origin. It manifests with initial alveolitis progressing to fibrosis, resulting in symptoms such as dyspnea, extensive interstitial fibrosis, and impaired gas exchange. The diagnosis of IPF relies on a history of escalating exertional dyspnea and a persistent dry cough. The pathological hallmark confirming IPF is the presence of usual interstitial pneumonia upon biopsy. It is slightly more common in men than women [2]. As the lung tissue thickens, it is less able to carry oxygen into the circulation. As a result, patients suffer from shortness of breath. All vital organs were deprived of the oxygen necessary for survival [3].
The therapeutic experience of generations of physicians operating in indigenous medical systems has been synthesized into traditional medicine. The use of plant material as an indigenous treatment in folklore or traditional medical systems led to the introduction of plant-derived pharmaceuticals into contemporary medicine. Examining the rich history of traditional medicine is crucial, and since plant-based medications are inexpensive and rarely cause negative side effects, research is being done to determine their effectiveness in traditional medicine [4].
Even though a number of strategies were envisaged to protect the lungs against toxic agents, treatment options were also often tricky because of the profound side effects of the medicine used. Hence, lung disease remains one of the most serious health problems today, and there has been no effective drug for the treatment of lung disease. Limitations to such conventional treatment have encouraged the development of new treatment modalities [5].
Bleomycin is a chemotherapeutic antibiotic that has been commonly prescribed for Hodgkin lymphoma and testicular germ-cell tumors. The major limitation of bleomycin therapy is pulmonary toxicity. The patient’s symptoms and signs of bleomycin lung toxicity were reported such as dyspnea, cough, sputum, fever, thoracic pain, cyanosis, tachypnea, pleuritic pain, and pleural rubbing. It was widely recognized as one of the most commonly used inducers for creating a PF animal model, providing the advantage of rapid PF development. The control group treated with bleomycin exhibited notable thickening of alveolar walls, alveolar collapse, deposition of inflammatory cells, fibrotic changes, and collagen accumulation [6,7].
Phyllanthus acidus (L.) Skeel, a member of the Phyllanthaceae family, can be found across tropical and subtropical regions around the world. The fruits of P. acidus, popularly called as star gooseberry, Malay gooseberry, Otaheite gooseberry, Country gooseberry, Tahitian gooseberry, West India gooseberry, and Grosella, or simply gooseberry. The plant grows to a height of 2–9 m, and is a shrub-tree intermediate. The broad, bushy crown of the tree is made up of thick, sturdy main branches that terminate in clusters of 15–30 cm long, deciduous, greenish branchlets. The branchlets have alternating, lanceolate, or ovate-shaped leaves with short petioles and pointy tips. The thin, 2–7.5 cm-long leaves have a smooth, green upper surface and a blue-green underside. The bilimbi tree and the Otaheite gooseberry tree resemble each other quite a bit. The blooms may be hermaphrodite, male, or female. They are tiny, pinkish, and grouped together in panicles that are 5–12.5 cm long. At the top of the tree, in the leafless sections of the main branches, flowers develop. The fruits are many, oblate, tightly grouped, and have six to eight ribs. They are quite sour, crisp, juicy, waxy, pale yellow or white, and juicy. Every fruit has a stone in the middle that holds four to six seeds [8].
This plant serves a dual purpose, as its delectable fruits are edible, and traditionally, the peppered leaves are used to make a poultice to treat lumbago, sciatica, and rheumatism; seeds as cathartic; and roots as purgative. The various parts of the plant scientifically address a diverse range of health concerns, such as anti-inflammatory [9], cystic fibrosis [10], antinociceptive [11], diabetes [12], and hepatic disorders [13], antioxidant and cytotoxic activity [14], antibacterial activity [15], boosting of immunity [16] in Asia, Central and South America, and the Caribbean region.
Any pulmonary protective medicine’s efficacy depends on its capacity to either lessen side effects or maintain normal lung physiology after a drug or hazardous substance has upset it. In recent times, the exploitation of various drugs as pulmonary toxic models has gained importance. The reason underpinning this research work may be that the drugs in common use can impose noxious effects on the lungs, which can mimic almost every naturally occurring lung disease in man. In this study, the positive effects of P. acidus (L) Skeel fruits were evaluated in relation to the prevention of bleomycin-induced lung fibrosis in rats.
