INTRODUCTION
The International Diabetes Federation reported in 2021 that the global number of individuals affected by diabetes was 537 million. Based on prevalence, the Southeast Asia area was ranked fourth globally in terms of the highest occurrence of diabetes in adult patients. In addition to that, China, India, and Pakistan occupied the top three positions. Meanwhile, Indonesia would be fifth with the highest number of diabetes patients in 2021–2045 [1]. These results demonstrated the importance of effectively preventing and treating diabetes to mitigate the escalating financial strain on healthcare in Asia.
Multiple studies linked race/ethnicity to diabetic kidney disease [2,3]. A diabetes patient cohort study found that Asians were more likely than whites to acquire end-stage renal disease (ESRD) [3]. This further stresses the significance of adequate diabetes care because the risk of kidney problems in Asian diabetes patients is higher. Other investigations have found differences in stroke, Heart Failure (HF), total mortality, leg amputation, peripheral arterial disease, and cardiovascular mortality between South Asian and white Type 2 Diabetes Mellitus (T2DM) patients [4]. Gijsberts et al. [5] found that diabetic Chinese and Malay people have more severe cardiovascular events than white people. The observed gap between races/ethnicities highlights the need for specialized diabetes management studies.
Sodium-Glucose Cotransporter-2 Inhibitors (SGLT2Is), including dapagliflozin, empagliflozin, canagliflozin, ertugliflozin, and sotagliflozin, have undergone extensive research to evaluate their efficacy and safety over a range of measurement criteria. Multiple systematic reviews and meta-analyses demonstrate the efficacy of SGLT2Is in improving the health of the heart and kidneys in individuals with type T2DM and those without diabetes [6,7]. In the meta-analysis conducted in 2022, researchers analyzed 13 large-scale randomized controlled trials (RCTs) involving 90,413 participants. The findings revealed that using SGLT2I therapy resulted in a 38% reduction in the progression of renal disease. Additionally, it reduced the risk of acute kidney injury by 21% and lowered the composite risk of Cardiovascular death (CV death) or hospitalization due to HF (CV death/HHF) by 23% in patients with T2DM who had a high risk of atherogenic cardiovascular disease (CV), a history of HF, or a history of chronic kidney disease (CKD) [6]. Nevertheless, it is essential to note that the higher susceptibility of Asian communities to diabetic kidney disease [8] and the potential impact of racial/ethnic disparities on outcomes prevent the generalization of these findings to all populations with T2DM. Meanwhile, from the various studies included accordingly, there are conflicting results regarding the outcome of Major Adverse Cardiovascular Events (MACEs), a composite of CV death/HHF, and progression of renal disease, especially for the Asian subgroup [9–20].
Multiple meta-analyses have been carried out to evaluate the efficacy of SGLT2Is on cardiovascular outcomes in Asia. All three meta-analyses indicated that SGLT2Is did not significantly reduce the incidence of MACE [21–23]. However, two meta-analyses yielded contradictory findings for the composite outcome of CV death/HHF [21,22]. After the publication of the meta-analyses, several further RCTs were conducted, and a few yielded significant findings that differed from those of the three meta-analyses [13,17,18,24]. Hence, there is a want for an up-to-date meta-analysis incorporating further RCTs examining the effects of SGLT2Is on cardiovascular and renal outcomes. Therefore, this study aims to evaluate the influence of SGLT2Is on cardiorenal outcomes in the Asian population. This study also evaluates the safety profile and certainty of the findings derived from the included RCT results, which were not done in previously published meta-analyses.
METHODS
The protocol was registered with Prospero under the registration number CRD42023480072. The reporting of this meta-analysis adhered to the principles set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).
Searching strategies
This study conducted a comprehensive search on PubMed, Embase, and CENTRAL databases and manually searched for related publications to include all relevant research until December 12, 2023. The keywords utilized for the PubMed and CENTRAL databases were initially refined in the MeSH database. Subsequently, other synonyms were incorporated based on the terms found in the title or abstract to enhance the search sensitivity. Meanwhile, for the Embase database, the keywords were modified to align with Emtree, and synonyms of words in the title or abstract were also incorporated. This step is applied to every Patient and Intervention criterion derived from PICO (Patient, Intervention, Comparator, and Outcome). This meta-analysis only included papers that followed a parallel RCT design and utilized keywords as per the guidelines provided by Cochrane. The text word menu was used to specify the parameters for the Asia region or Asian ethnicity. There were no limitations regarding language or publishing date. The full keywords can be seen in the supplementary file (Appendix 1).
Selection of studies
PICO framework eligibility criteria and RCT study design were used to select studies. This study adjusted patient selection criteria to match SGLT2I treatment guidelines. T2DM patients who were at least 18 years old and lived in Asia or were of Asian heritage were the focus of this meta-analysis. These patients were at a high risk of developing Atherosclerotic Cardiovascular Disease (ASCVD), and had a history of HF or CKD. The inclusion criteria for this trial were all types and doses of SGLT2Is, whether used alone or in combination therapy, with the placebo as the comparator. The primary outcomes evaluated in this study included a 3-point MACE, a composite of CV death, or worsening HF (including urgent visits owing to HF). Additionally, the progression of renal disease was also measured. The evaluated secondary outcomes included cardiovascular mortality, overall mortality, and adverse effects such as drug-related adverse events, serious adverse events, discontinuation of therapy due to adverse events, genital mycotic infection, urinary tract infection, documented hypoglycemia, major hypoglycemia, renal-related adverse events, volume depletion, bone fractures, diabetic ketoacidosis, amputation, thrombotic event, and cancer. The eligibility criteria of each study are narrated in Appendix 2. The database’s article inventory was loaded into the Zotero program. Following the elimination of duplicate articles, FC and RS conducted a screening procedure to choose studies based on the title and abstract. Subsequently, FC and RS individually conducted a thorough analysis of the full text of the selected studies. Opinions were discussed until a consensus was reached. The article selection process was performed using the Covidence tool, accessible at https://www.covidence.org/.
