INTRODUCTION
Fixed-dose combinations (FDCs), also known as polypills, are defined as a combination of two or more active ingredients within a single form of pharmaceutical administration (i.e., dosage form) [1–6]. By simplifying medication administration they have been shown to improve treatment adherence, which is particularly important in patients with chronic diseases [7]. Patients with chronic diseases such as hypertension and diabetes often require multiple drugs to treat their conditions. Complicated drug regimens may pose accessibility and affordability challenges for patients, while the burden of taking multiple medicines daily may affect patient adherence and clinical outcomes. FDCs have the potential to reduce these difficulties.
In several countries, including Vietnam, FDCs are considered a new medicine, even though the single ingredients are quite familiar and covered by health insurance. The cost of FDCs is often more expensive than a single-ingredient drug, but less expensive than the sum cost of its constituent active ingredients when purchased separately when all drugs are either branded or generic. When the FDC is branded and the constituent single-ingredient drugs can be purchased separately as generics, the FDC is typically more expensive. Given the controversies surrounding the use of FDCs, there is an urgent need to discuss both their advantages and disadvantages including their cost effectiveness. This is particularly important in low- and middle-income countries (LMICs) given their high prevalence of both infectious diseases and noncommunicable diseases (NCDs), their considerably limited resources, and the continued growth in both morbidity and mortality from NCDs [8].
Economic evaluations can inform decision-making regarding health resource allocation. In the pharmaceutical sector, cost-effectiveness analyses are considered when establishing drug coverage policies, pricing, and rebate negotiations. Some studies have found that FDCs may be cost-effective [9–12]. However, there are many variations between studies regarding the conditions studied, methodology, applied assumptions, and the comparators of choice. These might limit the generalizability and transferability of findings into other contexts. To the best of our knowledge, there has not been any specific guidance related to conducting economic evaluation of FDCs. This study aimed to review and assess the quality of published evidence on the cost-effectiveness of FDCs and summarize key methodologic assumptions. We also aim to provide recommendations for future economic evaluations of FDCs.
METHODS
This review was conducted in accordance with the proposal registered in PROSPERO (CRD42021295388). This review was also conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13].
Location of studies
The literature search was conducted in MEDLINE (using PubMed), EMBASE, Web of Science, Health Technology Assessment database of INAHTA. The initial search was carried out in December 2021 and an updated search was done in October 2023. The search terms were combinations of the following terms and synonyms: “fixed dose combination,” “FDC,” “economic evaluation,” “cost-effectiveness analysis,” and “cost-utility analysis.” The detailed search terms and search strategy for each database are described in Supplementary Material S1. Reference lists of included studies were explored. We also contacted experts in this field for potentially relevant studies.
Selection of studies
Two authors (T.L.P and T.T.T.P) independently selected studies. Separate authors (D.T.O and H.N.T) mediated all disagreements following discussion. Economic evaluation studies published in English were eligible if the intervention included FDCs of at least two active drugs and the comparators were either: 1) a regimen of two or more single active drug forms that together comprise the FDC, 2) a single active drug, or 3) a placebo. The main outcomes of interest included incremental cost-effectiveness ratio (ICER), cost per quality-adjusted life year (QALY), cost per disability-adjusted life year (DALY), and cost per life-year gained. Reviews, systematic reviews, meta-analyses; pilot studies, case reports/case series, open letters, editorials, commentaries, letters to the editor, research protocols, notes, book chapters, and conference abstracts not published in peer-reviewed journals were excluded. Research not published in the full text was also excluded.
Data abstraction
Two authors (T.L.P and N.T.N.N) independently abstracted data including author, year of publication, country, study design, the objective of the study, model characteristics (i.e., model type, model structure, and simulation technique; model assumptions, data sources for parameters, modeled complications/events, outputs from the model, results from sensitivity analyses, perspective, time horizon), name of FDCs and constituent ingredients, indications, comparators, types of costs included, total costs, year of costing, outcomes, and discount rate. Data abstraction was performed using a pre-designed data extraction form. Disagreements of data extraction between the two authors were resolved by discussion with the third-party authors (K.N.C.D, H.T.N).
