INTRODUCTION
Oral anticoagulants like warfarin and acenocoumarol under vitamin K antagonists have been used for several decades. Its interactions with the diet and regular monitoring of laboratory parameters have been replaced with other alternatives such as directly acting oral anticoagulants (DOACs) which are shown to be safer and effective than warfarin in a few disease conditions. The DOACs such as dabigatran, apixaban, edoxaban, and rivaroxaban are approved for the treatment of stroke, atrial fibrillation (AF), systemic embolism (SE), venous thromboembolism (VTE) as a preventive measure in orthopaedic surgery, acute VTE management, and recurrent VTE treatment [1]. The scientific studies have comprehensively demonstrated the safety and effectiveness of oral anticoagulants and various clinical international normalized ratio (INR) goal ranges have been fixed for oral anticoagulant therapy in a variety of disease states [2,3]. Studies have demonstrated that most patients do not have sufficient knowledge about the oral anticoagulant (OAC) they are taking, despite the fact that their usage is common and that there is a possibility of severe adverse reactions [4,5]. Based on various studies, individuals who are more knowledgeable about OAC medications likely to have better long-term outcomes [6,5].
Individuals with concurrent illnesses who know less about medical conditions find it challenging to comprehend prescription medicines and other medication-related instructions. Patients have difficulty learning and remembering instructions due to a variety of variables, including distracting environments, emotional and physical suffering, and a lack of motivation. Healthcare experts and clinicians look into numerous approaches to make the instructions more accessible because these diverse variables cannot be ruled out at the time of discharge. Few studies have shown that the process of creating pictures enhances the perception of written treatment instructions [7,8]. According to a study by Austin et al. [9] adding visuals to the instructions boosted comprehension of the discharge instructions by a factor of 1.5. Since, it is not possible to completely exclude these other influences at the time of discharge, researchers and doctors research with multiple strategies to improve the readability of the instructions.
Patients should be knowledgeable about their medicines, as it is linked to better safety and adherence. Patients might need more medication details and understand what they have been informed about [10]. Even while healthcare professionals are crucial in giving patients spoken medical information, there may be restrictions like workload and time constraints that could make this provision difficult. Patients might not only be given insufficient details on medications, but they may also misunderstand what they have been informed.
Patient education is essential to every aspect of healthcare. Typically, patients who wished to better understand health information more clearly on medication use, administration, risks, storage, and disposal, were used to review the patient information leaflet (PIL) [11,12]. PILs that are included with medications have the potential to improve patient education about their medications, promote safe medication use, and serve as a resource for patients to use when making decisions about their medication use, thereby supporting patient-centered care [8,13]. PILs with user-friendly designs with simple language designed specifically for patients. They can assist patients in maintaining updated with crucial information that enhances understanding and minimizes adverse outcomes [7,8,14,13,15].
Pharmacists’ or fellow pharmacists can also assist in educating the patients regarding warfarin therapy by managing the drug interactions among the patients [15]. Patient information handouts, advice at the commencement of the therapy, re-education during prescription refilling, anticoagulation alert cards, and specialized assistance that make the patients alert regarding their medicine intake improve the adherence toward DOACs and other anticoagulants [16].
Here, a variety of criteria that offer a thorough evaluation of written content, communication, and presentation were used to assess the readability and understandability of real patient-facing files. This was done in light of the significance of making sure that patients comprehend information leaflets and an intricacy of measures for their evaluation. The main aim of the present research is the development and assessment of psychometric properties of patient education tools on oral anticoagulants and warfarin pharmacogenetics.
MATERIALS AND METHODS
A prospective observational study was carried out at a Tertiary Care Teaching Hospital, with Institutional Ethics Committee (IEC) reference number 582/2020. The IEC approval was attained before initiating the research. The detailed study procedure was described under the following headings.
Development and validation of patient education tools
The patient education tools in the form of an information leaflet on oral anticoagulants, warfarin, and warfarin pharmacogenetics were developed by searching and referring through evidence-based literature. Initially, the patient education tool was prepared in English version. The patient education tool consists of information regarding indication, use, monitoring parameters, medications to be avoided, and adverse effects of anticoagulants. The content of the educational tool was validated with the experts (n = 9) consisting of physicians (n = 3) and academic pharmacists (n = 6). The validated patient education tool was corrected according to the experts’ opinions and the recommendations were taken into consideration, and any gaps in knowledge or modifications were updated in the education tool and further subjected to readability by using “Flesch Reading Ease” (FRE) and “Flesch-Kincaid Grade-Level” (FKGL) [17], design and layout “Baker Able Leaflet Design” (BALD) [18]. The linguistic translation method was used to convert the education tool final version into Kannada. The final version was tested for design, content validity, and reliability.