Data extraction
Two reviewers, FC and RA, extracted data from included papers separately. The reviewers discussed their differences in results until a consensus was established. The primary outcomes included 3-point MACE, a composite of CV death/worsening of HF, and the progression of renal disease. The 3-point criteria define MACE as cardiovascular mortality, nonfatal myocardial infarction, and stroke. A drop in eGFR (estimated glomerular filtration rate) of >30%–50%, ESRD, or death from kidney failure are common signs of kidney disease progression. ESRD is defined as dialysis, transplantation, or persistent glomerular filtration rate (GFR) <10–15 ml/minute/1.73 m2. Furthermore, worsening of HF is defined as hospitalization due to heart failure (HHF) or urgent visit for HF, where HHF refers to the occurrence of hospitalization due to HF as the primary diagnosis, with a minimum 24-hour stay, and urgent visits for HF are unscheduled emergency department visits where the patient’s condition does not meet hospital admission requirements. The outcome data being evaluated consists of dichotomous data derived from the count of events and participants in each group. If the necessary data were unavailable, data conversion was performed using survival analysis results from the generic inverse variance option in RevMan Manager. This study evaluated outcomes by analyzing data from the Asia region and individuals of Asian ethnicity as separate entities. The complete list of countries included in each study can be found in Appendix 3. The authors were contacted via email to clarify the data.
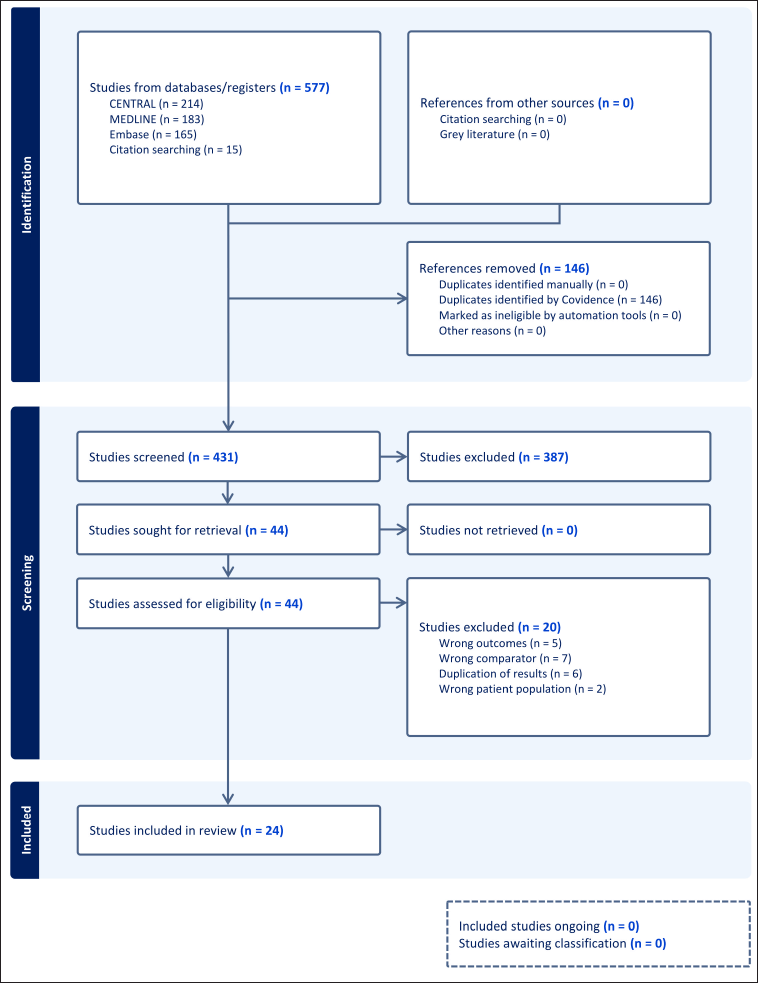 | Figure 1. PRISMA Flowchart [Click here to view] |
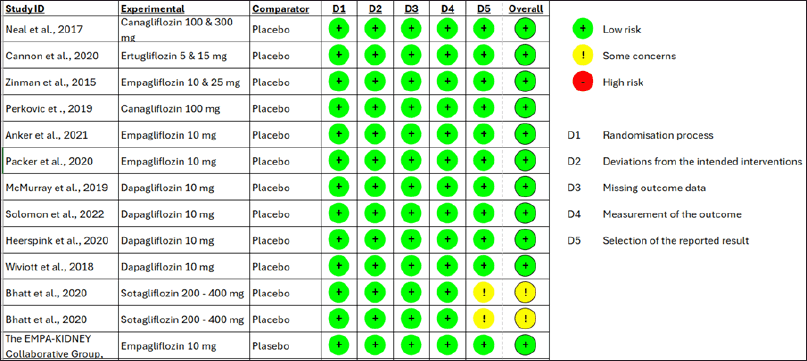 | Figure 2. Risk of Bias Assessment [Click here to view] |
Risk of bias assessment
Two reviewers, FC and RS, conducted this stage independently. The Cochrane Risk-of-bias2 (RoB2) tool was utilized to examine the data. Any discrepancies in the assessment results were discussed until a consensus was achieved.