MATERIALS AND METHODS
Collection and identification
The fruits of P. acidus (L.) Skeel were collected, washed with water to eliminate any dust, and air-dried under shade. With the use of a hand mill, the material was turned into a coarse powder. Prof. (Dr.) K. Madhava Chetty, taxonomist, SV University, Tirupati, Andhra Pradesh, India, verified the plant material’s authenticity. Voucher specimen number: 1132 and reserved in the Department of Pharmacognosy, CMR College of Pharmacy, Hyderabad, India.
Extraction
The P. acidus fruit (100 g) powder was extracted with absolute alcohol (1,200 ml) at room temperature by maceration for 7 days. The container was shaken or agitated occasionally to ensure better extraction. Afterward, the filtrate was concentrated by a rotary flash vacuum evaporator. Finally, it was dried in desiccators and stored in airtight containers.
Qualitative phytochemical analysis
The PAE was subjected to the detection of phyto components present in the extract [17].
Acute toxicity study
The albino mice (4-5 weeks old, 15–20 g) were used for the confirmation of the maximum tolerable dose of PAE. The acute toxicity study was performed using OECD Guideline 425 (up-and-down method).
Pulmonary fibrosis
Male Wistar Albino rats (8–10 weeks old, 150–200 g), were procured from Jeeva Life Sciences (CPCSEA license approval No.1757/PO/ReBiBt/S/14/CPCSEA), Hyderabad, and were housed as per CCSEA, New Delhi, India.
Treatment schedule
Randomly, animals (N = 36) were divided into six groups with six animals in each group under identical conditions.
Group-I (Normal): Administered normal saline intra-tracheal injection.
Group II (Toxic control): Bleomycin (5 mg/kg in Normal saline) in a single intra-tracheal injection.
Group-III (Standard NAC): N-acetyl cysteine (NAC) (3 mmol/kg) orally for 7 days after bleomycin exposure.
Groups IV, V, and VI animals received PAE of 100, 200, and 400 mg/kg doses orally for 7 days after bleomycin exposure.
Following animal sacrifice by cervical dislocation under mild anesthesia, the lungs were separated, and a tiny portion of the lung was preserved in 10% formalin for histopathology. The lung’s remaining portion was ready for oxidative stress parameter evaluation.
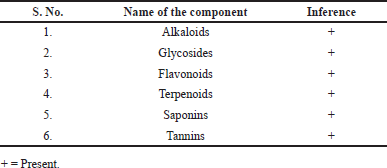 | Table 1. Qualitative phytochemical constituents of P. acidus (L) Skeel fruit extract. [Click here to view] |
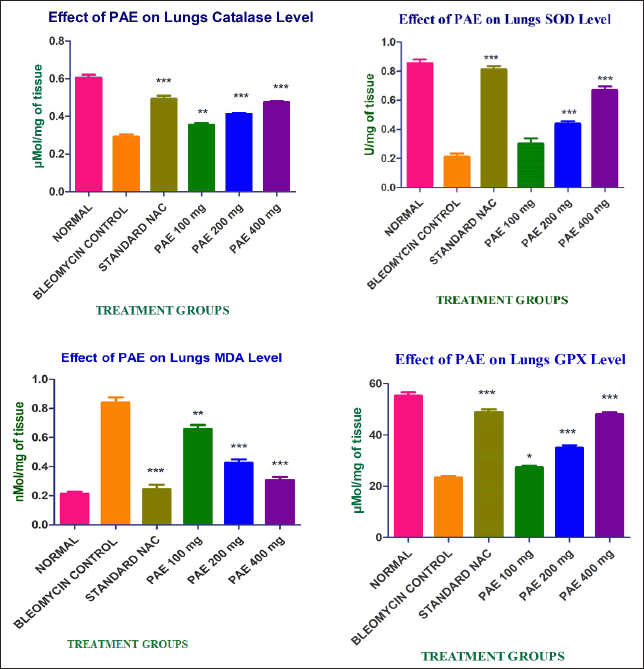 | Figure 1. Effect of PAE on lung tissue oxidants and antioxidants. [Click here to view] |
Collection of the bronchoalveolar lavage
A syringe, connected to a blunt needle, was employed to gently introduce a cannula into the trachea, facilitating the preparation of the lungs for lavage. The lung lavage was achieved by rinsing the lungs four times with 4 ml aliquots of saline through the tracheal cannula. Subsequent to this, the cell suspensions were concentrated using low-speed centrifugation, and the resulting cell pellet was then resuspended for further studies.
