INTRODUCTION
Traditional medical systems have used medicinal plants for centuries to treat various ailments [1]. According to WHO guidelines, herbal medicines are a sustainable alternative solution for detoxification [2]. Traditional medical systems have used Plumbago indica Linn, a wild medicinal plant, for centuries [3]. Tropical and subtropical areas of Asia and Africa are home to this plant, which belongs to the genus Plumbago [4,5]. Its botanical, traditional, and pharmacological features have led to extensive scientific attention, and the plant is a significant species within the Plumbaginaceae family [6].
Plumbago indica has a unique physical appearance, with its distinctive flower form and lanceolate leaves [7]. Its morphology varies depending on environmental conditions, with the foliage lustrous green and the blooms small, cylindrical, white, or light blue [8]. The plant’s taxonomic profile includes its taxonomic classification, morphological analysis, cytology, molecular biology, and conservation genetics [9]. It has a long history of use in traditional herbal therapy, particularly in Ayurveda, for its digestive, anti-inflammatory, anthelmintic, expectorant, diaphoretic, diuretic, purgative, and so on, effects. People use its roots and leaves to treat various ailments, such as gastrointestinal disorders, dermatological conditions, respiratory ailments, and rheumatic disorders [10]. It has a lot of different plant chemicals, such as plumbagin,6-hydroxy plumbagin, plumbagic acid, 3, 3 – biplumbagin, droserone, elliptinone, and so on, among these maximum amount of plumbagin (5-hydroxy-2-methyl-1,4-napthoquinone) is present which is a therapeutic naphthoquinone found in P. indica roots. Plumbagin helps in fighting inflammation, bacteria, free radicals, and cancer [11,12]. The increasing recognition of the medicinal benefits of Plumbago indica has led to its higher demand in the pharmaceutical and herbal product sectors. However, concerns about excessive harvesting and unsustainable methods have arisen, necessitating measures to control commerce and encourage ethical acquisition [13].
In addition, it contains bioactive substances that can be harmful when consumed in large amounts, making accurate dosage and administration instructions crucial [14]. Plumbago indica is at risk due to habitat loss, excessive use, and forest destruction. Preserving this species requires implementing conservation strategies such as habitat restoration, designated protected areas, and ex situ cultivation [9]. In vitro propagation techniques can help preserve the plant by facilitating large-scale reproduction of superior genetic varieties. Sustainable agricultural practices, such as agroforestry, organic farming, and biodiversity-friendly agriculture techniques, can increase the productivity of P. indica while reducing environmental impacts [15].
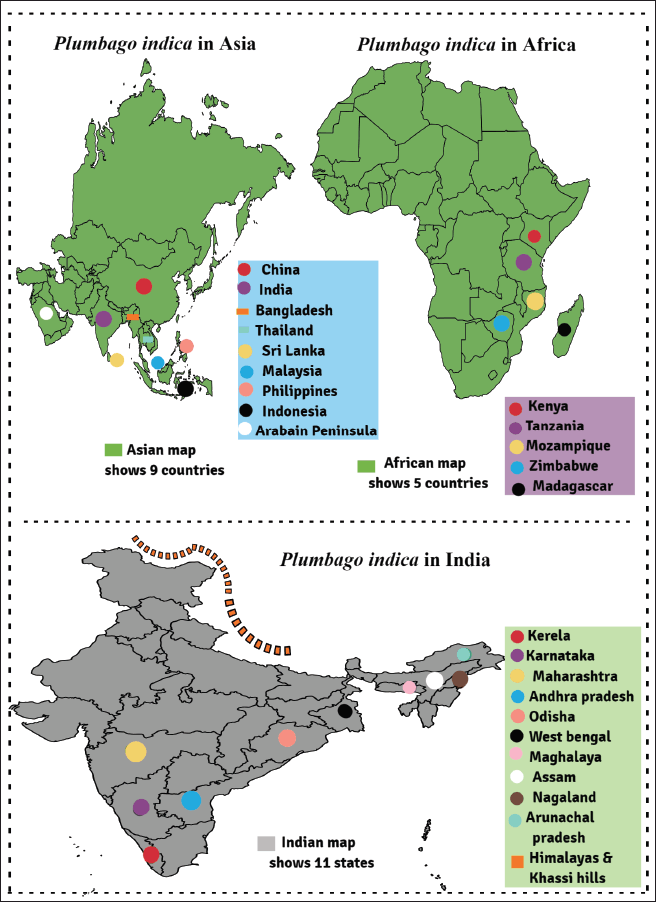 | Figure 1. Worldwide and Indian geographical distribution of P. indica. [Click here to view] |
This review focuses on the botanical, traditional, and pharmacological significance of Plumbago indica, addressing conservation challenges and promoting sustainable practices. Prospects include exploring its phytochemistry, pharmacology, and genetic diversity, combining traditional knowledge with scientific methodologies for safe herbal products.
MATERIAL AND METHODS
Literature searches were conducted using scientific search engines and databases such as Google Scholar, PubMed, Scopus, Web of Science, Science Direct, and Wiley Online Library. A comprehensive investigation was conducted by examining various publications, theses, dissertations, and published floras to acquire comprehensive information.
ECOLOGY AND DISTRIBUTION
The field of ecology encompasses the study of the relationships between organisms and their environment, including the distribution patterns of species. Plumbago indica is a botanical species that is cultivated on a large scale in various Asian nations, including India particularly in the Eastern Himalayas and Sikkim region, as well as in the South, encompassing Andhra Pradesh, Kerala, Maharashtra, Nagaland, Manipur, Assam, Meghalaya, Arunachal Pradesh, Odisha, Khasi Hills, and West Bengal), Sri Lanka, Philippines, China, Malaysia, Indonesia, Bangladesh, and the Arabian Peninsula. The cultivation of this crop is also observed in various African nations, including Kenya, Tanzania, Zimbabwe, Mozambique, and Madagascar, as well as in Europe [16–19]. The geographical distribution of P. indica around the world and in India is shown in (Fig. 1).
BOTANICAL DESCRIPTION
Taxonomy
The classification of Plumbaginaceae is ambiguous due to their affinities with Centrospermae and Primulales. However, the taxonomical account provided is properly formatted and includes all relevant information [20]. Plumbago indica is classified according to its taxonomic characteristics, as shown in (Table 1).
