INTRODUCTION
Osteoporosis is a systemic disease in which a decrease in bone mineral density increases the fragility of bone causing increased susceptibility to fracture. According to the WHO, osteopenia is defined as a T-score between –1 and –2.5 peak bone mass in the control population, and osteoporosis is defined as a T-score below –2.5 peak bone mass in healthy controls [1]. In humans, peripheral blood monocytes have been shown to have osteoclastogenic effects because they can differentiate into osteoclasts and express cytokines such as IL-6, IL-1, TGF-β, and TNF-α [2]. Osteoblasts have predominantly used macrophage colony-stimulating factor (M-CSF) to activate osteoclast precursors, and M-CSF enhances the survival and proliferation of granulocyte-macrophage colony-forming unit (GM-CFU). The tumor necrosis factor family molecule RANKL is expressed by osteoblasts, T cells, and endothelial cells, and conjugation with RANK commits GM-CFU to the osteoclast lineage, upregulating critical markers including TRAP. Continued exposure to these molecules causes preosteoclasts to fuse, and once activated, they adhere to the bone surface and produce osteoclast-specific markers such as cathepsin K. Osteoprotegerin (OPG), a RANKL decoy receptor that suppresses osteoclastogenesis, can decrease RANKL binding to RANK, determines whether and how much resorption occurs [3–6]
Annexin A2 (ANXA2) is a calcium-dependent phospholipid-binding protein that can stimulate osteoclasts to increase osteoclastic activity. Besides this, ANXA2 is found to increase monocyte transepithelial migration and is also involved in the elimination of compromised bone matrix by osteoclasts [7]. The proinflammatory ANXA2 has been linked to several immune-mediated illnesses, including pannus formation seen in rheumatoid arthritis [8]. Recently, we have established the reciprocal regulation of hormone receptors and proinflammatory ANXA2 in various cancer models like prostate and breast cancer and also confirmed the proinflammatory function of ANXA2 in different inflammatory disorders like rheumatoid arthritis [8]. Based on this knowledge, we have hypothesized in the current study that the decrease in the estrogen level in postmenopausal conditions augments the expression of ANXA2 and this becomes the reason for the increased osteoclastic activity during osteoporosis.
In this respect, after referring to earlier research work [8], we thought of determining the role of upstream pro-inflammatory ANXA2 and also its association with estrogen levels in postmenopausal women suffering from osteoporosis. Despite the constructive and successful ramifications of several therapies, the possibility of the paramount role of ANXA2 and its association with estrogen levels in the future treatment endeavors of osteoporosis goes unacknowledged. Millions, if not billions of women could benefit greatly from the evolution in the implicated theme, thus decreasing the economic and physical burden on postmenopausal females all over the world.
MATERIALS AND METHODS
Study population
This single-center study consists of 42 women subjects who visited our teaching hospital for the treatment of bone or hip-related problems. This sample size of 21 in each group was obtained using nMaster software (version 2.0) by considering the ANXA2 standard deviation of 1.76 in pre-menopausal and 1.45 in the post-menopausal group, the mean difference as 1.4 and effect size as 0.87 at 5% level of significance with an 80% power. The study protocol was approved by the Institutional Ethics Committee, K.S. Hegde Medical Academy Mangalore, India with approval no. EC/NEW/INST/2020/834 on Dec 07, 2020. The post-menopausal women with osteoporosis and pre-menopausal women without osteoporosis, who were not on treatment were recruited for the study after obtaining the written informed consent. The subjects with bone marrow diseases including multiple myeloma and connective tissue disorders, endocrinopathies such as hyperparathyroidism, hypothyroidism, type 1 diabetes, and Cushing’s syndrome, and with Paget’s disease, and who are on glucocorticoid therapy, anticonvulsant drugs, cytotoxic drugs, and cyclosporin were excluded from this study.
Bone mineral density and T-score determination
Both the study groups (pre-menopausal, n = 21, and post-menopausal, n = 21) were first thoroughly examined clinically by the orthopaedician, and subjected to dual-energy X-ray absorptiometry (DXA scan) using GE-Lunar Prodigy® DXA machine. Every day, the equipment was calibrated and the repeated measurements had a coefficient of variation of 1.87%. Values for bone mineral density (BMD) were calculated and expressed in g/cm2, and then, converted further into values related to the average female peak bone mass, i.e., T score. In general, the osteoporosis was diagnosed based on the T-score.