Estimation of cell counts (Total number of cells, Macrophages, Neutrophils, and Eosinophils)
Total cell counts were determined using a hemocytometer, while from cytospin preparations, the calculation of differential cell counts was carried out by assessing 300 cells stained with May-Grunwald-Giemsa [18].
Estimation of oxidants and antioxidants
The oxidative stress (OS) parameters such as Malondialdehyde (MDA) [19], Superoxide dismutase (SOD) [20], Catalase (CAT) [21], and Glutathione peroxidase (GPx) [22] were measured by analyzing the lung tissue homogenate with standard procedures.
Histopathological changes in lungs
The animals’ removed lungs were promptly cleaned with buffer and preserved in 10% buffered formalin upon sacrifice. They were then embedded in paraffin wax, cleaned in xylene, and dehydrated using a series of progressively stronger alcohols. Hematoxylin-eosin stain was applied after microtome cutting of sections with a thickness of 5–7 μm [23].
Statistical analysis
The Graph Pad Prism 5.0 software was used for Statistical analysis. Values are represented as Mean ± SEM (n = 6). Statistical analysis was performed using one-way ANOVA followed by post hoc Dunnett’s test, ***p < 0.001 versus; **p < 0.001, *p < 0.01 versus toxic group. Results are considered statistically significant where p < 0.05.
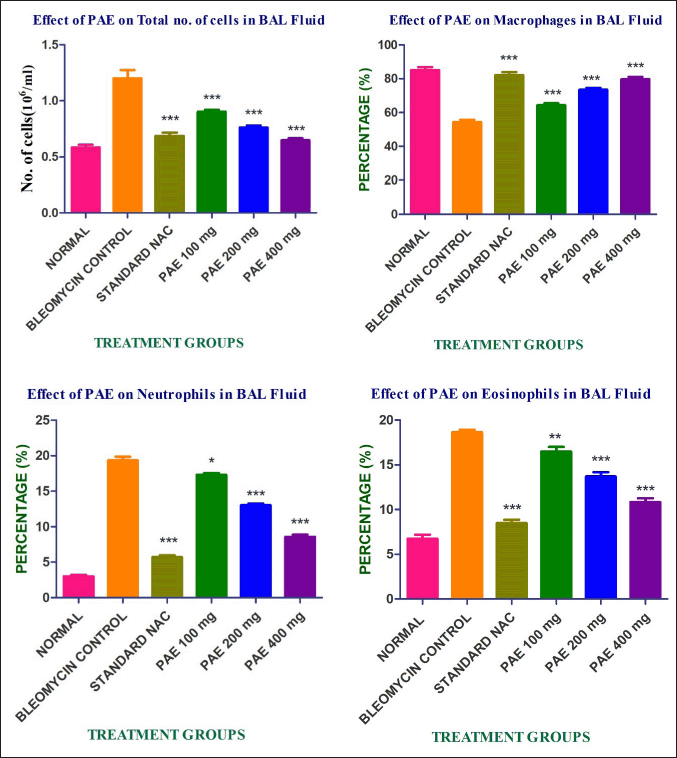 | Figure 2. Effect of PAE on bronchoalveolar lavage fluid cell profile. [Click here to view] |
RESULTS
Preparation of the P. acidus fruit extract
The dried powder of P. acidus fruit was extracted with ethanol by maceration at room temperature. The yield of the ethanolic extract of fruits was 8.25%.
Qualitative phytochemical analysis
The Qualitative phytochemical analysis of extract was found to be following secondary metabolites such as alkaloids, glycosides, flavonoids, terpenoids, saponins, and tannins. The results are shown in Table 1.
Acute toxicity study
There were no indications of vocalization, stereotypy, or passivity in the mice administered with 2,000 mg/kg of PAE orally. Additionally, they showed no symptoms of depression, and their motor activity, secretory indicators and also behavior were normal. The 1/5th, 1/10th, and 1/20th doses of PAE were chosen as treatment doses for evaluating the protective effect because the PAE did not cause any behavioral signs or death up to a dose level of 2,000 mg/kg.
Effect of PAE on lung tissue oxidants and antioxidants
The effect of PAE on lung tissue oxidants and antioxidants is shown in Figure 1.