Morphological description
Plumbago indica is a perennial botanical species characterized by its vertical stem, which can reach heights ranging from 1 to 5 m. The plant exhibits prominent elongated spikes that bear red, pentamerous, bisexual flowers measuring between 10 and 30 centimeters in length. The roots exhibit a distinct dark brown hue and emit a strong, pungent odor [17]. The identification of the genus is based on the presence of a pilose calyx, and the specimens within this group have the potential to exhibit perennial shrub characteristics.
The dimensions of the bracts are approximately 2–3 mm in length, exhibiting an oval morphology. The length of the peduncles ranges from 2 to 10 cm. Bisexual flowers frequently exhibit a symmetrical morphology. The calyx exhibits a length of 8–9 mm and possesses a tubular shape. It is characterized by glandular features and displays a crimson hue. The height of the corolla tube ranges from 0 to 1 mm. The calyx exhibits a tubular morphology with a diameter measuring 1.5 cm. The apex of the structure exhibits a rounded shape with a mucronate tip that is characterized by a purple to crimson coloration. Notably, this particular structure is devoid of stamens [21].
Cytology
Several researchers have conducted investigations on the chromosomal characteristics of P. Indica and have determined that the meiotic chromosome count is 2n = 14 [19]. It exhibits two distinct chromosomal counts, namely 2n = 28 and 2n = 12, which can be categorized as diploid and tetraploid, respectively, based on the fundamental chromosomal number of x = 7. However, the equation 2n = 12 necessitates additional investigation. This species exhibits polyploidy, and its chromosome number is variable [22,23].
Molecular and genetic diversity
The topic of molecular and genetic diversity encompasses the examination of variations at the molecular and genetic levels within a population or species. The recognition of genetic variation holds significant importance in the efficient management and utilization of genetic resources. The utilization of molecular analysis of plant DNA is highly advantageous when conducting assessments of genetic stability. The utilization of Polymerase Chain Reaction (PCR)-based molecular markers has been employed in the evaluation of genetic fidelity in plantlets derived from cry storage. PCR and Random Amplified Polymorphic DNA (RAPD) technology play a crucial role in the evaluation of plant stability resulting from in vitro conservation. PCR has the capability to amplify a specific region of the genome, as defined by a pair of primers, when the gene sequence is already known. The utilization of a solitary oligonucleotide primer possessing an arbitrary nucleotide sequence, or the combination of arbitrarily primed oligonucleotides with PCR, results in the generation of DNA (RAPDs) fragments. These fragments serve as valuable markers for the identification and analysis of genetic alterations. The evaluation of genetic stability in micro propagated Plumbago rosea plants has been documented by multiple researchers [9,34].
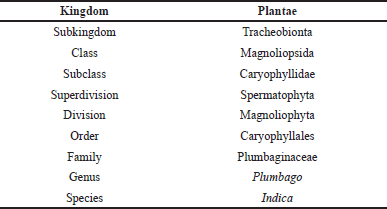 | Table 1. Taxonomic classification of Plumbago indica. [Click here to view] |
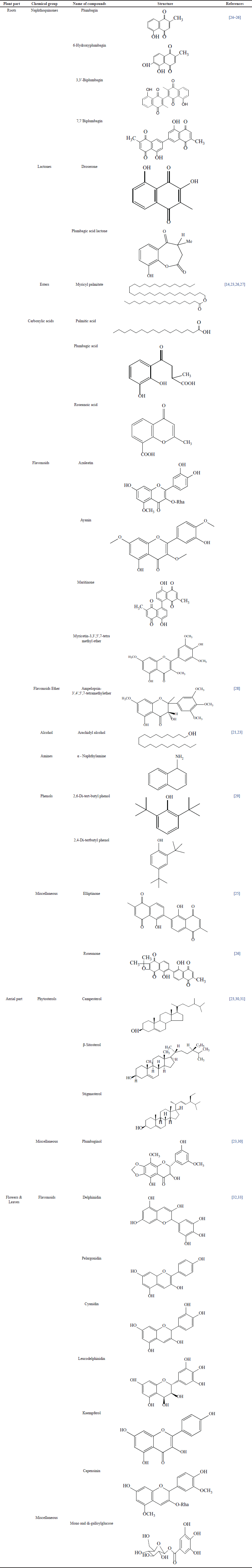 | Table 2. Phytoconstituents and structures found in several parts of P. indica. [Click here to view] |
TRADITIONAL APPLICATIONS
Plumbago indica, a widely recognized plant in the Indian System of Medicine, is used in traditional therapy to treat a variety of illnesses. Ayurveda and Siddha medicine prescribe the roots, bark, and leaves of the plant for numerous diseases. The root is known for its anthelmintic, expectorant, diaphoretic, diuretic, purgative, alterant, antioxidant, and aphrodisiac effects. It is used as a tonic to treat amenorrhoea and dysmenorrhea in Unani medicine. The leaves and bark have expectorant, diuretic, emmenogogue, and alterant properties that can be used to treat rheumatism, headaches, and splenopathy [23,35,36].
Medications derived from P. indica have undergone rigorous scientific testing and have demonstrated efficacy in treating various medical problems. People use poultices made from scraped bark to treat headaches and they apply the bark to the spine as a blistering plaster to treat fevers. In addition, they use roots along with black pepper for jaundice in the form of pills as well as used by Chakma for stomach pain. People use the root as an abortifacient and antifertility medication and they use the extracted juice as a potent sudorific [37].
The leaves and roots are also used for the treatment of dyspepsia, piles, dysentery, diarrhea, and to enhance the appetite. Myanmar uses Ayurveda to treat leprosy and syphilis due to its ability to inhibit vata and pitta. West Bengal uses a decoction of fresh roots as an abortifacient and in Bangladesh, they treat skin diseases by applying leaf and root juice topically. To produce sterility in women, 1 tablespoon every day for 3 days is prescribed [38].