Quality assessment
Two authors (D.T.O and K.N.C.D) independently performed the quality assessment using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS 2022) statement checklist [14]. The CHEERS 2022 checklist specifies 28 items for assessing the quality of reporting economic evaluations. Each item was scored with 1 (fully completed), 0.5 (partially completed), or 0 points (not completed or not reported) based on the criteria. A percentage was calculated to compare scores between studies. The denominator was calculated by summing the number of applicable items per study, and the numerator was calculated by summing the scores. Studies were deemed to be of high (>75%), moderate (50%–75%), or low (<50%) reporting quality. After grading the studies, both authors (D.T.O and K.N.C.D) shared their results, and the final CHEERS grade was obtained as an average of both evaluations.
RESULTS
Selection of studies
A total of 1,563 records were identified from database searches and 28 articles were retrieved from other sources. 232 studies were removed as duplicated studies, and 1,359 studies were screened with titles and abstracts. 97 out of 1,359 studies were selected for full-text screening. Eventually, 39 studies met the eligibility criteria and were included in this systematic review. Figure 1 shows the PRISMA flowchart of study selection.
Characteristics of the eligible studies
A detailed description of the included studies and CHEERS assessment results are presented in Table 1. The studies were published between 1996 and 2023 with most study samples recruited from Europe (n = 19), America (n = 10), Asia (n = 5), Australia (n = 2), and Africa (n = 1). There are two studies conducted on a large scale which involved 20 countries [15] and five countries [16].
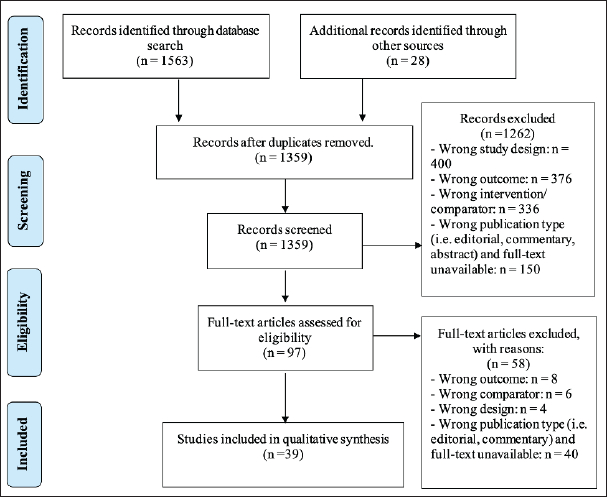 | Figure 1. PRISMA flowchart of study selection. [Click here to view] |
Most of the studies (n = 34) were of FDCs indicated for the treatment of chronic diseases. Of these, 11 studies investigated the cost-effectiveness of FDCs in chronic obstructive pulmonary disease (COPD) [9,10,17–26], one study in metabolic syndrome [27], two studies in type-2 diabetes [15,28], 21 studies in cardiovascular diseases including hypertension and heart failure [11,12,16,26,29–45], one study in rheumatoid arthritis [46], one study in benign prostatic hyperplasia [47], one study in cancer [48], and one study in preventing nausea and vomiting [49].
Most of the studies (n = 37) utilized cost-effectiveness analysis (CEA) and cost-utility analysis (CUA) approaches. One study by Price et al. [10] used the cost-minimization analysis (CMA) and one study used the cost-benefit analysis (CBA) [38]. The comparators of included studies can be categorized into three groups: (1) regimens of multiple separate components that belong to FDCs (n = 6), (2) mono-components that belong to FDCs with or without other drugs (n = 13), and (3) no treatment/placebo/usual care (n = 17). In studies comparing FDCs with usual care, seven studies specified that usual care involved separate medications, which were components of FDCs [16,32,38,41–43,49], and three studies indicated that usual care entailed either no treatment or the absence of FDC [33,34,40]. There are three studies [26,27,36] with two sets of comparators: the first comparator is a mono-component that belongs to FDCs and the second comparator is a regimen of multiple separate components that belong to FDCs.
Regarding the discount rate, 31 out of 39 studies were discounted for cost and effectiveness. Five studies analyzed the costs and outcomes within a short time horizon and did not apply discount rates [28,46]. The discount rate is generally identical between cost and outcomes, except for two studies by Van Boven et al. [19] and van Gils et al. [32], where the discount rates for costs and outcomes were 4% and 1.5%, respectively.