Comparing the bleomycin-treated group to the normal control, there was a substantial increase in MDA levels. When PAE was administered instead of the usual medication NAC, MDA levels were reduced in a dose-dependent manner with a notable effect. The outcome suggests that PAE has an excellent line of defense against lung injury. The depletion activity of SOD levels in rats treated with bleomycin was compared to that of control animals in the current study. The SOD level was dramatically improved by the therapeutic treatment using the natural substance PAE in a dose-dependent manner when compared with toxic control. This finding suggests that dose-dependent free radical scavenging activity was enhanced by the PAE. In the current investigation, the administered PAE boosted lung tissue’s catalase activity and shielded it from OS brought on by free radicals after bleomycin exposure. When PAE was compared to the standard NAC, this finding validates its antioxidant capabilities in a dose dependent. In the context of bleomycin toxicity, there was a reduction in the activity of glutathione peroxidase in lung tissues, presumably as a response to counterbalance the free radical scavenging effect facilitated by glutathione as the substrate in comparison to the normal group of animals. Notably, the administration of PAC resulted in a dose-dependent increase in GPx activity.
Effect of P. acidus fruit extract on bronchoalveolar lavage fluid cell profile
Total and differential cell counts
Rats treated with bleomycin had significantly higher total and differential cell counts in their bronchoalveolar lavage fluid as compared to control rats. Administration of PAE dramatically reduced the cell numbers in a dose-dependent manner when compared to the toxic control and showed in Figure 2.
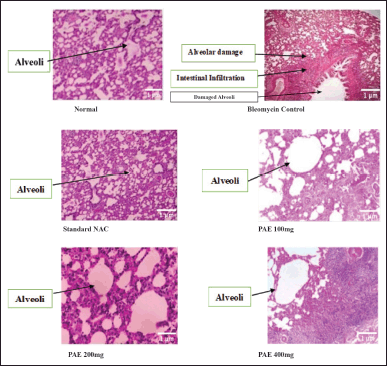 | Figure 3. Histopathological changes of lungs. [Click here to view] |
Histopathology study
Histological examination of lung tissue from the normal control group showed no pathological changes. The lung tissue from the bleomycin group showed a patchy, dense collection of lymphocytes and plasma cells, the alveoli showed prominent pneumocytes with widened intestibula, and severe acute interstitial pneumonia was observed in toxic control. The lung tissue from the standard drug NAC group showed macrophages and lymphocytes, and the alveoli showed mild widening of the interstitium. Mild acute interstitial pneumonia was observed. The lung tissue from the PAE 100 mg treatment showed mild recovery from acute interstitial pneumonia. PAE 200 mg treatment lung tissue showed moderate recovery from acute interstitial pneumonia. PAE 400 mg group showed a collection of macrophages and lymphocytes with normal widening of the interstitium with mild interstitial pneumonia observed. The results are shown in Figure 3.
DISCUSSION
The human lung stands out as the sole organ within the entire human body that experiences the most significant exposure to atmospheric oxygen. This heightened exposure results from the lung’s extensive surface area and rich blood supply, making it vulnerable to oxidative damage due to a wide range of reactive oxygen species and free radicals [24].
Bleomycin is a chemotherapy option for treating neoplastic diseases. Its primary drawback lies in its potential to cause pulmonary toxicity, a condition that can alter lung structure and result in a fatal outcome. The pulmonary damage triggered by bleomycin begins with the initial generation of oxidant species through an iron-dependent process. Subsequent harm likely stems from elevated levels of reactive species produced by activated inflammatory cells that are recruited to the injured lung [25].
Plant defense mechanisms include a combination of enzymatic as well as non-enzymatic systems to manage the excessive production of reactive oxygen and nitrogen species, as well as to enhance their tolerance to harmful stress. Furthermore, plants activate signaling molecules such as proline, which trigger a range of physiological and molecular responses essential for coping with cytostatic, metal, or salt-induced toxic conditions. In the present study, the salubrious effect of PAE on PF was evaluated. The ethanolic extract of P. acidus (L) Skeel fruits reported alkaloids, glycosides, flavonoids, terpenoids, and saponins as secondary metabolites, which closely resemble the findings in our study [26]. Quercetin, the most abundant flavonol, accounts for 70% of this intake and has proven its effectiveness as a potent phenolic antioxidant in both experimental and real-life settings. Through its ability to efficiently counteract harmful oxidants, quercetin directly safeguards against oxidative damage and inflammation, two crucial contributors to the initiation of lung fibrosis. The dietary antioxidant quercetin (800 mg/kg diet) supplement moderately reduces the risk of PF caused by bleomycin through Nrf2-dependent restoration of redox imbalance [26,27]. In our study, phyto investigation of PAE was found to be a good source of flavonoids. So, it was additional support for screening the protective effect of PAE. The PAE was found harmless up to 2,000 mg/kg in an acute toxicity study. Based on that, we selected 100, 200, and 400 mg/kg doses as treatment doses for screening the attenuating effect against bleomycin-induced PF. NAC, an antioxidant and mucolytic agent, was used as a standard drug.