PHYTOCHEMISTRY
Plumbago indica exhibits a high abundance of various alkaloids, flavonoids, saponins, glycosides, coumarins, alkylated phenols, triterpenes, fatty acids, sterols, and tannins. Plumbagin (5-Hydroxy-2-methyl-1, 4-naphthoquinone), a bioactive organic compound, is derived from the roots [12,17,29]. The structures of the phytoconstituents that are found in various sections of P. indica plant are presented in (Table 2).
PHARMACOLOGICAL IMPORTANCE
Plumbago indica, a botanical species, is known for its diverse bioactive compounds, and traditional medicine applications are highlighted in table 3 detailing its pharmacological effects and relevant references. Its medicinal properties are also outlined in (Table 3).
TRADE AND MARKET DEMAND
The subject of trade and market demand is of significant academic interest; hence P. indica is highly valuable in the pharmaceutical and natural medicine industries because of its bioactive component, plumbagin, which covers its market dynamics, utilization, and cost.
Market demand and utilization
Plumbago indica is medically significant because it contains a substantial amount of plumbagin, a bioactive chemical. Herbal medication formulations should utilize new harvests of plumbago species to maintain appropriate concentrations of plumbagin. This will help ensure the intended therapeutic effects. Chitrakadi Vati is an Ayurvedic formulation that contains 1.65%–10% plumbagin, emphasizing the therapeutic significance of this component [39,40].
The need for medicinal products is increasing dramatically, resulting in a surge in demand for bioactive substances such as plumbagin. India had a demand of 1,285 metric tonnes of Plumbago indica roots between 2004 and 2006, and it is expected to increase by 10% annually. In India alone, the annual demand for plumbagin amounts to 7 metric tonnes. Commercial exploitation of P. indica is a significant concern because of its high demand, leading to overexploitation and posing a threat to its natural population [41,42].
Trade name and pricing
The trade name for P. indica is “Chitrak.” The price per kg ranges from 90 to 150 Indian rupees. The anticipated yearly trade volume for this plant is between 100 and 200 kg [43]. However, the price of the roots varies from Rs. 200 to Rs. 350 per kg, indicating its significant economic worth [44]. In addition, P.indica, known as Chitrak Root Extract (1 kg), costs around 2,230.00 Indian rupees [45].
Commercial sustainability
The cultivation of P. indica has the potential to be economically profitable, with the possibility of yielding up to Rs. 5 lakh per hectare over 3 years [44].
Economic feasibility
Growing P. indica offers a chance to make a significant income, particularly due to the potential for numerous harvests moreover, the plant can be harvested again from the same root system, increasing its economic value [44].
Commercialization and trade
Plumbago indica is one of the 242 plant species utilized for the production of herbal drugs in India, highlighting its importance in the pharmaceutical and herbal medicine sectors [46].
TOXICITY
The extract of the root of the P. indica plant has been categorized as a drug that poses the least risk to hamsters, falling into category 5. High doses of the ethanolic extract caused adverse effects in mice, including diarrhoea, skin rashes, leukocytosis, and raised blood phosphatase levels. The LD50 of the ethanolic extract was determined to be 1,250 mg/kg [47,48]. Mice showed higher tolerance to oral administration of the extract compared to intraperitoneal administration. Rat autopsy findings showed liver discoloration, enlarged spleen, decreased liver, thymus, testes, and kidney weight changes. Cytotoxicity studies showed a lower LD50 value for the extract than other species such as Plumbago zeylanica and Plumbago auriculata [48,63]. The extract caused an imbalance in oxidant-antioxidant substances in the liver, leading to chronic inflammation and cellular damage [58]. Niosomal plumbagin administration reduced mortality in BALB/c mice compared to free plumbagin [76]. Overall, the P. indica root extract has been found to be non-toxic and should be considered for further research.
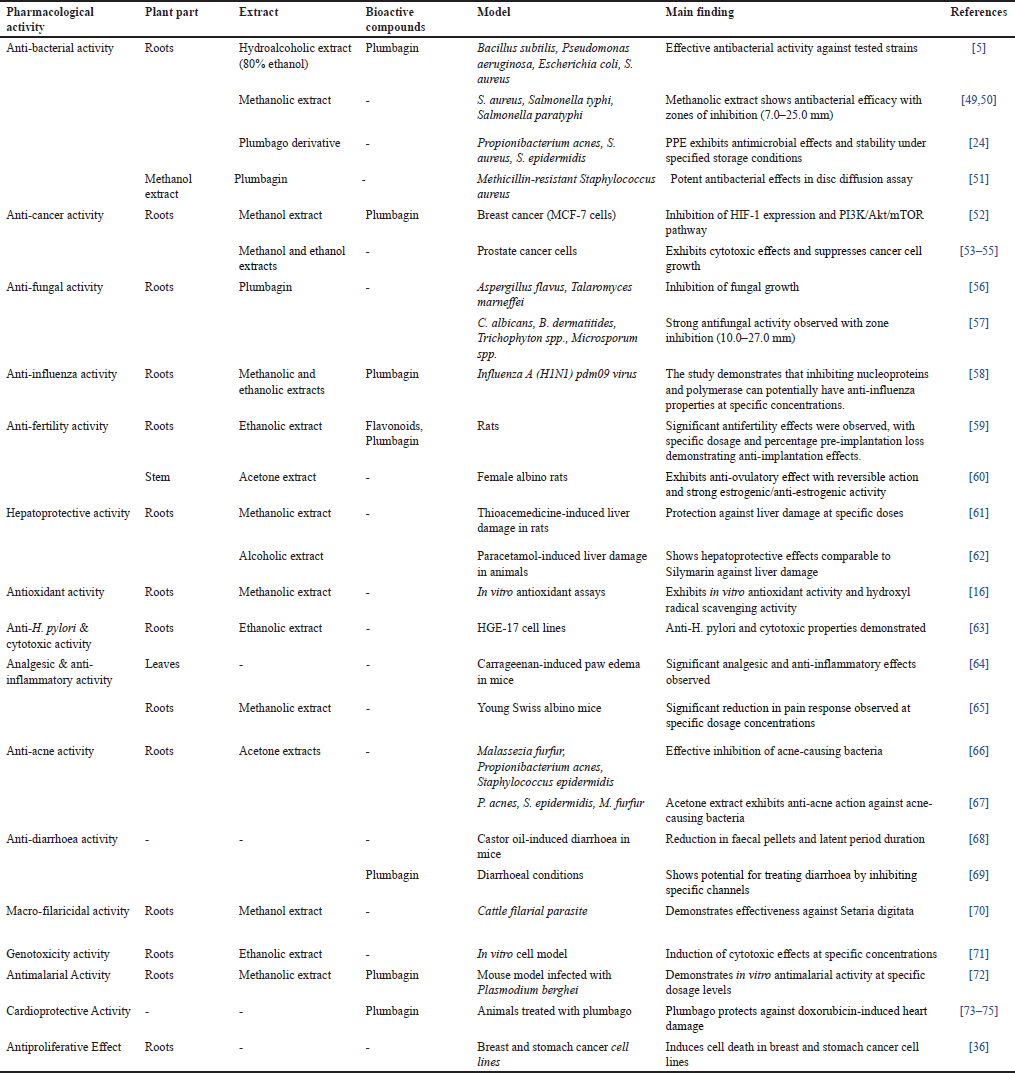 | Table 3. Pharmacological activities, plant parts, extracts, bioactive chemicals, model, and main findings [Click here to view] |
THREAT AND CONSERVATION STATUS
The limited accessibility of the P. indica plant can be attributed to various factors, including the absence of a fruiting stage, slow growth, and extensive harvesting. It is special that over 30% of Ayurvedic formulations incorporate this particular species as an ingredient. However, it is currently on the IUCN Red List of Endangered Species, but its status may be different in different parts of the world [77–79].