Data synthesis
The data synthesis findings were displayed using Risk Ratio (RR) and 95% Confidence Interval (95% CI). The outcomes obtained from the generic inverse variance method were shown as Hazard Ratio (HR) and 95%CI. The I2 approach was utilized to evaluate heterogeneity, represented as a percentage. The Mantel-Haenszel method with a fixed effect model synthesized dichotomous data with minor variation between studies (I2<50%). In contrast, the random effect model was used when the study variation was large (I2≥50%). Data was analyzed using RevMan 5.3 and shown as a forest plot. Based on data availability, diabetic patients at high risk of ASCVD, HF patients, and CKD patients were subgrouped. The CV death/HHF composite was sensitivity-analyzed by adding or removing urgent hospital visits. This study also used GRADE to assess the certainty of its findings.
RESULTS
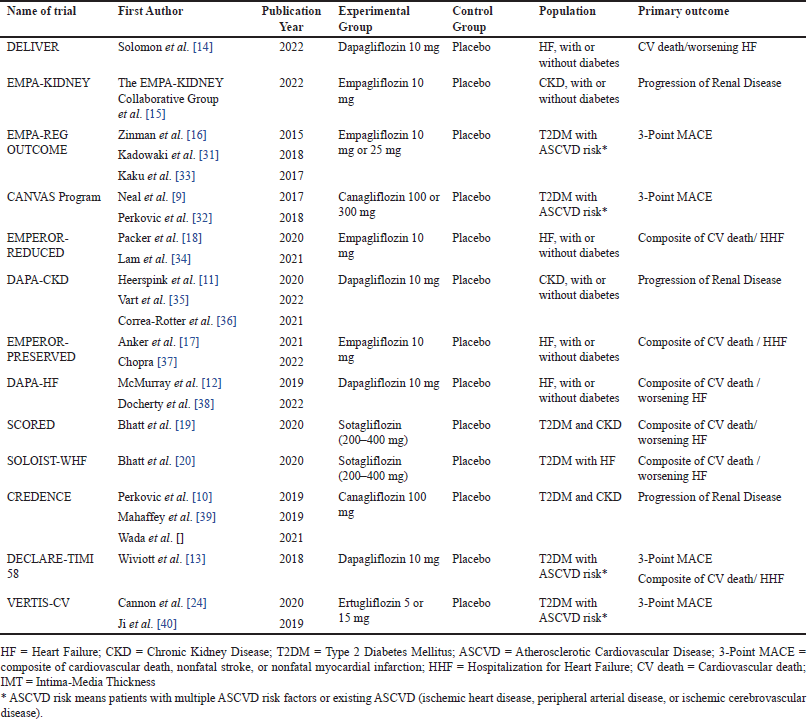
The authors discovered 577 papers by a comprehensive search of three databases and citation searching. One hundred forty-six publications were excluded, leaving 431 papers for screening. From these, 44 publications underwent a thorough evaluation, with 387 studies excluded due to inappropriate patient criteria (n = 50), inappropriate intervention (n = 9), inappropriate comparison (n = 77), inappropriate outcome (n = 106), and non-RCT trials (n = 145). Ultimately, 24 publications (comprising 13 trials) met the inclusion criteria, involving 12,758 participants. Fig 1 displays the PRISMA flowchart, while Table 1 provides the characteristics of the included research. For a more comprehensive understanding of the features of the studies covered, please refer to the Supplementary Appendix (Appendix 4 and Appendix 5).