Pulmonary damage was inflicted by a sublethal dosage of bleomycin administered intratracheally. This strategy’s basic assumption is that chronic, persistent inflammation both precedes and ultimately causes the progressive fibrosis that characterizes IPF. It is believed that aggressively suppressing this inflammation will stop any scarring from developing later on [28].
Bleomycin induces toxicity through OS mechanisms. Bleomycin can instigate cell damage that is independent of its DNA-related actions, primarily through the initiation of lipid peroxidation. This phenomenon is especially relevant in the context of lung damage and may partially elucidate its capacity to injure alveolar cells, subsequently resulting in pulmonary inflammation. The reduced antioxidant capacity triggered by BLM leads to a significant upsurge in fibro proliferation and the deposition of the extracellular matrix. This fosters the expression of inflammatory mediators such as NF-κB, TNF, IL-1, IL-6, IL-18, IL-22, IL-17a, and inducible nitric oxide synthase, ultimately resulting in the extensive disruption of lung architecture and the development of fibrosis (PF). The formation of aldo and keto aggregates from protein carbonylation results in a sudden buildup of these compounds within cells, leading to oxidative modifications and dysfunction in pulmonary cells. Protein carbonylation stands as a significant end product in various oxidative processes within cells. The OS experienced by cells and tissues is brought about by an imbalance between antioxidant and pro-oxidant processes, and the physiological system’s inability to effectively cleanse formed reactive substances. The increased myofibroblast synthesis due to fibrotic injury and OS contributes to the deposition of extracellular matrix proteins (ECM), including type I collagen and fibronectin, in the lungs [29–32].
The PAE reduces the maximum level of MDA in a dose-dependent manner. This result indicates that PAE has a good protective effect against lung damage. The depletion of superoxide dismutase activity in bleomycin-treated rats on treatment with PAE in a dose-dependent manner, this result indicates that PAE promoted free radical scavenging activity. Similar to SOD, catalase and glutathione peroxidase levels also significantly declined in the bleomycin-treated animals. The PAE was regulated to normalize the catalase and glutathione peroxidase levels. This result supports the antioxidant properties of PAE. The MDA levels were significantly increased in the bleomycin-treated rats. The current study has correlated the reports of Turgut et al. 2016 [33].
The alveolar macrophage is believed to have a pivotal role in the progression of lung injury caused by bleomycin, primarily because of its capability to stimulate the release of various active substances, such as cytokines, lipid metabolites, and oxygen radicals. However, the exact mechanism responsible for the activation of alveolar macrophages remains unidentified. The presence of bleomycin receptors on the surfaces of rat alveolar macrophages has been detected, implying that macrophage activation could potentially be initiated through a second messenger system [30,34].
In the bleomycin-treated group, a significant elevation in total cells, macrophages, neutrophils, and eosinophils was observed. The PAE regulates the levels of total and differential cells to normal. These results induct the PAE to have activity against the inflammatory response. The effect of PAE was further evaluated by the histological studies, where the lung tissue sections showed a decrease in patchy, dense collections of lymphocytes and plasma cells around bronchioles, and the alveoli showed decreased interstitium widening as comparison with normal and standard drug treatments.
CONCLUSION
The protective potential of P. acidus (L.) Skeel fruits against bleomycin-induced PF in rats, supported by both biochemical assays and histopathological evaluation. Consumption of these fruits notably enhanced lung morphology, reducing malonaldehyde formation and boosting levels of antioxidant defense enzymes. The significant protective effect of the fruits was observed at a dose of 400 mg/kg dose, potentially attributed to their capacity to lower inflammatory cytokine levels, prevent the generation of oxygen free radicals, and/or eliminate them from the medium, thanks to their antioxidant properties. Integrating antioxidants or inhibitors of oxidant generation, along with anti-inflammatory properties, into existing therapies could enhance the efficacy of treatments for IPF. Nonetheless, comprehensive studies are essential to fully elucidate the molecular mechanisms behind the protective effects of Phyllanthus acidus (L.) Skeel fruits against bleomycin-induced pulmonary fibrosis.