Factors affecting population loss
The global decline in population and the increasing demand for herbal remedies are exerting pressure on medicinal plants on a global scale [5]. Plumbago indica, an endangered species, is currently being utilized in the pharmaceutical, nutraceutical, and herbal sectors [29]. The degradation of habitats and competition for resources are being caused by urbanization, invasive species, and illegal harvesting. A significant portion of the respondents exhibit a lack of awareness regarding the diminishing number of domesticated species resulting from the decline of wild medicinal plants. The proliferation of human endeavors and illegal extraction exacerbate the detrimental impact on indigenous populations [78].
Conservation measures
Cryopreservation is the only viable long-term conservation method for the propagation of P.indica species, requiring an effective in vitro regeneration system. It efficiently utilizes preserved propagules for future large-scale regeneration [80]. It potentially benefits from constitutional protections and regulatory measures, which serve to safeguard its natural habitats against activities such as deforestation and development. The promotion of cultivation in botanical gardens and nurseries has the potential to decrease the size of wild populations and mitigate the demand for specimens [15]. Conducting regular research and monitoring is of utmost importance to accurately evaluate the conservation status of plants and to implement measures that are effective [81].
In vitro propagation
Propagation of P. indica by explant culture and hormonal treatments in an organized laboratory environment. In vitro propagation involves cultivating plants from tissue cells and organs using explants from the mother plant. Auxins such as IBA facilitate rooting, while cytokinins such as BAP, KN, and TDZ stimulate shoot induction and proliferation [82]. Nodal explants and leaf explants successfully induce bud initiation [83]. When treated with BAP during shoot multiplication, nodal explants are the most effective starting material for P. indica shoots. Callus regeneration is achieved by cultivating nodal segments in Murashige and Skoog medium supplemented with kinetin and 2,4-dichlorophenoxyacetic acid [84,85]. Acetic Acid, Indole Butyric Acid, and Putrecine result in the highest root production and regenerated shoots with a growth rate of 4.5 cm within 30–40 days [86].
Techniques and applications for large-scale plumbagin production from P indica
The production of plubagin from Plumbago indica root cultures has led to better ways to make chemicals on a large scale. Plumbagin can be extracted from the tissue-cultivated roots of Plumbago indica, but specific conditions must be met for high-level production. Adding chitosan to root cultures along with either β-alanine or methyl-β-cyclodextrin greatly increases the production of plumbagin. This makes it a cheap way to make a lot of therapeutic chemicals [87]. It is possible for the hairy root culture of P. indica to be a useful source of plumbagin because it grows shoots easily and can be changed into genetically modified plants [88]. Supplementing with l-alanine and adsorbing in situ greatly enhances plumbagin production in root cultures, making it an economical method for producing valuable therapeutic chemicals on a large scale [89]. Adding jasmonic acid to the culture conditions and stimulating them greatly raises the plumbagin levels in adventitious root cultures of Plumbago rosea, a plant that is used in ayurvedic medicine to treat a number of illnesses [90]. Regenerating transgenic plants and making P. indica roots hairy can produce consistent amounts of plumbagin. This implies that destroying the plant species in large numbers is not necessary, thereby simplifying its protection [91]. The addition of chitosan and methyl jasmonate significantly increases the generation of plumbagin in P. indica hairy root cultures and releases plumbagin into the culture medium [92].
The application adding of the outside hormones GA3 and NAA to the hairy roots of P. indica plants makes them grow faster and store more plumbagin, but adding 2, 4-D stops growth and production. Synthetic seeds prove to be an efficient method for preserving superior root clones [81]. Gamma-ray irradiation at a low dose of 20 Gy has a substantial effect on increasing plumbagin production in root cultures, with the highest output observed when the cultures are 10 days old [93]. Heat shock and in situ adsorption using Diaion® HP-20 greatly increase the production of plumbagin in root cultures, which has the potential to be used in commercial applications [94,95].
CULTIVATION PRACTICES
Cultivation practices refer to the various methods and techniques utilized in the cultivation and care of plants or crops. Plumbago indica, a highly adaptable angiosperm, exhibits robust growth in regions characterized by tropical and subtropical climates. This versatile plant species can be cultivated in various settings, including gardens, borders, and containers. Notably, container gardening proves advantageous in colder regions during the winter season [96]. This plant exhibits a preference for full sunlight rather than partial sunlight, and it is suitable for cultivation in various settings such as gardens, borders, or pots, particular plant exhibits resistance to both pests and diseases and can be propagated through either stem cuttings or seeds [97,33]. The exploitation and conservation status of the species highlights the difficulties in balancing market demand with the need to maintain its sustainability in its natural environment. Cultivation is a viable alternative to gathering plants from the wild because it offers economic prospects while simultaneously alleviating the strain on natural populations [98].