Development and validation of anticoagulation knowledge questionnaire
The anticoagulation knowledge questionnaire was developed based on the content of the patient education tool. Initially, 20-item questions were built based on knowledge, attitude, and practice of anticoagulants. The questionnaire was validated by the expert panel (n = 7) who signed an informed consent form and participated in the study. In this research, the content validity of the anticoagulation knowledge questionnaire is measured using “4-point” content validity index (CVI) [19] which was provided to physicians and academicians from pharmacy. The researcher provided a duplicate of the anticoagulation knowledge questionnaire, and CVI assessment form and gave each of them a detailed description of the goals and purposes of the research objectives. The experts were asked to rank each item according to its relevance, clarity, simplicity, and ambiguity using a four-point rating system. The expert panel checked each line for errors and/or improper item selection. A qualitative response was given, and several items had instructions such that the assessors would be familiar with certain particular questions ought to be responded to.
Performing content validation and assigning scores
The CVI of the questionnaire as well as each item was determined using proportions. Each item’s CVI was calculated as the percentage of judges who thought the item was legitimate, or deserving score of 3 or 4. First, it had to be determined which proportion of judges had given an item a score of 3 or 4 on each of the four parameters (ambiguity, relevance, clarity, and simplicity). The percentage of experts who gave each item’s content a legitimate rating (score of 3 or 4) is its CVI [20]. Alternatively, it is calculated by dividing the total number of judges by the number of judges who gave a valid score to an item.
Validity evidence for the response process
Following a response process validity assessment of ten questions that had reached an acceptable level of I-CVI, the remaining 10 items were removed in accordance with the results of the study on content validity. The minimum permissible value for I-CVI and S-CVI/Avg was 0.80 because the assessment employed more than five experts. After removing items from the list that scored an I-CVI less than 0.80, the response process validity of the remaining items was assessed. Following the item elimination procedure, the 10-item Anticoagulant questionnaire’s S-CVI/Avg was compared with the S-CVI/Avg of removed items, which was recomputed using the scores of the remaining items.
Pilot-testing and responsiveness
In a pilot study, the anticoagulation knowledge questionnaire was administered to 20 randomly selected patients of any age who can understand English or Kannada language. Before administering the questionnaire, the patients’ consent was obtained. The questionnaire comprises of 10 multiple choice questions (MCQs) with four options each. Each correct answer scored 1 while an incorrect answer recorded as zero. After the baseline knowledge assessment of the patients. Following the patients’ 10–20 minute use of the educational tool, a post-knowledge assessment was conducted. The education tool was explained and informed about the oral anticoagulants dose, diet, adverse effects, precautionary measures, and reporting to healthcare professionals about anticoagulation laboratory reports.
Statistical analysis
The reliability was assessed by using Cronbach’s alpha based on the questionnaire scored by the experts and 20 randomly selected patients using the Kuder and Richardson formula as follows: [21].
The change in knowledge score was analyzed using the paired-“t” test. A p-value less than 0.05 was considered as statistically significant. The Statistical Package for the Social Sciences (SPSS) Version 20.0 was used to analyze the data.
RESULTS
Readability score for the patient education tools
The best FRE and FK-GL scores achieved for anticoagulants education tool was 62.3 and 8.1 and warfarin pharmacogenetics education tool scored 60.7 and 8.6, respectively. Both the scores rate that the developed education tools were “standard”.
Layout and design scores for the patient education tools:
The layout and design of anticoagulants, warfarin, and warfarin pharmacogenetics education tools developed in English and Kannada languages were good and their individual BALD scores are 28, 28, 29, 26, 26, and 28, respectively.
CVI assessment for anticoagulation knowledge questionnaire
Based on the expert panel rating score, the questions added, modified, and deleted. Content validity was supported by a scale CVI of 0.84 for 10 MCQs questionnaire with a Cronbach’s value more than 0.80 were included in the final anticoagulation knowledge questionnaire. By eliminating the questions in the knowledge questionnaire based on the rating scale, the number of questions decreased from 20 to 10.
Scoring for the content validity
Out of 20 questions, 10 questions were judged for relevance, clarity, simplicity, and ambiguity and CVI was determined for each item. Table 1 depicts the I-CVI range for ten items, which is 0.7–1.0. The SCVIs/Avg scores ranged from 0.79 to 0.90 and SCVIs/UA ranged between 0.6 and 0.8.