The risk of bias assessment revealed that 11 studies exhibited a low risk of bias, as shown in Fig 2. However, SCORED [19] and SOLOIST-WHF [20] trials had “some concern” risk of bias due to changing the primary endpoint once the advantage of SGLT2Is is discovered. Meanwhile, The quantitative data synthesis conducted using RevMan indicated that using SGLT2Is did not provide any significant benefit in terms of 3-point MACE outcomes for individuals of Asian race and the Asia region. The HR for the Asian race was 0.82 [95% confidence interval (CI) = 0.66–1.01], while for the Asia region, it was 0.88 (95% CI = 0.76–1.01). However, the use of SGLT2Is demonstrated a positive effect on cardiovascular outcomes, namely the composite occurrence of CV death/worsening of HF; this advantage was observed in both individuals of Asian race and the Asia region, with the HR for Asian race was 0.64 (95%CI = 0.52–0.80), while for the Asia region it was 0.66 (95% CI = 0.58–0.75), as depicted in Fig 3. The key cardiovascular outcomes were combined using a general inverse variance method due to the lack of data on the number of events and participants in certain trials (CANVAS [9], SCORED [19], and SOLOIST-WHF [20] trials). As a result, the findings were reported as HR and 95%CI. Except for the composite outcome of CV death/worsening of HF in the Asian race (I2 = 56%), all other cardiovascular events exhibited acceptable heterogeneity. Therefore, the data with high heterogeneity was synthesized using a random-effect model. The Wiviott et al. [13] trial (DECLARE-TIMI 58) did not provide specific values for the HR and 95%CI for all cardiovascular outcomes. The data for the 3-point MACE and progression of renal disease in the Asia region were obtained from a previous meta-analysis [25]. However, this trial was excluded from the composite outcome of CV death/worsening of HF in the Asia region because the exact HR value was unavailable. Regarding CV death/worsening of HF in Asian Race and Asia Region, the results of sensitivity analysis produce similar outcomes (HR = 0.61 (95%CI = 0.50–0.74); I2 = 0%) and (HR = 0.63 (95%CI = 0.51–0.77; I2 = 0%) respectively, without an urgent visit for HF, thus, did not change the conclusion of significant effect of SGLT2Is use. Additionally, the utilization of SGLT2Is positively impacted the progression of renal disease, as evidenced by the data synthesis findings on renal outcomes. The HR for the Asian race was 0.64 (95%CI = 0.51–0.80), while for the Asia region, it was 0.64 (95% CI = 0.56–0.74). The renal results exhibited minimal heterogeneity, as depicted in Fig 4.
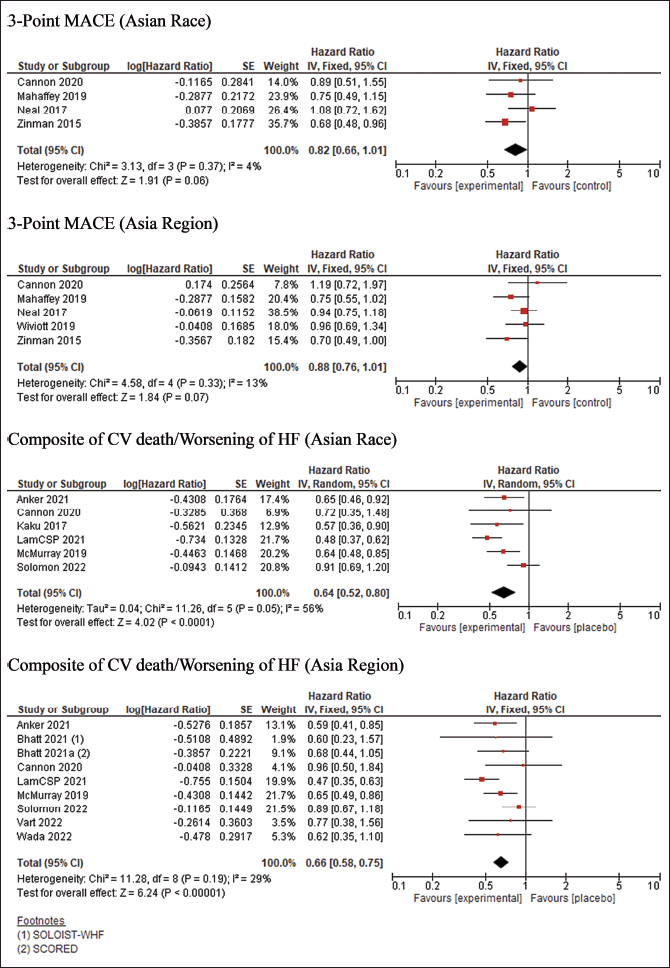 | Figure 3. Cardiovascular Primary Outcome [Click here to view] |
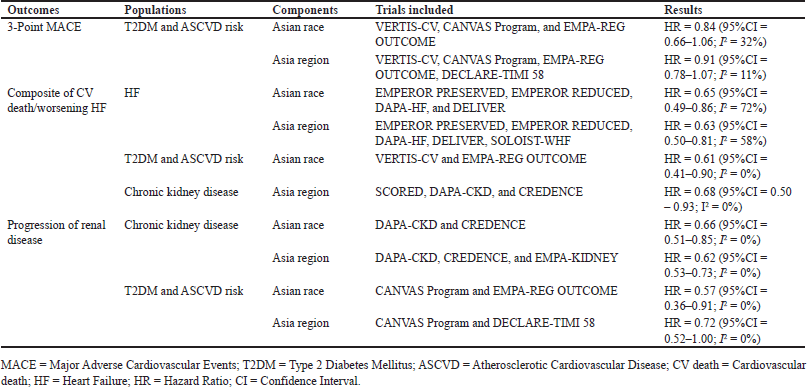
Subgroup analysis results in primary outcomes are shown in Table 2, with the forest plot can be seen in Appendix 6. The data showed that most results supported the use of SGLT2Is, except for the 3-point MACE and progression of renal disease outcomes in the T2DM and ASCVD risk populations. For the Asian race and the Asia region, the results of HR for a 3-point MACE outcome were 0.84 (95%CI = 0.66–1.06; I2 = 32%) and 0.91 (95% CI = 0.78–1.07; I2 = 11%), respectively. Meanwhile, the HR for the progression of renal disease in T2DM and ASCVD risk, especially in Asia region was 0.72 (95%CI = 0.51–1.00; I2 = 0%). Moreover, certain data had considerable heterogeneity in the HF subgroup population on a composite of CV death/worsening of HF outcome. Thus, a random effect model was utilized, especially in this subgroup. Subgroup analysis could not be conducted for all subgroup populations toward all key outcomes due to a shortage of trial numbers.