FUTURE PROSPECTS
Plumbago indica exhibits a wide range of potential applications in various fields such as horticulture, medicine, biodiversity conservation, sustainable agriculture, phytoremediation, traditional knowledge preservation, and the production of herbal products and cosmetics. The plant’s vibrant blue or white flowers and lush green foliage contribute to its widespread popularity in both gardens and public areas. Ongoing research is being conducted with the aim of discovering novel bioactive compounds that may have promising implications in the fields of contemporary medicine, herbal remedies, and dietary supplements. The drought tolerance and pest resistance exhibited by P.indica make it an appropriate candidate for sustainable agriculture, phytoremediation, and the preservation of traditional knowledge.
CONCLUSION
In conclusion, P. indica, which is known as Indian leadwort or Chitrak, is well-known for its potential as a medicinal and possesses a wide range of applications in both traditional and modern medicine. The focus of this review is on the botanical, traditional, phytochemical, pharmacological, and conservation approaches used to understand the plant’s therapeutic potential. The presence of bioactive chemicals like plumbagin, as well as other phytoconstituents like alkaloids, flavonoids, and terpenoids, found in different parts
of the plant, may enhance the therapeutic efficacy. It is a valuable natural medicine resource that holds potential for the development of novel treatments and alternative therapies. To manage chronic diseases like diabetes, further research is necessary. We recommend investigating its potential in combination with other plants or traditional drugs, and in the preparation of formulations to significantly improve its efficacy.
ACKNOWLEDGEMENT
The authors thank the Department of Pharmaceutical Sciences, GNDU, Amritsar for providing support during the preparation of the manuscript.
AUTHOR CONTRIBUTION
The authors, including Abdulkadir Abdu, Akhilesh Prakash, Rishav Kondal, Ritu Pal, Sudhir Sharma and Mani Bhagat, contributed to the initial idea conception, literature search, data collection, drafting, manuscript preparation, figure creation, and critical review. Hasandeep Singh, Balbir Singh, and Sarabjit Kaur reviewed and edited the manuscript for scientific accuracy and coherence. All authors have read and approved the final version of the manuscript submitted to the Journal of Applied Pharmaceutical Science. They can be authors per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
CONSENT TO PARTICIPATE
It is a review article, thus it is not applicable.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
The data that support the findings of this study are available in standard research databases such as PubMed, Science Direct, or Google Scholar, and/or on public domains that can be searched with either key words or DOI numbers.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Ahirwar RK, Gupta VK. Quantitative ethnomedicinal investigation of medicinal plants used by traditional healers to treat various diseases in the district Dindori, Madhya Pradesh, India. Ethnobo Res and App. 2024;28:28. CrossRef
2. WHO launches guidelines for traditional, alternative medicines. PharmacoEco Outco News. 2004;456(1):5–5. CrossRef
3. Haque F, Hassan AS, Jahan MAA, Roy SK. In vitro shoot proliferation and plant regeneration of Plumbago indica L. (Ractochita), a rare medicinal shrub of Bangladesh. Bang J of Sci and Ind Res. 2012;47(2):197–202. CrossRef
4. Lenora RDK, Dharmadasa RM, Abeysinghe DC, Arawwawala LDAM. Investigation of plumbagin content in Plumbago indica Linn. Grown under different growing systems. Pharmacologia. 2012;3(2):57–60. CrossRef
5. Bashir SF, Kumar G. Preliminary phytochemical screening and in vitro antibacterial activity of Plumbago indica (Laal chitrak) root extracts against drug-resistant Escherichia coli and Klebsiella pneumoniae. Open Agri. 2021;6(1):435–44. CrossRef
6. Singh K, Naidoo Y, Baijnath H. A Comprehensive review on the genus plumbago with focus on Plumbago auriculata (Plumbaginaceae). Afr J of Trad Comp and Alt Med. 2017;15(1):199–215. CrossRef
7. Anitha C, Basil RMR, Arokya GPT, Prakash SS, Josephine PC. In vitro antimicrobial activity of flower extracts of Plumbago indica L. against pathogenic bacteria and fungi. Int J of Zoo Invest. 2023;9(1):954–60. CrossRef
8. Li Y, Cheng X, Lai J, Zhou Y, Lei T, Yang L, et al. ISSR molecular markers and anatomical structures can assist in rapid and directional screening of cold-tolerant seedling mutants of medicinal and ornamental plant in Plumbago indica L. Front Plant Sci. 2023;3:14. CrossRef
9. Prakash AV. Cryopreservation of chethikoduveli (Plumbago rosea L.) and assessment of genetic fidelity of regenerated plantlets using molecular markers [Doctoral dissertation]. Vellayani, India: Department of Plant Biotechnology, College of Agriculture; 2014.
10. Study of the Pretreatment (Shodhana) of Roots of Plumbago indica L. in Ayurveda. 2015. Available from: http://dr.lib.sjp.ac.lk/handle/123456789/4584
11. Eldhose B, Notario V, Latha MS. Evaluation of phytochemical constituents and in vitro antioxidant activities of Mentha arvensis. Int J Biol Pharm All Sci. 2021;10:157–61. CrossRef
12. Bhatia H, Ahuja D. Phytochemical evaluation of Plumbago indica L. root extracts. J of Popu Ther and Cli Pharmaco. 2022;29:1538–47. CrossRef
13. Jaisi A, Panichayupakaranant P. Increased production of plumbagin in Plumbago indica root cultures by biotic and abiotic elicitors. Biotech Let. 2015;38(2):351–5. CrossRef
14. Manyaem S, Samosorn S. The isolation of bioactive compounds from the branches of Plumbago indica L. 2023. Available from: http://ir-ithesis.swu.ac.th/dspace/handle/123456789/2042
15. Bhadra SK, Akhter T, Hossain MM. In vitro Micropropagation of Plumbago indica L. through induction of direct and indirect organogenesis. Pla Tis Cul and Biotech. 1970;19(2):169–75. CrossRef
16. Eldhose B, Notario V, Latha MS. Evaluation of phytochemical constituents and in vitro antioxidant activities of Plumbago indica root extracts. J Pharmacog and Phytochem. 2013;2(4):157–61.