ACKNOWLEDGMENT
The authors are thankful to Secretary and Correspondent, Dr. Ch. Gopal Reddy, CMR College of Pharmacy, Kandlakoya (V), Medchal, Hyderabad, India.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Institutional Animal Ethic Committee of the CMR College of Pharmacy, Hyderabad, India (Approval No.: CPCSEA/ 1657/IAEC/CMRCP/COL-22/105).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Gupta RS, Koteci A, Morgan A, George PM, Quint JK. Incidence and prevalence of interstitial lung diseases worldwide: a systematic literature review. BMJ Open Resp Res. 2023;10:e001291. CrossRef
2. Amaral AF, Colares PFB, Kairalla RA. Idiopathic pulmonary fibrosis: current diagnosis and treatment. J Bras Pneumol. 2023;49(4):e20230085. CrossRef
3. American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000;161(2 Pt1):646–64.
4. Zeeshan S, Misra V, Singh S, Arora G, Sharma S, Sharma S. Current status of herbal drugs and their future perspective. Biological forum. 2009;1(1):12–7.
5. Schwaiblmair M, Behr W, Haeckel T, Märkl B, Foerg W, Berghaus T. Drug induced interstitial lung disease. Open Respir Med J. 2012;6:63–74. CrossRef
6. Ahn J, Joo H, Park J, Park JW, Kim KI, Jung HJ et al. The effects of lung-moistening herbal medicines on Bleomycin-induced pulmonary fibrosis mouse model. Processes. 2020;8(1):1–7. CrossRef
7. Hosseini S, Imenshahidi M, Hosseinzadeh H, Karimi G. Effects of plant extracts and bioactive compounds on attenuation of bleomycin-induced pulmonary fibrosis. Biomed Pharmacother. 2018;107:1454–65. CrossRef
8. Rymbai H, Deshmukh NA, Jha AK, War GF, Paul D, Patel RS, et al. Stargoose berry. Breeding of Underutilized Fruit Crops. 2015;33:485–94.
9. Hossen MJ, Jeon SH, Kim SC, Kim JH, Jeong D, Sung NY et al. In vitro and in vivo anti-inflammatory activity of Phyllanthus acidus methanolic extract. J Ethnopharmacol, 2015;168:217–28. CrossRef
10. Sousa M, Ousingsawat J, Seitz R, Puntheeranurak S, Regalado A, Schmidt A et al. An extract from the medicinal plant Phyllanthus acidus and its isolated compounds induce airway chloride secretion: a potential treatment for cystic fibrosis. Mol Pharmacol. 2007;71(1):366–76. CrossRef
11. Chakraborty R, Biplab D, Devanna N, Sen S. Anti-inflammatory, antinociceptive and antioxidant activities of Phyllanthus acidus L. extracts. Asian Pac J Trop Biomed. 2012 ;2(2):S953–61.
12. Siddiqui Z, Khan MI, Akhtar J, Ahmad M. Multifunctional role of Phyllanthus Acidus L. As a therapeutic agent for management of diabetes and associated complications: a review. Biomed Pharmacol J. 2022;15(4):1821–31.
13. Shruthi G, Karur AK. Evaluation of hepatoprotective activity of Phyllanthus acidus in wistar rats. J Popul Therap Clin Pharmacol. 2022;29(04):1536–47. CrossRef
14. Andrianto D, Widianti W, Bintang M. Antioxidant and cytotoxic activity of Phyllanthus acidus fruit extracts. IOP Conf Ser: Earth Environ Sci. 2017;58(1):`012022.
15. Rahman MM, Habib MR, Hasan SR, Sayeed MA, Rana MS. Antibacterial, cytotoxic and antioxidant potential of methanolic extract of Phyllanthus acidus L. Int J Drug Dev Res. 2011;3(2):154–61.