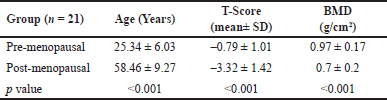 | Table 1. Age and bone characteristics of study subjects. [Click here to view] |
 | Figure 1. The levels of ANXA2 (A) and its comparison with Dexa T-score (B), and Estrogen E2 (C) serum levels in pre- and post-menopausal individuals. [Click here to view] |
 | Table 2. Statistical analysis of T-score, BMD, E2, and ANXA2 levels in pre-and post-menopausal women using independent t-test. [Click here to view] |
Blood collection and serum estradiol estimation
Two-milliliter peripheral venous blood was collected using a 21-gauge needle from the subjects in a plain vacutainer, left for 2 hours at room temperature to coagulate, centrifuged at 5,000 rpm for 3 minutes to separate serum, and stored at –80°C till use. Serum estradiol (E2) levels were determined by an automated hematology analyzer (Vitoss XT 7600).
Measurement of serum ANXA2 levels
ANXA2 levels in the serum of pre- and post-menopausal women were determined using a double antibody-based sandwich ELISA kit (FineTest®-Human, Wuhan). In brief, the wells were pre-coated with human ANXA2 monoclonal antibodies, then filled with the test serum and standards, and incubated at 37°C for 2 hours. After the rinse and blocking, biotin-labeled ANXA2 antibodies coupled with streptavidin–horseradish peroxidase to produce an immunological complex, were added and incubated for 30 minutes. Unbound material is removed, and chromogen reagent is added for turbidimetric reaction, and finally added with stop solution to halt the reaction. This colorimetric change was immediately recorded at 450 nm using an automated ELISA reader (Tecan Spark) and the results were expressed as ng/ml.
Statistical analysis
GraphPad Prism 7 software was used for all statistical analyses. The preponderance of continuous variables was reported as mean ± standard deviation. The differences in ANXA2, E2, and BMD among the two groups were analyzed using a two-sided Student’s t-test or unpaired T-test and compared using 2 way-ANOVA. A p-value less than 0.05 was considered statistically significant.
RESULTS
The age and bone characteristics of the study population of pre- and post-menopausal women have been provided in Table 1. The BMD determined using DXA scan in pre-menopausal women was found to have a normal T-score, compared to post-menopausal women, who had a T-score suggestive of severe osteoporosis (Table 1). However, the serum ANXA2 levels observed in pre-menopausal women was 62.97 ± 7.11 ng/ml, which is significantly lower, compared to the post-menopausal women, who had observed a significantly (p < 0.001) high level of 76.4 ± 10.07 ng/ml ANXA2 in the serum (Fig 1A). Therefore, the comparative analysis of these results indicates that the exceptionally high BMD subjects will have very low levels of ANXA2 in the serum, conversely, extremely low BMD subjects will have higher levels of ANXA2 in their serum (Fig 1B, Table 2). These data imply that ANXA2 was found to be high among osteoporotic post-menopausal individuals, in whom there will be more ANXA2-mediated osteoclastic activity and hence the low BMD score when compared to high BMD pre-menopausal individuals.
To understand the role of decreased levels of estrogen hormone in post-menopausal women, the serum levels of estrogen (E2) were determined and found that the E2 levels were significantly lower in post-menopausal women (5.52 ± 1.35 pg/ml), compared to pre-menopausal women (90.18 ± 52.76 pg/ml). These results indicate that E2 and BMD are directly proportional to each other, i.e., as the serum E2 level decreases (seen in postmenopausal women), the BMD also decreases as expressed by the DXA T-score (Fig 1C). In the current study, the physiological decrease in serum estradiol following menopause is significantly related to low BMD, with a p-value < 0.001(N = 42, r = 0.563). So as mentioned earlier, with the decrease in estrogen, osteoclastogenesis is favored and imbalance is seen in bone remodeling.
DISCUSSION
This study primarily established the relationship between serum proinflammatory ANXA2 and estrogen in post-menopausal individuals assessing their BMD status. WHO considers the DXA scan the gold standard for distinction among normal BMD, osteopenia, and osteoporosis. DXA monitors bone mass over time and aids in the patient selection for treatment [9]. The T-score between pre-menopausal and post-menopausal women in the study by Kadam et al. [10] was comparable to ours and statistically significant with a p-value < 0.001. In comparison to premenopausal women without osteoporosis, osteoporotic post-menopausal women had considerably higher serum ANXA2 levels. Similar observations have been made in earlier studies that the ANXA2 protein was significantly upregulated in those with poor BMD as opposed to high BMD [2,11]. In an in vitro study, it has been shown how ANXA2 protein concentrations might influence osteoblastic activity, where they have observed a substantial difference in the levels of serum ANXA2 protein between subjects with/without fracture histories; ANXA2 levels in plasma were inversely related to BMD [12]. ANXA2 is a family of calcium-binding proteins that are involved in many biological processes including bone remodeling. The ANXA2 was reported in bone development in both bone formation and bone resorption [13,14]. In a study done by Deng FY et al. [2,11]. ANXA2 protein expression level in the peripheral blood monocytes was significantly up-regulated in low vs. high BMD subjects in postmenopausal individuals. Furthermore, ANXA2 is an upstream biological marker postulating that soluble ANXA2 can be a useful indicator of an inflammatory process and also it has a reciprocal regulation with anti-inflammatory Annexin A1 in the pathogenesis of rheumatoid arthritis [8]. Overall, this study represented the pioneering efforts in assessing the ANXA2 protein level in plasma and analyze its association with BMD in pre- and post-menopausal women, and observed that ANXA2 was significantly elevated in post-menopausal women with extremely low BMD versus pre-menopausal women with high BMD.