17. Priyanjani HASA, Senarath RMUS, Senarath WTPSK, Munasinghe MLAMS. Propagation, Phytochemistry and Pharmacology of Plumbago indica—a review. J of Pharmaceu Res Int. 2021;188–202. CrossRef
18. Sharma K, Yadav P. Forensic analysis of roots of an abortifacient plant Plumbago rosea L. Int J Sci Res Rev. 2019;7(5):1156–59.
19. Khatun R, Dash CK, Alam SS, Sultana SS. Chromosomal characterization in Plumbago zeylanica and P. indica from Bangladesh. Cytologia. 2019;84(2):153–5. CrossRef
20. Chaudhari S. Anatomy of two species of Plumbago: a traditional medicinal plant and its relevance for taxonomy. Flora Fauna. 2021;2(2):197–208. CrossRef
21. Satheeshkumar K, Jose B, Pillai D, Krishnan PN. Prospects of Plumbago rosea L. hairy root culture in traditional preparations: a phytochemical comparison with tuberous roots. Pla Root. 2014;8(0):13–23. CrossRef
22. Borah SP. Cytogenetics of Plumbago Linn studies on karyomorphology and nuclear DNA content in certain species. 2015. CrossRef
23. Kurian A, Sankar MA. Medicinal plants. New Delhi, India: New India Publishing; 2007. vol. 2, 147–51 pp.
24. Harborne JB. Comparative biochemistry of the flavonoids-IV. Phytochem. 1967;6(10):1415–28. CrossRef
25. Kaewbumrung S, Panichayupakaranant P. Antibacterial activity of plumbagin derivative-rich Plumbago indica root extracts and chemical stability. Nat Prod Res. 2014;28(11):835–7. CrossRef
26. Dinda B, Das SK, Hajra AK, Bhattacharya A, De K, Chel G, et al. ChemInform abstract: chemical constituents of Plumbago indica roots and reactions of Plumbagin: part 2. ChemInfo. 1999;30(43). CrossRef
27. Khosla G, Sharma V, Shukla VK. Isolation, Characterization and antioxidant activity of Plumbago indica L. Extract. Int J Drug Del Tech. 2022;12(03):936–46. CrossRef
28. Ariyanathan S, Saraswathy A, Rajamanickam GV, Connolly JD. ChemInform abstract: polyphenols from the roots of Plumbago rosea. ChemInfo. 2010;41(29). CrossRef
29. Tripathi T, Singh A, Dhobi M, Kalaiselvan V. Comparative metabolic profiling, isolation of alkylated phenols and antioxidant activity of roots of Plumbago species using GC-MS and NMR based metabolomics study. Nat Pro Res. 2022;36(23):6126–31. CrossRef
30. Dinda B, Chel G, Achari B. A dihydroflavonol from Plumbago indica. Phytochem. 1994;35(4):1083–4. CrossRef
31. Melk MM, El-Hawary SSE, Melek FR, Saleh DO, Selim NM. Cytotoxic Plumbagin-5-O-α-l-rhamnopyranoside from Plumbago indica. Revi Bras de Farmacog. 2021;31(6):838–41. CrossRef
32. Harborne JB. Plant polyphenols. Arch Biochem Biophy. 1962;96(1):171–8. CrossRef
33. Khatun A. Plumbago indica L. A review of its medicinal uses, phytochemistry, pharmacology, and toxicology. Int J Herb Med. 2023;11(4):31–7. CrossRef
34. Plumbago indica—Useful Tropical Plants. Available from: https://tropical.theferns.info/viewtropical.php?id=Plumbago+indica
35. Manager. Plumbago indica L. Nature. Available from: https://www.asia-medicinalplants.info/plumbago-indica-l/
36. Jayanthi M, Gokulanathan A, Haribalan P, Ashakiran K, Dinesh Kumar C, Kamal D, et al. Plumbagin from two Plumbago species inhibits the growth of stomach and breast cancer cell lines. Ind Cro and Prod. 2020;146:112147. CrossRef
37. Laurel Plumbago rosea: Philippine Medicinal Herbs / Philippine Alternative Medicine. Available from: http://www.stuartxchange.org/Laurel.html
38. Yusuf M, Wahab M, Yousuf M, Chowdhury JU, Begum J. Some tribal medicinal plants of Chittagong Hill Tracts, Bangladesh. Bang J of Pla Tax. 1970;14(2):117–28. CrossRef
39. Mallavadhani UV, Sahu G, Muralidhar J. Screening of Plumbago species for the bio-active marker Plumbagin. Pharmaceu Bio. 2002;40(7):508–11.
40. Dutta DN. Wealth of medicinal and aromatic crops of North East India and their commercial exploitation. Guwahati, India: Assam Department of Agriculture; 2019. 12–68 pp.
41. Jose B, Dhanya BP, Silja PK, Krishnan PN, Satheeshkumar K. Plumbago rosea L.-A review on tissue culture and pharmacological research. Int J Pharma Sci Rev Res. 2014;25(1):246–56.
42. Padumadasa C, Abeysekera AM, Meedin SDK. A Preliminary Investigation of the Shodhana (Detoxification) of Roots of Plumbago indica L. In Ayurveda. 2015.
43. Goraya GS, Ved DK. Medicinal plant species in commercial demand. Consolidated inventory and analysis. New Delhi, India: echarak; 2015. 257–91 pp.
44. Ghate U, Britto J, Dubey P, Verma H, Kashyap V. Good agriculture/collection practices (GACP) package of practices (POP) of important medicinal plants for quality Council of India (QCI). New Delhi, India: CCD- the Covenant Centre for Development, Madurai & Durg, GMCL- Gram Mooligai Co. Ltd; 2020. 1–26 pp.