16. Kamble MT, Yakupitiyage A, Salin KR, Chavan BR. Effect of Psidium guajava and Phyllanthus acidus leaf extract on immunostimulant response of Nile tilapia against Streptococcus agalactiae infection. Isr J Aquacult—Bamid. 2018;70:1–9. CrossRef
17. Rai B, Bhutia S, Pal P, Kakoti BB. Phytochemical analysis and antibacterial evaluation against selected gram strains by Oroxylum indicum (L.) Kurz stem bark extract, a folklore medicine of Sikkim Himalaya. J Pharmacogn Phytochem. 2020;9(1):11–16.
18. Punyashetty KB, Solanki PS, Anand SA. Cellular analysis of bronchoalveolar lavage fluid in various lung lesions. Trop J Pathol Microbiol. 2020;6(2):167–73. CrossRef
19. Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chimica Acta. 1978;90(1):37–43. CrossRef
20. Kakkar P, Das B, Viswanathan PN. A moditometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21(2):131–2.
21. Naz H, Akram NA, Ashraf M, Hefft DI, Jan BL. Leaf extract of neem (Azadirachta indica) alleviates adverse effects of drought in quinoa (Chenopodium quinoa Willd.) plants through alterations in biochemical attributes and antioxidants. Saudi J Biol Sci. 2022;29(3):1367–74. CrossRef
22. Prakash J, Gupta S, Kochupillai V, Singh N, Gupta Y, Joshi S. Chemopreventive activity of Withania somnifera in experimentally induced fibrosarcoma tumours in Swiss albino mice. Phytother Res. 2001;15(3):240–4. CrossRef
23. Narota A, Kumar S, Kaur R, Kaur S, Aggarwal R, Agnihotri N. Althea rosea seed extract ameliorates 1, 2-dimethylhydrazine induced preneoplastic lesions in mouse model of colon cancer by modulating oxidative stress and inflammation. Pharmacogn Mag. 2020;16(70):360–70. CrossRef
24. Chaudhary P, Janmeda P, Docea AO, Yeskaliyeva B, Abdull Razis AF, Modu B et al. Oxidative stress, free radicals and antioxidants: potential crosstalk in the pathophysiology of human diseases. Front chem. 2023;11:1158198. CrossRef
25. Carter SK, Blum RH. New chemotherapeutic agents -- bleomycin and adriamycin. CA cancer J clin. 1974;24(6):322–31.
26. Veith C, Drent M, Bast A, van Schooten FJ, Boots AW. The disturbed redox-balance in pulmonary fibrosis is modulated by the plant flavonoid quercetin. Toxicol Appl Pharmacol. 2017;336:40–8. CrossRef
27. Boots AW, Veith C, Albrecht C, Bartholome R, Drittij MJ, Claessen SMH et al. The dietary antioxidant quercetin reduces hallmarks of bleomycin-induced lung fibrogenesis in mice. BMC Pulm Med. 2020;20(1):112. CrossRef
28. Bringardner BD, Baran CP, Eubank TD, Marsh CB. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2008;10(2):287–301. CrossRef
29. Allawzi A, Elajaili H, Redente EF, Nozik-Grayck E. Oxidative toxicology of bleomycin: role of the extracellular redox environment. Curr opin in toxicol. 2019;13:68–73. CrossRef
30. Reinert T, Baldotto CS, Nunes FA, Scheliga AA. Bleomycin-induced lung injury. J Cancer Res. 2013;2013:1–9. CrossRef
31. Hay J, Shahzeidi S, Laurent G. Mechanisms of bleomycin-induced lung damage. Arch toxicol. 1991;65(2):81–94. CrossRef
32. Karamalakova Y, Stefanov I, Georgieva E, Nikolova G. Pulmonary protein oxidation and oxidative stress modulation by Lemna minor L. in progressive bleomycin-induced idiopathic pulmonary fibrosis. Antioxidants (Basel). 2022;11(3):523. CrossRef
33. Turgut NH, Kara H, Elagoz S, Deveci K, Gungor H, Arslanbas E. The protective effect of naringin against bleomycin-induced pulmonary fibrosis in Wistar rats. Pulm Med. 2016;2016:7601393. CrossRef
34. Liborio-Ramos S, Barbosa-Matos C, Fernandes R, Borges-Pereira C, Costa S. Interstitial macrophages lead early stages of bleomycin-induced lung fibrosis and induce fibroblasts activation. Cells. 2023;12(3):402. CrossRef