Reliability score for anticoagulation knowledge questionnaire
The knowledge assessment questionnaire was finalized based on Cronbach’s α value which was presented in Table 2. The reliability scores using Cronbach’s alpha for the anticoagulant knowledge questionnaire completed by the experts and 20 randomly selected patients were 0.816 and 0.704.
The anticoagulant knowledge questionnaire completed by experts and 20 randomly chosen patients had Cronbach’s alpha reliability values of 0.816 and 0.704, respectively.
Pilot User-testing
The pilot user-testing was done by selecting 20 patients randomly and the patients’ average age was 48 ± 2.82. Table 3 illustrates demographic characteristics.
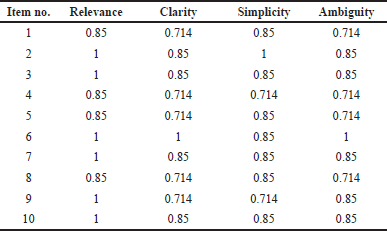 | Table 1. CVI Scores of each item for relevance, clarity, simplicity, and ambiguity for the knowledge questionnaire. [Click here to view] |
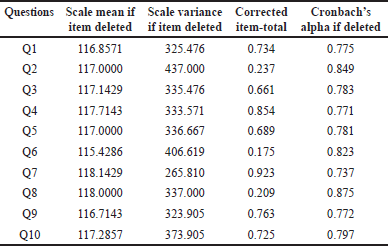 | Table 2. Reliability score for knowledge questionnaire. [Click here to view] |
Pilot-testing and responsiveness
The knowledge scores before and after using the patient education tool were 55 ± 21.21 and 80 ± 14.14, respectively. The anticoagulation knowledge questionnaire ability to detect the changes and the knowledge level has significantly improved by 25 (p < 0.001) after using the patient education tool.
DISCUSSION
To improve clinical outcomes and treatment adherence, patients can better understand their disease with the help of cost-effective written and printed patient education resources, such as leaflets [22,15]. To help patients understand and remember the information better, it is presented in a patient education tool with the appropriate pictograms [5,23]. In the earlier research, leaflets were created for rheumatoid arthritis, diabetes, hypertension, diabetic foot ulcers, asthma, peptic ulcers, chronic kidney disease, coronary heart disease, and tuberculosis diseases [24,25]. This is the first study to analyze the psychometric characteristics of an oral anticoagulant medication patient education tool. In this study, patient education tools were prepared in a simple language with fairly good readability. The readability scores matched the outcomes of related research projects [24,25,26]. The results of the BALD were better than those of earlier studies conducted in India [24,25,27].
For healthcare practitioners to efficiently evaluate patients on both warfarin and DOACs, there is not currently an anticoagulant knowledge tool available in Kannada and English. For patients in South India receiving warfarin or DOACs, this study effectively developed and validated an anticoagulant knowledge questionnaire. The anticoagulant knowledge questionnaire for patients receiving warfarin and/or DOACs in South India was effectively created and validated by this study. The CVI scores of the questionnaire developed in the present study were comparable to those obtained in a similar study [20,26].
There is a correlation between the items and the overall scale, and the reliability of the questionnaire was found to be relatively exceptional. The patient group had a lower level of reliability. This could be the result of the patients receiving exceptionally low scores on a few items. Due to the COVID pandemic impeding the study’s practicality, test-retest reliability was not evaluated in this pilot study. Since the patients were hesitant to come back to the hospital for follow-up appointments, it was challenging to get in touch with them. Low test-retest reliability can also result from patients’ tendency to look up the right answer online or from their doctors after the initial test, which could have given them a higher knowledge score on subsequent assessments. However, the questionnaire showed acceptable reliability and was comparable to the findings of earlier research [27,28] on obstructive airway diseases [28] and hemodialysis patients [8].