The majority of included studies (n = 19) were funded by the pharmaceutical industry. Other studies (n = 11) were funded by nonindustry sources such as a foundation, university, or research institution; two studies reported that they received funding from both industry and nonindustry sources [15,27]. Three studies reported that they received no funding [28,32,47] and three studies did not report the funding source [29,35,38].
In general, the methodological quality of included economic evaluations was classified as high (16 studies), although it varied across studies (Table 1). None of the studies fulfilled all 28 criteria. The maximum score was 98.2% [9,16,41], and the minimum score was 71.4% [28,49]. Two studies were graded as moderate quality [28,49]. These two studies did not report properly for 9 items of the CHEERS 2022 checklist. Three items in the CHEERS 2022 checklist have the lowest score (1) effect of engagement with patients and others affected by the study (item No.25), (2) characterizing heterogeneity (item No.18), and (3) characterizing distributional effects (item No.19). Details of the quality assessment results were shown in Supplementary Material S2.
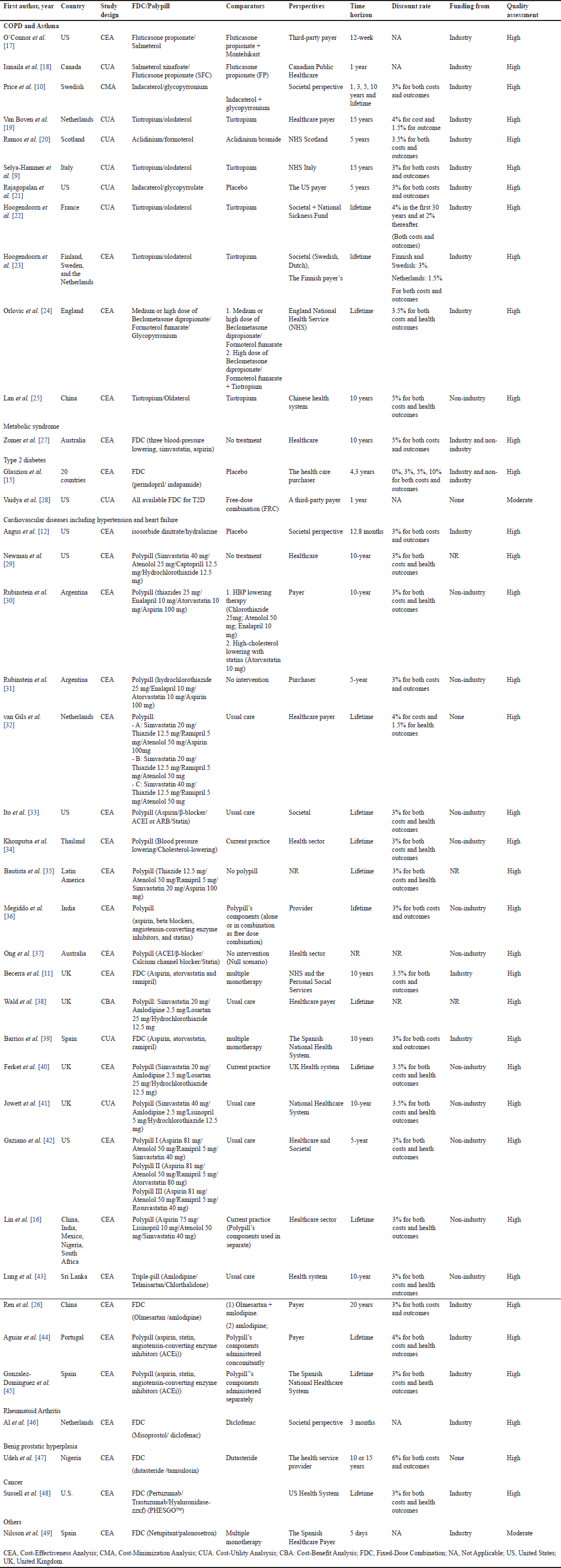 | Table 1. General characteristics of included studies. [Click here to view] |
Methodological characteristics of included studies
The methodological characteristics of the included studies are presented in Table 2. The majority of studies were model-based economic evaluations in which the Markov model was most commonly applied. A few studies were economic evaluations alongside a clinical trial [12,15,17] or based on real-world data [28]. Regarding the costs, most studies used direct medical costs from the payer’s perspective. Some studies [10,22,23] applied a societal perspective that required the include both direct costs and indirect costs. Ito et al. [33] also claimed that their study applied the societal perspective, but only the direct medical and direct nonmedical costs were included without involving the indirect cost. The sources of cost data were mainly from literature and local documents such as the regulated price of drugs and medical services, clinical guidelines, and local databases. Out of the majority of studies (n = 36), they explicitly specified the year of costing and adjusted for inflation, except for three studies [28,32,47].