The ability of ANXA2 to be released by both osteoclasts and monocytes, acting as an autocrine factor to induce monocyte trans-endothelial migration and/or osteoclastogenesis, provides a rationale for these data [4]. In human marrow cultures, ANXA2 has been shown to increase the number of osteoclast precursors and promote osteoclast formation [13]. Nesbitt and Horton demonstrated the critical function ANXA2 played in the removal of compromised bone matrix by osteoclasts. These findings corroborated the hypothesis that ANXA2 protein stimulates osteoclast activity and formation [15,16]. The active osteoclast itself may be a source of an activity that helps to fine-tune the coupling process, according to earlier research in genetically altered mice [17]. This coupling activity is generated from active osteoclasts by Nakamura et al. [18]. Consistently, a review of the communication between osteoclasts and osteoblasts suggested mature osteoclasts may interact not only with bone lining cells but also with osteoblasts to remove the bone collagen left by osteoclasts in resorption lacunae [19,20]. In light of the presence of ANXA2 in plasma its association with BMD, and its promotive roles in osteoclastogenesis, herein we propose a new concept as follows. ANXA2 protein, secreted into plasma by PBMs and osteoclasts, plays significant roles in the regulation of osteoporosis by taking part in the process of bone remodeling. The action of ANXA2 and E2, as observed in this study, implies that there is a reciprocal regulation of estrogen and ANXA2. The above integrative evidence strongly supports the concept that ANXA2 is involved in the pathogenesis of osteoporosis in humans.
The currently established relation of estradiol and proinflammatory ANXA2 in the development of osteoporosis was similar to our earlier observations in various hormone-dependent cancer models, where investigators identified the reciprocal regulation between E2 and ANXA2. Previously, we have demonstrated that ANXA2 expression was null when androgen receptor expression was very high in hormone-dependent prostate cancer. Similarly, we have shown that estrogen receptor-positive breast cancer had a very low or null expression of ANXA2. This established reciprocal regulation of ANXA2 encouraged us to check its relation with E2 in an osteoporotic clinical setup. As hypothesized, ANXA2 and E2 were reciprocally regulated in post-menopausal osteoporotic women, in whom when estrogen levels go down that induces the expression of proinflammatory ANXA2, which could be the reason for the occurrence of osteoclastic activity in osteoporosis and thereby decreased the bone density. However, the molecular mechanisms underlying these observations have to be further proved with in-vitro and in-vivo studies are warranted.
CONCLUSION
This study results conclude that in post-menopausal individuals with osteoporosis, plasma ANXA2 protein level is inversely related to hip BMD and has a very poor level of serum estrogen, which accounts for the occurrence of increased plasma ANXA2 levels in individuals with poor BMD. Overall, this research revealed that greater levels of ANXA2 have a role in promoting osteoclastogenesis and are linked to osteoporosis development in post-menopausal individuals.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Institutional Ethics Committee, K.S. Hegde Medical Academy Mangalore, India with approval number EC/NEW/INST/2020/834 on December 07, 2020. The post-menopausal women with osteoporosis and pre-menopausal women without osteoporosis, who were not on treatment were recruited for the study after obtaining the written informed consent.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Sözen T, Özisik L, Basaran NÇ. An overview and management of osteoporosis. Eur J Rheumatol [Internet]. 2017 Mar 1 [cited 2024 Jun 26];4(1):46.
2. Sun Y, Li J, Xie X, Gu F, Sui Z, Zhang K, Yu T. Macrophage-osteoclast associations: origin, polarization, and subgroups. Front Immunol [Internet]. 2021 Dec 1 [cited 2024 Jun 26];12:778078.