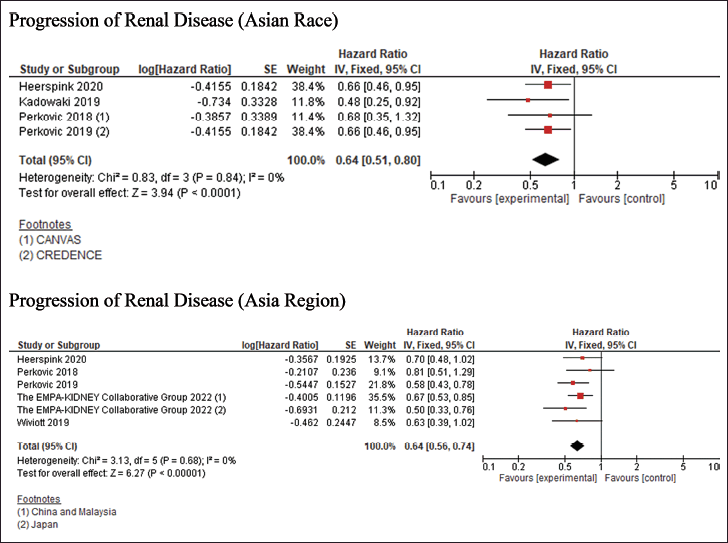 | Figure 4. Renal Primary Outcome [Click here to view] |
 | Table 1. Characteristics of included studies. [Click here to view] |
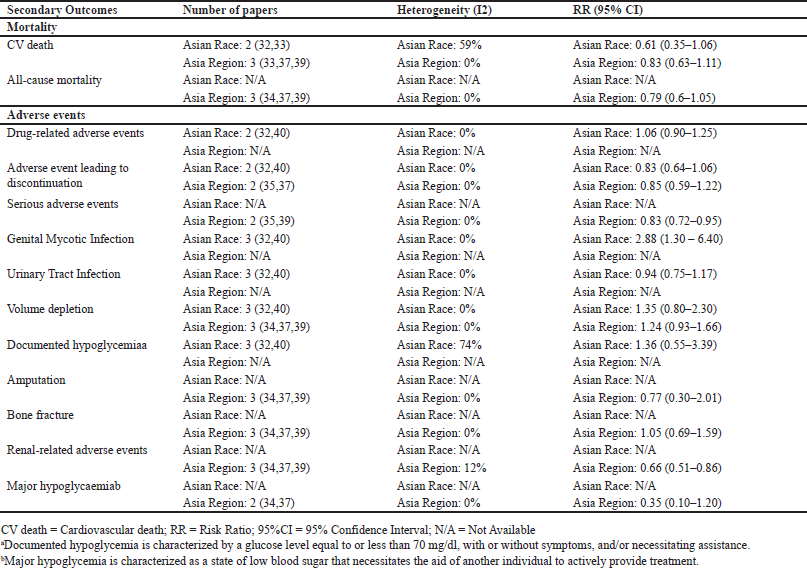
A secondary analysis of the outcomes demonstrated conclusive findings for cardiovascular mortality (Appendix 7). In the Asia region, the use of SGLT2Is did not show a significant reduction in CV death, with a RR of 0.83 (95%CI = 0.63–1.11). However, in the Asian race, the RR was 0.61 (95%CI = 0.35–1.06), but there was a high level of heterogeneity in the results. Therefore, we employed a random effect model for the Asian race. The intervention did not significantly reduce all-cause mortality in the Asia region, with RR = 0.79 (95% CI = 0.6–1.05). Regarding safety considerations, the intervention raised the Genital Mycotic Infection occurrences in the Asian race with RR = 2.88 (95% = 1.30–6.40; I2 = 0%). Interestingly, this intervention lowered the renal-related adverse events in the Asia region with RR = 0.66 (95%CI = 0.51–0.86; I2 = 12%). Data synthesis for thrombotic events and cancer could not be carried out because only one trial examined this outcome in the Asian race, and no studies were found in the Asia region.
The findings of GRADE analysis showed a moderate to high level of certainty, as demonstrated in Table 4. All three outcomes, with a moderate level of certainty, demonstrated indirectness. Some trials categorized the Asia region as part of the rest of the world or Asia-Pacific, whereas others only included East and Southeast Asia, excluding other regions of Asia. In addition, some trials did not particularly address T2DM and included non-diabetic patients; therefore, they did not directly answer the purpose of this study. In addition, the 3-point MACE in the Asia region exhibited a broad range of CI that led to varying conclusions, thus creating an imprecision. Inconsistency was discovered in a composite of CV death/worsening of HF in Asian results due to considerable heterogeneity. Finally, an asymmetrical funnel plot was identified on a composite of CV death/worsening HF in the Asia region, thus leading to the chance of publication bias. Additional findings from the GRADE analysis can be found in the supplementary appendix (Appendix 8).