The majority of studies used QALYs and life years (LYs) as the effectiveness outcomes. Clinical outcomes were also reported in some studies such as exacerbation rate in COPD [9,19,22,23], cardiovascular events prevented [11,39], or symptomatic ulcers [46]. The data sources for outcomes were mainly from literature and well-known clinical trials in the study’s condition. For example, many studies [19,20,22,23] referred to the UPLIFT study as the source for outcome data in the COPD indication of FDCs. For studies that used QALY as the outcome, the EQ-5D was mentioned as the most popular tool to elicit the utility of patients [50].
The results of economic evaluations are dependent on their assumptions. The main assumptions in the included studies can be categorized into three groups: (1) assumptions relating to costs, (2) assumptions relating to drug efficacy, and (3) assumptions relating to treatment adherence. Due to the unavailability of cost or price information for FDCs in certain countries at the time of the studies, authors had to make assumptions regarding FDC prices based on available components or other FDCs. In their studies, Megiddo et al. [36] assumed that the costs of FDCs were less than the additive costs of every single drug in the free combination, and Zomer et al. [27] assumed that FDC’s price was 25% less than the additive price of each drug in the free combination [27] and Bautista et al. [35] estimated the average cost of the FDC was $50 per subject per year. Selya-Hammer et al. [9] investigated the cost-effectiveness of a new FDC tiotropium/olodaterol (Respimat ®) in COPD treatment. As this FDC was not marketed in Italy at the time of the study, the authors assumed that the price of the FDC was a parity price to other LAMA/LABA FDCs [9]. Jowett et al. [41] applied the cost of Trinomia1® for the cost of FDC used in their model, as the specific cost of the FDC used was not available in the UK at that time. Notably, Trinomia1® had different compositions compared to the FDC used in their study [41]. For the drug’s efficacy, the assumption of equal efficacy between FDCs and the free combination was applied in two studies by Price et al. [10], Ito et al. [33], and Lin et al. [16]. Khonputsa et al. [34] assumed that the efficacy of three drugs in the FDC (in half standard dose) were 20% lower than those in standard doses and the FDC’s effect was equal to the multiplication of the individual components’ effects.
While the adherence rate is the main advantage of FDCs, it was poorly reported among many of the included studies. Some studies reported different assumptions regarding the adherence rate. Price et al. (2014) assumed that the adherence rate was similar between FDCs and comparators [10]. Six studies assumed the adherence rate was 100% among those treated with FDC [18,19,21,29,38,46]. Specifically, three studies indicated equal adherence rates between the FDC and the comparator group [18,19,21], while the remaining three studies did not provide information on adherence rates in the comparator group. Some studies cited data on the adherence rates which were different between FDCs and comparators. Barrios et al. [39] assumed based on prior research that 76% of patients treated with the FDC were adherent while only 49% of those adhered to regimens with the separate monocomponents. Becerra et al. [11] assumed an adherence rate of 86% in FDCs and 65% in regimens of separate monocomponents, based on results from the UMPIRE study. Ren et al. [26] assumed an adherence rate of 56.55% in FDC and 50.83% for a regimen of separate monocomponents based on prior research. Notably, two out of three studies which are trial-based economic evaluations reported a lower adherence rate for the FDC groups compared with placebo [12,15]. Angus et al. [12] reported that the adherence rate was 84.6% in FDCs and 85.2% in placebo based on the A-HeFT study. Glasziou et al. [15] used adherence rates of 73% in FDC and 74% in placebo. Other studies also reported that the adherence in the FDC group was 83% in Wald’s study [38], 84% in Jowett’s [41], and 85% in the first year in van Gils’s study [32]. Gaziano et al. [42] reported that adherence was decreasing over time both in the FDC and usual care groups (from 81.9% to 37.8% in the FDC group and from 65% to 30% in usual care) [42]. Lin et al. [16] estimated an adherence rate based on a prior study of only 41%–55% in the compared group and 58% in the FDC group [16].