45. Chitrak Root Extract—Plumbago indica-Extract@TheWholesalerCo. TheWholesalerCo. Available from: https://thewholesaler.in/products/chitrak-root-extract-plumbago-indica
46. Gowthami R, Sharma N, Pandey R, Agrawal A. Status and consolidated list of threatened medicinal plants of India. Gen Res Cro Evo. 2021;68(6):2235–63. CrossRef
47. Sukkasem N, Chatuphonprasert W, Tatiyaaphiradee N, Jarukamjorn K. Imbalance of the antioxidative system by plumbagin and Plumbago indica L. extract induces hepatotoxicity in mice. J Intercul Ethnopharma. 2016;5(2):137. CrossRef
48. Solomon FE, Sharada AC, Devi PU. Toxic effects of crude root extract of Plumbago rosea (Rakta chitraka) on mice and rats. J Ethnopharma. 1993;38(1):79–84. CrossRef
49. Kaewbumrung S, Panichayupakaranant P. Isolation of three antibacterial naphthoquinones from Plumbago indica roots and development of a validated quantitative HPLC analytical method. Nat Prod Res. 2011;26(21):2020–3. CrossRef
50. Saha D, Paul S. Antibacterial activity of Plumbago indic . Turk J Pharm Sci. 2014;11(2):217–22.
51. Dissanayake DMIH, Perera DDBD, Keerthirathna LR, Heendeniya S, Anderson RJ, Williams DE, et al. Antimicrobial activity of Plumbago indica and ligand screening of plumbagin against methicillin-resistant Staphylococcus aureus. J Biomol Struc Dyna. 2020;40(7):3273–84. CrossRef
52. Jampasri S, Reabroi S, Tungmunnithum D, Parichatikanond W, Pinthong D. Plumbagin suppresses breast cancer progression by downregulating HIF-1α expression via a PI3K/Akt/mTOR independent pathway under hypoxic condition. Mole. 2022;27(17):5716. CrossRef
53. Powolny AA, Singh SV. Plumbagin-induced apoptosis in human prostate cancer cells is associated with modulation of cellular redox status and generation of reactive oxygen species. Pharma Res. 2008;25(9):2171–80. CrossRef
54. Yin Z, Zhang J, Chen L, Guo Q, Yang B, Zhang W, et al. Anticancer effects and mechanisms of action of Plumbagin: review of research advances. Troiano G, editor. BioMed Res Int. 2020;2020:1–10. CrossRef
55. Hafeez BB, Fischer JW, Singh A, Zhong W, Mustafa A, Meske L, et al. Plumbagin inhibits prostate carcinogenesis in intact and castrated PTEN knockout mice via targeting PKC?, Stat3, and epithelial-to-mesenchymal transition markers. Canc Prev Res. 2015;8(5):375–86. CrossRef
56. Manyaem S, Samosorn S. The isolation of bioactive compounds from the branches of Plumbago indica l [Doctoral dissertation]. Bangkok, Thailand: Srinakharinwirot University; 2022.
57. Saha D, Paul S. In vitro screening of antifungal activity of methanol extract of Plumbago indica L. against some pathogenic species of fungi. J Res Pharma Sci. 2012;2(2):55–7.
58. Chavan R, Shinde P, Girkar K, Madage R, Chowdhary A. Assessment of anti-influenza activity and hemagglutination inhibition of Plumbago indica and Allium sativum extracts. Pharmaco Res. 2016;8(2):105. CrossRef
59. Savadi R, Alagawadi K. Antifertility activity of ethanolic extracts of Plumbago indicaandAerva lanataon albino rats. Int J Green Pharma. 2009;3(3):230. CrossRef
60. Sheeja E, Joshi S, Jain D. Antiovulatory and estrogenic activity of Plumbago rosealeaves in female albino rats. Ind J of Pharmacol. 2009;41(6):273. CrossRef
61. Binil E, Jayesh K, Sankunni LM. Curative effect of Plumbago indica root extract on thioacetamide induced hepatotoxicity in experimental rats. Int J of Phytomed. 2017;9(1):138. CrossRef
62. Rajasekaran A. Protective effect of ethanolic root extract of Plumbago indica L. on paracetamol induced hepatotoxicity in rats. Afr J Pharma Pharmacol. 2011;5(20). CrossRef
63. Paul AS, Islam A, Yuvaraj P. Anti-Helicobacter pylori and cytotoxic activity of detoxified root of Plumbago auriculata, Plumbago indica and Plumbago zeylanica. J Phytopharmacol. 2013;2(3):4–8. CrossRef
64. Ittiyavirah SP, Jobin KV, Jissa MS, Jomy M, Josmi TJ, Littin B. Anti-inflammatory and analgesic activities of Plumbago capensis and Plumbago indica . Adv Pharmacol Tox. 2012;13(1):47.
65. Paul S, Saha D. Analgesic activity of methanol extract of Plumbago indica (L.) by acetic acid induced writhing method. JPharma Tech. 2012;2(2):74–6.
66. Kaur DA, Prasad SB. Anti-acne activity of acetone extract of Plumbago indica root. J Pharmaceu Cli Res. 2016;9(2):285–7.
67. Kaur D, Prasad SB, Verma S. Formulation and evaluation gel from extract of Plumbago indica for acne. Int J Drug Del Tech. 2016;6(3):95–8.
68. Ibrahim M, Baura J, Ahasan Q, Islam T, Homa Z, Chowdhury MM, et al. Preliminary phytochemical and pharmacological investigations of Alpinia conchigera Griff. and Plumbago indica L. Bang Pharm J. 2012;15(2):153–7.