 | Table 2. Subgroup analysis results. [Click here to view] |
 | Table 3. Secondary outcomes of SGLT2Is compared to control in Asian Race/Asia Region. [Click here to view] |
 | Table 4. Results of GRADE analysis on primary outcomes. [Click here to view] |
DISCUSSION
The findings from the cardiovascular primary outcomes demonstrated that SGLT2Is enhanced the composite measure of CV death/worsening of HF, both among individuals of Asian race and within the Asia region. The meta-analysis conducted by Lee et al. [21] focused on DAPA-HF and EMPEROR-Reduced trials specifically for individuals of Asian race, and found that these trials yielded comparable findings for this composite outcome without the need for urgent medical visits [21]. Therefore, with some additional trials included, the result for SGLT2Is benefit did not change. The sensitivity analysis results from this meta-analysis confirmed this benefit, with or without including urgent visits of HF to the analysis. Nevertheless, using SGLT2Is does not reduce MACE outcomes. Prior meta-analyses that specifically examined individuals of Asian race have yielded consistent findings for MACE [21,23]. This consistency remains even after incorporating the results of the CREDENCE and VERTIS-CV trials. In a separate meta-analysis conducted by Singh [22], similar findings were observed about the impact of the Asian race on MACE. However, it appears that this particular meta-analysis merged the outcomes of both the Asian race and the Asia region into a single analysis, potentially producing bias. Regarding cardiovascular primary outcomes, most of the results produce acceptable heterogeneity, except for the composite of CV death/worsening HF in Asian race; however, sensitivity analysis including only the composite of CV death/HHF (without an urgent visit for HF), results showed similar conclusion, with highly acceptable heterogeneity (I2 = 0%). Subgroup analysis revealed that SGLT2Is provided a favorable outcome in all population groups on composites of CV death/worsening of HF. However, significant heterogeneity, particularly in the HF subgroup, indicated that caution was required when interpreting and implementing these findings. Subgroup studies did not demonstrate any advantageous effects of SGLT2Is utilization in the Asian race and Asia region regarding MACE outcomes for individuals with T2DM and at risk for ASCVD. The findings diverged from the global conclusions on MACE, which indicated that SGLT2Is positively impacted MACE outcomes [6]. Therefore, it is plausible that the effects of administering SGLT2Is provide disparate results across various races and ethnicities. The determinants that impact this disparity are undeniably intriguing for future investigation. Although Asia is becoming a prominent center of the T2DM pandemic, none of the cardiovascular outcome trials for glucose-lowering medications in T2DM patients have been undertaken specifically on Asian patients [25]. Thus, it is likely that the differential results can be attributed to an insufficient statistical power resulting from the underrepresentation of Asians [22]. The lack of sufficient representation of racial and ethnic minorities (such as Asian race) among patients hinders the ability to apply cardiovascular research findings to a broader population, and it contributes to an inadequate comprehension of these disparities. Thus, it is necessary to undertake real-world investigations on the Asian race or the Asia region to observe the outcomes in everyday healthcare practice. It is crucial to identify strategic possibilities to tackle the social and structural variables that contribute to racial and ethnic disparities to achieve better results for these patients [26]. An RCT showed that non-White race contributed to MACE at a higher risk [27]. Because diabetes tends to develop at an earlier age and a lower body mass index in Asian subgroups [21], MACE may be more significant in Asian populations. Because the pharmacological properties of drugs can be influenced by genes, the effectiveness of SGLT2 inhibitors in reducing MACE may be influenced by pharmacogenomics related to race. In this case, carriers of wild-type alleles have shown an increase in plasma levels of the SGLT2 inhibitor canagliflozin due to the presence of UGT1A9*3 and UGT2B4*2 polymorphisms [28], while another gene, such as SLC5A2 and its genetic variants may have further impact on plasma glucose concentration [29]. However, the correlation between race and these polymorphisms has not been studied further, including in the Asian race, and it would be quite intriguing to acquire further knowledge about the subject.
Renal outcome data showed that SGLT2Is slowed renal disease development in Asians. Subgroup analysis conducted on people with CKD and T2DM at risk of ASCVD further supports these findings, except for T2DM with ASCVD risk in the Asia region, which could be due to a number of events and trial limitations. The positive findings of renal outcomes in this study are consistent with those of a previous meta-analysis [6]. However, no previous meta-analysis has specifically examined the effects of this intervention on renal outcomes in the Asian race or Asia region subgroup for comparison purposes.
CV death outcomes revealed clear results for both the Asian race and Asia region, where the usage of SGLT2Is did not produce any advantage. Due to the low number of available trials, it was impossible to undertake subgroup analysis to determine the root cause of the heterogeneity in the data, despite the indication of significant heterogeneity and resulting inconsistency in the outcome related to the Asian race. Furthermore, SGLT2Is did not improve all-cause mortality, which is similar to a global meta-analysis [12], but this result was non-specific and might be caused by many factors.
Safety analysis results suggest that SGLT2I use is considerably safe, with genital mycotic infection being the only concern needing attention for SGLT2Is. One of the two trials on diabetic ketoacidosis had no events in intervention or control groups, therefore it cannot be statistically studied. Hence, no conclusion can be made for this particular outcome. The random effect method was employed for hypoglycemic outcomes with substantial heterogeneity, but the small number of trials prevented subgroup analysis to determine the causes.