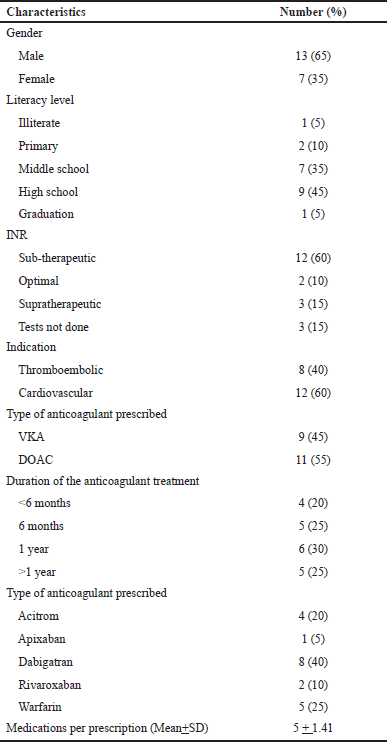 | Table 3. Demographic characteristics. [Click here to view] |
The patients had a knowledge gap on anticoagulation information, according to the results of our pilot study, which had a bigger impact on the INR value. Patients who have inadequate INR management are more vulnerable to hemorrhagic or ischemic stroke due to an increased risk of overall mortality [29]. This education tool has the ability to be used in healthcare facilities to identify patients’ misconceptions or knowledge gaps about oral anticoagulants.
The patient’s knowledge significantly improved as evidenced by the knowledge scores obtained both before and after using the patient education tool. A number of other studies, both in India and elsewhere, involving different ailments, have similarly demonstrated improvements in post-test scores, indicating a substantial benefit in knowledge regarding medications, diseases, and lifestyle modifications [29,30].
There were fewer items in the anticoagulant knowledge questionnaire compared to the other tools, and it only contained closed-ended questions [30,31]. Use of it in healthcare settings, particularly in public institutions, may therefore be more practical. In general, the questionnaire may serve as a useful screening tool for identifying individuals who lack information about anticoagulants.
On a positive note, the questionnaire showed some ability to distinguish between the patients who took part in the study based on their degree of knowledge. A larger-scale study is necessary in the future to investigate the impact of patient knowledge on ensuing clinical outcomes using the questionnaire as the assessment method, since there is evidence that anticoagulation knowledge is positively correlated with adherence to the treatment and anticoagulant control. We admit that the size of our validation sample was limited. But there is no hard-and-fast rule when it comes to sample size in validation studies.
CONCLUSION
The validated patient education tools had standard readability and a good layout and design. The study findings showed that the anticoagulation knowledge questionnaire developed is reliable, valid, and ability to detect the changes. In the present study, the anticoagulation knowledge questionnaire was systematically translated into the Kannada version and it was used to evaluate the knowledge level of OAC in patients. In the study, the developed patient education tools had good psychometric properties.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Tertiary Care Teaching Hospital, with Institutional Ethics Committee with approval number 582/2020.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Lee LH. DOACs—advances and limitations in real world. Thromb J. 2016;14(Supp 1):17. CrossRef
2. Rudasill SE, Liu J, Kamath AF. Revisiting the International Normalized Ratio (INR) threshold for complications in primary total knee arthroplasty: an analysis of 21,239 Cases. J Bone Joint Surg Am. 2019;101(6):514–22. CrossRef
3. Schwebach AA, Waybright RA, Johnson TJ. Fixed-dose four-factor prothrombin complex concentrate for vitamin K antagonist reversal: does one dose fit all? Pharmacotherapy. 2019;39(5):599–608. CrossRef
4. Shrestha S, Sapkota B, Kumpakha A, Acharya U, Sharma R. Evaluation of patients’ knowledge on warfarin in outpatient pharmacy of a tertiary care cardiac center. BMC Res Notes. 2015;8:1–5. CrossRef
5. Kagansky N, Knobler H, Rimon E, Ozer Z, Levy S. Safety of anticoagulation therapy in well-informed older patients. Arch Intern Med. 2004;164(18):2044–50. CrossRef
6. Tang EOY, Lai CS, Lee KK, Wong RS, Cheng G, Chan TY. Relationship between patients’ warfarin knowledge and anticoagulation control. Ann Pharmacother. 2003;37(1):34–9. CrossRef
7. Koo M, Krass I, Aslani P. Enhancing patient education about medicines: factors influencing reading and seeking of written medicine information. Health Expect. 2006;9(2):174–87. CrossRef
8. Mateti UV, Nagappa AN, Attur RP, Bairy M, Nagaraju SP, Vilakkathala R, et al. Preparation, validation and user-testing of pictogram-based patient information leaflets for hemodialysis patients. Saudi Pharm J. 2015;23(6):621–5. CrossRef
9. Austin PE, Matlack R, Dunn KA, Kesler C, Brown CK. Discharge instructions: do illustrations help our patients understand them?. Ann Emerg Med. 1995;25(3):317–20. CrossRef
10. O’Reilly M, Mohamed K, Foy D, Sheehan E. Educational impact of joint replacement school for patients undergoing total hip and knee arthroplasty: a prospective cohort study. Int Orthop. 2018;42:2745–54. CrossRef
11. Ventola CL. Social media and health care professionals: benefits, risks, and best practices. Pharm Ther. 2014;39(7):491.