69. Yu B, Zhu X, Yang X, Jin L, Xu J, Ma T, et al. Plumbagin prevents secretory diarrhea by inhibiting CaCC and CFTR channel activities. Front Pharmacol. 2019;10:1181. CrossRef
70. Mathew N, Paily KP, Abidha, Vanamail P, Kalyanasundaram M, Balaraman K. Macrofilaricidal activity of the plant Plumbago indica /rosea in vitro. Drug Develop Res. 2002;56(1):33–9. CrossRef
71. Thitiorul S, Ratanavalachai T, Tanuchit S, Itharat A, Sakpakdeejaroen I. Genotoxicity and interference with cell cycle activities by an ethanolic extract from Thai Plumbago indica roots in human Lymphocytes in vitro. Pac J Can Prev. 2013;14(4):2487–90. CrossRef
72. Sumsakul W, Plengsuriyakarn T, Chaijaroenkul W, Viyanant V, Karbwang J, Na-Bangchang K. Antimalarial activity of plumbagin in vitro and in animal models. BMC Comp and Alt Med. 2014;14(1). CrossRef
73. Li Z, Chinnathambi A, Ali Alharbi S, Yin F. Plumbagin protects the myocardial damage by modulating the cardiac biomarkers, antioxidants, and apoptosis signaling in the doxorubicin-induced cardiotoxicity in rats. Enviro Tox. 2020;35(12):1374–85. CrossRef
74. Zhang G, Ni X, Zhou Y. Cardioprotective effect of Plumbagin and Amelioration of pro-inflammatory cytokines through suppression of Na+/K+-ATPase on myocardial ischemia. Pharmaco Mag. 2021;17:643–8. CrossRef
75. Wang SX, Wang J, Shao JB, Tang W, Zhong JQ. Plumbagin mediates cardioprotection against myocardial Ischemia/Reperfusion injury through Nrf-2 signaling. Med Sci Mon. 2016;22:1250–7. CrossRef
76. Naresh RAR, Udupa N, Devi PU. Niosomal Plumbagin with reduced toxicity and improved anticancer activity in BALB/C Mice. J Pharma Pharmaco. 1996;48(11):1128–32. CrossRef
77. Ravindran A, Gayan J, Das BN. A standardized protocol for genomic DNA isolation from the species of Plumbago Linn. Int J Life-Sciences Sci Res. 2017;3(5):1345–9.
78. Bhattacharyya R, Ray A, Gangopadhyay M, Bhattacharya S. In vitro conservation of Plumbago indica —a rare medicinal plant. Plant Cell Biotechnol Mol Biol. 2007;8:39–46.
79. Reddy KN, Reddy CS. First Red List of Medicinal Plants of Andhra Pradesh, India—Conservation Assessment and Management Planning. Ethnobotanical leaflets. 2008;2008(1):12 Available from: https://opensiuc.lib.siu.edu/cgi/viewcontent.cgi?article=1046&context=ebl.
80. Gangopadhyay M, Bhattacharyya R, Bahttacharya S. Ex situ conservation of Plumbago indica using biotechnology. Planta Medica. 2009;75(09). CrossRef
81. Gangopadhyay M, Dewanjee S, Chakraborty D, Bhattacharya S. Role of exogenous phytohormones on growth and plumbagin accumulation in Plumbago indica hairy roots and conservation of elite root clones via synthetic seeds. Ind Cro and Prod. 2011;33(2):445–50. CrossRef
82. Khoa TT, Duy PM, Huong TT, Quynh NT. Effects of in vitro plant ages on the subsequent growth of Plumbago indica l. after ex vitro transplantation. Acad J Bio. 2021;43(1):109–18. CrossRef
83. Gopalakrishnan M, Janarthananm B, Lakshmi Sai G, Sekar T. Plant regeneration from leaf explants of Plumbago. Pla Tis Cult Biotech. 1970;19(1):79–87. CrossRef
84. Springob K, Samappito S, Jindaprasert A, Schmidt J, Page JE, De-Eknamkul W, et al. A polyketide synthase of Plumbago indica that catalyzes the formation of hexaketide pyrones. The FEBS J. 2006;274(2):406–17. CrossRef
85. Kumar KS, Bhavanandan KV. Micropropagation of Plumbago rosea Linn. Pla Cel, Tis Org Cult. 1988;15(3):275–8. CrossRef
86. Makhzoum A, Hefferon K. Applications in plant biotechnology. Boca Raton, FL, CRC Press; 2022. Available from: http://books.google.ie/books?id=U9eYEAAAQBAJ&pg=PT2&dq=9781003008866&hl=&cd=1&source=gbs_api
87. Jaisi A, Panichayupakaranant P. Enhanced plumbagin production in Plumbago indica root culture by simultaneous and sequential dual elicitations using chitosan with ?-alanine and methyl-β-cyclodextrin. Bioresources Bioprocessing. 2020 Feb 28;7(1). CrossRef
88. Gangopadhyay M, Sircar D, Mitra A, Bhattacharya S. Hairy root culture of Plumbago indica as a potential source for plumbagin. Biologia plantarum. 2008;52(3):533–7. CrossRef
89. Jaisi A, Panichayupakaranant P. Enhanced plumbagin production in Plumbago indica root cultures by ?-alanine feeding and in situ adsorption. PCTOC. 2016; 129(1):53–60. CrossRef
90. Silja PK, Satheeshkumar K. Establishment of adventitious root cultures from leaf explants of Plumbago rosea and enhanced plumbagin production through elicitation. Ind Crops Prod. 2015 Dec;76:479–86. CrossRef
91. Martin KP, Sabovljevic A, Madassery J. High-frequency transgenic plant regeneration and plumbagin production through methyl jasmonate elicitation from hairy roots of Plumbago indica L. J Crop Sci Biotechnol. 2011 Sep;14(3):205–12. CrossRef
92. Gangopadhyay M, Dewanjee S, Bhattacharya S. Enhanced plumbagin production in elicited Plumbago indica hairy root cultures. J Biosci Bioeng. 2011 Jun;111(6):706–10. CrossRef
93. Jaisi A, Sakunphueak A, Panichayupakaranant P. Increased production of plumbagin in Plumbago indicaroot cultures by gamma ray irradiation. Pharm Biol. 2013 Jun 7;51(8):1047–51. CrossRef
94. Jaisi A, Panichayupakaranant P. Simultaneous heat shock and in situ adsorption enhance plumbagin production in Plumbago indica root cultures. Eng Life Sci. 2016 Apr;16(5):417–23. CrossRef
95. Jaisi A, Panichayupakaranant P. Chitosan elicitation and sequential Diaion® HP-20 addition a powerful approach for enhanced plumbagin production in Plumbago indica root cultures. Proc Biochem. 2017 Feb;53:210–5. CrossRef
96. Regional cum facilitation centre (RCFC), southern region. Available from: https://rcfcsouthern.org/species_details.php?species_id=13
97. NParks | Plumbago indica. Available from: https://www.nparks.gov.sg/florafaunaweb/flora/2/3/2347
98. Plumbago indica | Species. India Biodiversity Portal. https://indiabiodiversity.org/species/show/230776