This meta-analysis has some limitations. The first concern is the inclusion of trials, such as the CREDENCE and CANVAS programs, that included the Asia region as the rest of the world, meanwhile, DECLARE-TIMI combined Asia as Asia-Pacific region. This may result in the inclusion of individuals from other ethnicities, not specific to Asians, in this subgroup, potentially introducing bias. Some other studies, e.g., Wada et al. [30] (subgroup analysis of CREDENCE) and EMPA-KIDNEY trial, only included East Asia Countries, with Malaysia in addition to the EMPA-KIDNEY trial. Thus, the generalisability of other parts of the Asia region is uncertain. The CREDENCE subgroup analysis also included India as another ethnic, apart from Asia. The various definitions of the Asia region utilized in different trials may introduce bias. Nevertheless, in this meta-analysis, we also performed primary outcome analyses specifically focusing on individuals of Asian race, enhancing the reliability and ethnic specificity of the study. The second constraint is the inclusion of non-diabetic patients in some trials. To address this limitation, the authors performed a sensitivity analysis by only including trials with participants specifically diagnosed with T2DM. This analysis yielded comparable results, indicating that SGLT2Is were beneficial for reducing the risk of CV death/worsening HF and improving the progression of renal disease. The supplementary appendix contains the comprehensive results of the sensitivity analysis (Appendix 9). The third constraint pertains to the insufficient number of events and trials to conduct additional subgroup analyses. The fourth limitation arises from the different definitions of the development of renal disease outcomes observed in several trials, as evidenced in the supplemental appendix (Appendix 9). The variation in the definition can lead to discrepancies in the findings of each study. However, the fundamental criteria must include a 40%–50% reduction in GFR or a two-fold increase in serum creatinine levels. Additionally, it should encompass ESRD, commencement of renal replacement therapy, and renal/cardiovascular mortality [10,11,15,31,32]. In addition, different studies (VERTIS-CV and EMPA-REG OUTCOME) have slightly different thresholds to define documented hypoglycemia, and this discrepancy in definitions may lead to somewhat different outcomes.
This meta-analysis has advantages over previous ones: its findings are backed by moderate to high certainty, showing quality and certainty. 2) This meta-analysis presents new evidence on the effects of SGLT2Is on cardiovascular and renal outcomes in Asian trials. 3) This meta-analysis also shows SGLT2I safety composite data.
CONCLUSION
SGLT2Is have demonstrated efficacy in enhancing renal outcomes among individuals of Asian race or residing in the Asia region. However, additional research is required to investigate their impact on cardiovascular outcomes, particularly their influence on MACE, as it did not decrease MACE in participants of both the Asian race and Asia region. Real-world studies in Asia are needed to see the effectiveness of SGLT2Is in natural conditions of standard practice in health facilities.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This study received no specific financing from government, commercial, or non-profit entities.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declare that they used Quillbot, an artificial intelligence (AI)-tool, for paraphrasing the sentences to enhance the readability of the manuscript, and no images were manipulated using AI.
SUPPLEMENTARY MATERIAL
Supplementary material can be accessed at the link here: [https://japsonline.com/admin/php/uploadss/4425_pdf.pdf].
REFERENCES
1. Home, Resources, diabetes L with, Acknowledgement, FAQs, Contact, et al. IDF Diabetes Atlas 2021 | IDF Diabetes Atlas [Internet]. [cited 2022 Oct 7].
2. Bhalla V, Zhao B, Azar KMJ, Wang EJ, Choi S, Wong EC, et al. Racial/Ethnic differences in the prevalence of proteinuric and nonproteinuric diabetic kidney disease. Diabetes Care. 2013 May;36(5):1215–21.
3. Kanaya AM, Adler N, Moffet HH, Liu J, Schillinger D, Adams A, et al. Heterogeneity of diabetes outcomes among Asians and Pacific Islanders in the U.S. Diabetes Care. 2011 Apr;34(4):930–7.
4. Yeo JL, Brady EM, McCann GP, Gulsin GS. Sex and ethnic differences in the cardiovascular complications of type 2 diabetes. Ther Adv Endocrinol Metab. 2021 Aug 4;12:20420188211034297.
5. Gijsberts CM, Seneviratna A, Carvalho LP de, Ruijter HM den, Vidanapthirana P, Sorokin V, et al. Ethnicity modifies associations between cardiovascular risk factors and disease severity in parallel Dutch and Singapore coronary cohorts. PLoS One. 2015 Jul 6;10(7):e0132278.
6. Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022 Nov 19;400(10365):1788–801.
7. Ma J, Lu J, Shen P, Zhao X, Zhu H. Comparative efficacy and safety of sodium-glucose cotransporter 2 inhibitors for renal outcomes in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Ren Fail. 2023;45(2):2222847.
8. Takeuchi M, Ogura M, Inagaki N, Kawakami K. Initiating SGLT2 inhibitor therapy to improve renal outcomes for persons with diabetes eligible for an intensified glucose-lowering regimen: hypothetical intervention using parametric g-formula modeling. BMJ Open Diabetes Res Care. 2022 Jun;10(3):e002636.
9. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017 Aug 17;377(7):644–57.
10. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019 Jun 13;380(24):2295–306.
11. Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020 Oct 8;383(15):1436–46.
12. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019 Nov 21;381(21):1995–2008.
13. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Eng J Med. 2019 Jan 24;380(4):347–57.
14. Solomon SD, McMurray JJV, Claggett B, De Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection Fraction. N Engl J Med. 2022 Sep 22;387(12):1089–98.