12. Hammar T, Nilsson AL, Hovstadius B. Patients’ views on electronic patient information leaflets. Pharm Pract (Granada). 2016;14(2):702. CrossRef
13. Karuniawati H, Putra ON, Wikantyasning ER. Impact of pharmacist counseling and leaflet on the adherence of pulmonary tuberculosis patients in lungs hospital in Indonesia. Indian J Tuberc. 2019;66(3):364–9. CrossRef
14. Rajpurohit S, Musunuri B, Mohan PB, Vani LR, Bhat G, Shetty S. Development and evaluation of patient information leaflet for liver cirrhosis patients. Clin Epidemiol Glob Health. 2023;24:101436. CrossRef
15. Mansoor LE, Dowse R. Effect of pictograms on readability of patient information materials. Ann Pharmacother. 2003;37(7-8):1003–9. CrossRef
16. Kimmel SE, Chen Z, Price M, Parker CS, Metlay JP, Christie JD, et al. The influence of patient adherence on anticoagulation control with warfarin: results from the International Normalized Ratio Adherence and Genetics (IN-RANGE) Study. Arch Intern Med. 2007;167:229–35. CrossRef
17. Flesch RF. How to Write Plain English: A Book for Lawyers, Consumers. 1st ed. New York, NY: Barnes and Noble; 1981.
18. Baker S. Who can read consumer product information? Aust J Hosp Pharm.1997;27:126–31. CrossRef
19. Yaghmaei F. Content validity and its estimation. J Med Educ 2003;(3):25-–7. CrossRef
20. Lynn MR. Determination and quantification of content validity. Nurs Res. 1986;35(6):381–5.
21. Kuder GF, Richardson MW. The theory of the estimation of test reliability. Psychometrika. 1937;2(3):151–60. CrossRef
22. Griffin J, McKenna K, Tooth L. Written health education materials: making them more effective. Aust Occup Ther J. 2003;50(3):170–7. CrossRef
23. Demir F, Ozsaker E, Ilce AO. The quality and suitability of written educational materials for patients. J Clin Nurs. 2008;17(2):259–65. Doi: 10.1111/j.1365-2702.2007.02044.x
24. Vooradi S, Acharya LD, Seshadri S, Thunga G, Vijayanarayana K. Preparation, validation and user-testing of patient information leaflets on diabetes and hypertension. Indian J Pharm Sci. 2018;80(1):118–25. Doi: 10.4172/pharmaceutical-sciences.1000336
25. Adepu R, Swamy MK. Development and evaluation of patient information leaflets (PIL) usefulness. Indian J Pharm Sci. 2012;74:174–8. Doi: 10.4103/0250-474X.103857.
26. Sebastian J, Parthasarathi G, Ravi MD. Development of knowledge, attitude and practice questionnaire of parents towards vaccination: process, challenges and solutions. Indian J Pharm Pract. 2017;10(2):115–20.
27. Sirimalla S, Mateti UV, Shenoy P, Shetty S. Health education for chronic kidney disease patients not on dialysis through the pictorial patient information leaflet. Pharm Technol Int. 2023;39(6):274–80. CrossRef
28. John JA, Mateti UV, Rajesh V. Impact of pharmacist education on knowledge, attitude, and practice of patients using inhaler medications in obstructive airway diseases. J Datta Meghe Inst Med Sci Univ. 2020;15(1):118–22. CrossRef
29. Briggs AL, Jackson TR, Bruce S, Shapiro NL. The development and performance validation of a tool to assess patient anticoagulation knowledge. Res Soc Adm Pharm. 2005;1(1):40–59. CrossRef
30. Chan SH, Sin PS, Lee MK, Fong WC, Cheung CY, Lee CP, et al. Development and validation of the Chinese oral anticoagulants knowledge tool (C-OAKT): a pilot study. PEC Innov. 2023;3:100210. CrossRef
31. Magon A, Arrigoni C, Roveda T, Grimoldi P, Dellafiore F, Moia M, et al. Anticoagulation knowledge tool (AKT): further evidence of validity in the Italian population. PLoS One. 2018;13(8):0201476. CrossRef