3. Kular J, Tickner J, Chim SM, Xu J. An overview of the regulation of bone remodelling at the cellular level. Clin Biochem [Internet]. 2012 Aug [cited 2024 Mar 6];45(12):863–73. Available from: https://pubmed.ncbi.nlm.nih.gov/22465238/
4. Hodge JM, Collier FM, Pavlos NJ, Kirkland MA, Nicholson GC. M-CSF potently augments RANKL-induced resorption activation in mature human osteoclasts. PLoS One [Internet]. 2011 [cited 2024 Mar 6];6(6):e21462. Available from: https://pubmed.ncbi.nlm.nih.gov/21738673/
5. Mun SH, Park PSU, Park-Min KH. The M-CSF receptor in osteoclasts and beyond. Exp Mol Med [Internet]. 2020 Aug 17 [cited 2024 Mar 6];52(8):1239–54. Available from: https://www.nature.com/articles/s12276-020-0484-z
6. Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, et al. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun [Internet]. 1998 Jun 29 [cited 2024 Mar 6];247(3):610–5. Available from: https://pubmed.ncbi.nlm.nih.gov/9647741/
7. Zhou X, Wu LF, Wang WY, Lu X, Jiang ZH, Zhang YH, et al. ANXA2 attenuates osteoblast growth and is associated with hip BMD and osteoporotic fracture in the Chinese elderly. PLoS One [Internet]. 2018 Mar 1 [cited2024 Mar 6];13(3):e0194781. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0194781
8. Haridas V, Shetty P, Sarathkumar E, Bargale A, Vishwanatha JK, Patil V, et al. Reciprocal regulation of pro-inflammatory Annexin A2 and anti-inflammatory Annexin A1 in the pathogenesis of rheumatoid arthritis. Mol Biol Rep [Internet]. 2019 Feb 1 [cited 2020 Mar 2];46(1):83–95. Available from: https://link.springer.com/article/10.1007/s11033-018-4448-5
9. Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi ML, et al. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone [Internet]. 2008 Dec [cited 2024 Mar 6];43(6):1115–21. Available from: https://pubmed.ncbi.nlm.nih.gov/18793764/
10. Kadam NS, Chiplonkar SA, Khadilkar AV, Khadilkar VV. Prevalence of Oosteoporosis in apparently healthy adults above 40 years of age in Pune City, India. Indian J Endocrinol Metab [Internet]. 2018 Jan 1 [cited 2024 Mar 6];22(1):67–73. Available from: https://pubmed.ncbi.nlm.nih.gov/29535940/
11. Deng FY, Liu YZ, Li LM, Jiang C, Wu S, Chen Y, et al. Proteomic analysis of circulating monocytes in Chinese premenopausal females with extremely discordant bone mineral density. Proteomics [Internet]. 2008 Oct [cited 2024 Mar 6];8(20):4259.
12. Zhao R. Immune regulation of osteoclast function in postmenopausal osteoporosis: a critical interdisciplinary perspective. Int J Med Sci [Internet]. 2012 Oct 27 [cited 2024 Mar 6];9(9):825.
13. Menaa C, Devlin RD, Reddy SV, Gazitt Y, Choi SJ, Roodman GD. Annexin II increases osteoclast formation by stimulating the proliferation of osteoclast precursors in human marrow cultures. J Clin Invest [Internet]. 1999 [cited 2024 Mar 6];103(11):1605–13. Available from: https://pubmed.ncbi.nlm.nih.gov/10359570/
14. Kirsch T, Harrison G, Golub EE, Nah HD. The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J Biol Chem [Internet]. 2000 Nov 10 [cited 2024 Mar 6];275(45):35577–83. Available from: https://pubmed.ncbi.nlm.nih.gov/10956650/
15. Takahashi S, Reddy SV, Chirgwin JM, Devlin R, Haipek C, Anderson J, et al. Cloning and identification of annexin II as an autocrine/paracrine factor that increases osteoclast formation and bone resorption. J Biol Chem. 1994 Nov 18;269(46):28696–701.
16. Genetos DC, Wong A, Weber TJ, Karin NJ, Yellowley CE. Impaired osteoblast differentiation in annexin A2- and -A5-deficient cells. PLoS One [Internet]. 2014 Sep 15 [cited 2024 Mar 6];9(9):e107482. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0107482
17. Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med [Internet]. 2005 [cited 2024 Mar 6];11(2):76–81. Available from: https://pubmed.ncbi.nlm.nih.gov/15694870/
18. Nakamura M, Udagawa N, Matsuura S, Mogi M, Nakamura H, Horiuchi H, et al. Osteoprotegerin regulates bone formation through a coupling mechanism with bone resorption. Endocrinology [Internet]. 2003 Dec [cited 2024 Mar 6];144(12):5441–9. Available from: https://pubmed.ncbi.nlm.nih.gov/14500574/
19. Everts V, Delaissié JM, Korper W, Jansen DC, Tigchelaar-Gutter W, Saftig P, et al. The bone lining cell: its role in cleaning Howship’s lacunae and initiating bone formation. J Bone Miner Res [Internet]. 2002 [cited 2024 Mar 6];17(1):77–90. Available from: https://pubmed.ncbi.nlm.nih.gov/11771672/
20. Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys [Internet]. 2008 May 15 [cited 2024 Mar 6];473(2):201–9. Available from: https://pubmed.ncbi.nlm.nih.gov/18406338/