15. The EMPA-KIDNEY Collaborative Group; Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR, et al. Empagliflozin in patients with chronic kidney disease. N Eng J Med. 2023 Jan 12;388(2):117–27.
16. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015 Nov 26;373(22):2117–28.
17. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021 Oct 14;385(16):1451–61.
18. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020 Oct 8;383(15):1413–24.
19. Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021 Jan 14;384(2):129–39.
20. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021 Jan 14;384(2):117–28.
21. Lee MMY, Ghouri N, McGuire DK, Rutter MK, Sattar N. Meta-analyses of results from randomized outcome trials comparing cardiovascular effects of SGLT2is and GLP-1RAs in Asian Versus white patients with and without type 2 diabetes. Diabetes Care. 2021 May;44(5):1236–41.
22. Singh AK, Singh R. Cardiovascular outcomes with SGLT-2 inhibitors and GLP-1 receptor agonist in Asians with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Diabetes Metab Syndr. 2020;14(4):715–22.
23. Qiu M, Ding L, Wei X, Wei W, Zhou H. Effects of glucagon-like peptide 1 receptor agonists and sodium glucose cotransporter 2 inhibitors on major adverse cardiovascular events in type 2 diabetes by race, ethnicity, and region: a meta-analysis. Medicine (Baltimore). 2020 Dec 4;99(49):e23489.
24. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020 Oct 8;383(15):1425–35.
25. Kadowaki T, Yamamoto F, Taneda Y, Naito Y, Clark D, Lund SS, et al. Effects of anti-diabetes medications on cardiovascular and kidney outcomes in Asian patients with type 2 diabetes: a rapid evidence assessment and narrative synthesis. Expert Opin Drug Saf. 2021 Jun;20(6):707–20.
26. Mital R, Bayne J, Rodriguez F, Ovbiagele B, Bhatt DL, Albert MA. Race and ethnicity considerations in patients with coronary artery disease and stroke. J Am Coll Cardiol. 2021 Dec 14;78(24):2483–92.
27. Rossello X, Ferreira JP, Caimari F, Lamiral Z, Sharma A, Mehta C, et al. Influence of sex, age and race on coronary and heart failure events in patients with diabetes and post-acute coronary syndrome. Clin Res Cardiol. 2021 Oct 1;110(10):1612–24.
28. Venkatachalapathy P, Padhilahouse S, Sellappan M, Subramanian T, Kurian SJ, Miraj SS, et al. Pharmacogenomics and personalized medicine in type 2 diabetes mellitus: potential implications for clinical practice. Pharmacogenomic PersMed. 2021 Nov 13;14:1441–55.
29. Heo CU, Choi CI. Current progress in pharmacogenetics of second-line antidiabetic medications: towards precision medicine for type 2 diabetes. J Clin Med. 2019 Mar;8(3):393.
30. Wada T, Mori-Anai K, Kawaguchi Y, Katsumata H, Tsuda H, Iida M, et al. Renal, cardiovascular and safety outcomes of canagliflozin in patients with type 2 diabetes and nephropathy in East and South-East Asian countries: results from the canagliflozin and renal events in diabetes with established nephropathy clinical evaluation trial. J Diabetes Investig. 2022 Jan;13(1):54–64.
31. Kadowaki T, Nangaku M, Hantel S, Okamura T, von Eynatten M, Wanner C, et al. Empagliflozin and kidney outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: results from the EMPA-REG OUTCOME® trial. J Diabetes Investig. 2019 May;10(3):760–70.
32. Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018 Sep;6(9):691–704.
33. Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ, et al. Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease - results from EMPA-REG OUTCOME®. Circ J. 2017 Jan 25;81(2):227–34.
34. Lam CSP, Ferreira JP, Pfarr E, Sim D, Tsutsui H, Anker SD, et al. Regional and ethnic influences on the response to empagliflozin in patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Eur Heart J. 2021 Nov 14;42(43):4442–51.
35. Vart P, Correa-Rotter R, Hou FF, Jongs N, Chertow GM, Langkilde AM, et al. Efficacy and safety of dapagliflozin in patients with CKD across major geographic regions. Kidney Int Rep. 2022 Apr;7(4):699–707.
36. Correa-Rotte R, Vart P, Jongs N, Hou FF, Chertow GM, Langkilde AM, et al. DAPA-CKD: a regional analysis of kidney and cardiovascular outcomes. In 2021.
37. Chopra V. Regional and ethnic influences on the response to empagliflozin in patients with heart failure and a preserved ejection fraction- results from the emperor-preserved trial. J Am Coll Cardiol. 2022 Mar 8;79(9_Supplement):336–336.
38. Docherty KF, Anand IS, Chiang CE, Chopra VK, Desai AS, Kitakaze M, et al. Effects of dapagliflozin in Asian patients with heart failure and reduced ejection fraction in DAPA-HF. JACC Asia. 2022 Apr;2(2):139–53.
39. Mahaffey KW, Jardine MJ, Bompoint S, Cannon CP, Neal B, Heerspink HJL, et al. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation. 2019 Aug 27;140(9):739–50.
40. Ji L, Liu Y, Miao H, Xie Y, Yang M, Wang W, et al. Safety and efficacy of ertugliflozin in Asian patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: VERTIS Asia. Diabetes Obes Metab. 2019 Jun;21(6):1474–82.