Cost-effectiveness results of fixed-dose combination drugs
The cost-effectiveness results of fixed-dose combination drugs are presented in Table 3. In these 39 studies, six studies did not report the cost of FDCs and/or comparators [18,31,32,36–38]. The overall cost of using FDCs was lower when the comparison group was a free combination of individual single substances as components in FDCs [10,26,28,30,48] (lower because the price of FDCs is lower than when taking combinations of individual drugs) and higher when the comparison group is a single substance (part of the FDCs) or placebo [9,11,15,19,21,23,25–27,35,45–47]. Two studies [12,39] reported total costs of treatment with FDC lower than comparators when the comparator is a single drug, placebo, or no treatment and one study reported cost of FDC was higher than usual care [33,40,43].
FDCs improved treatment efficacy compared to comparators which resulted in higher QALYs, and LYs, the incidence and number of COPD exacerbations decreased, the number of ulcers and deaths decreased, and adverse events [9,11,15,19,21–23,26,28,36,39,46,47]. The study by Angus et al. [12] showed that FDCs improved treatment efficacy but adherence rates when using FDCs were lower than placebo, namely 84.6% versus 85.2%. FDCs could significantly increase the DALY adverted compared to current practice for primary prevention of cardiovascular disease in different classified risk populations [30,34]. In addition, 3 studies did not explicitly mention the total number of results on clinical efficacy between the 2 treatment or comparator groups [10,18,20,27,31,32,37,38]. FDC was cost-saving in four studies [10,17,28,31], suggesting that better outcomes can be obtained at a cheaper cost, four studies [25,27,33,40] showed that FDC was not cost-effective. Regarding FDCs for cardiovascular diseases including hypertension and heart failure, Wald et al. [38] reported FDCs will be cost-effective if the cost of the FDC program were £1 per person per day and Jowett suggested when FDC’s price is lower than £150, it would be cost-effective for all population [41]. All 25 remaining studies showed that FDC was cost effective.
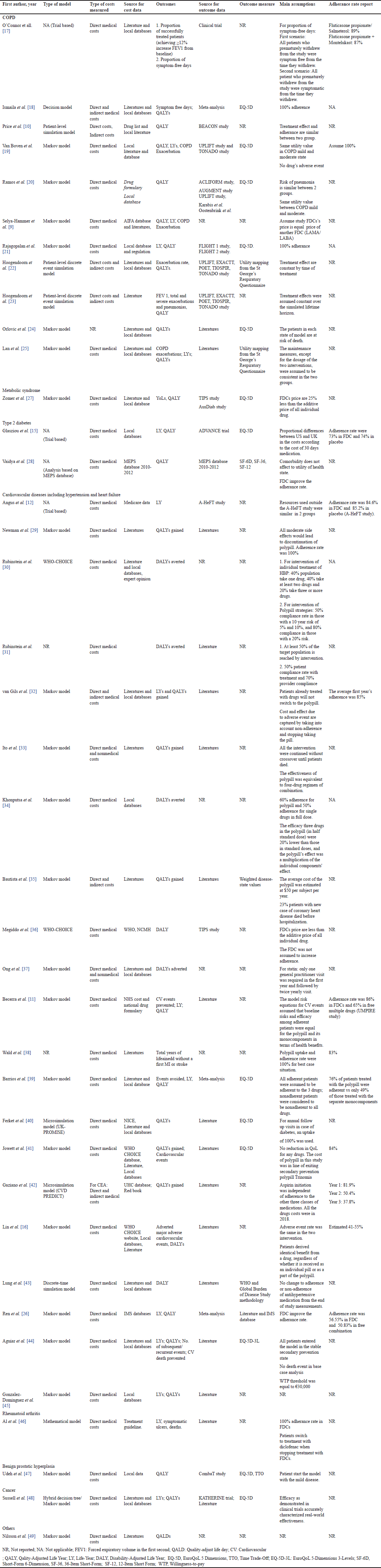 | Table 2. Methodological characteristics of included studies. [Click here to view] |
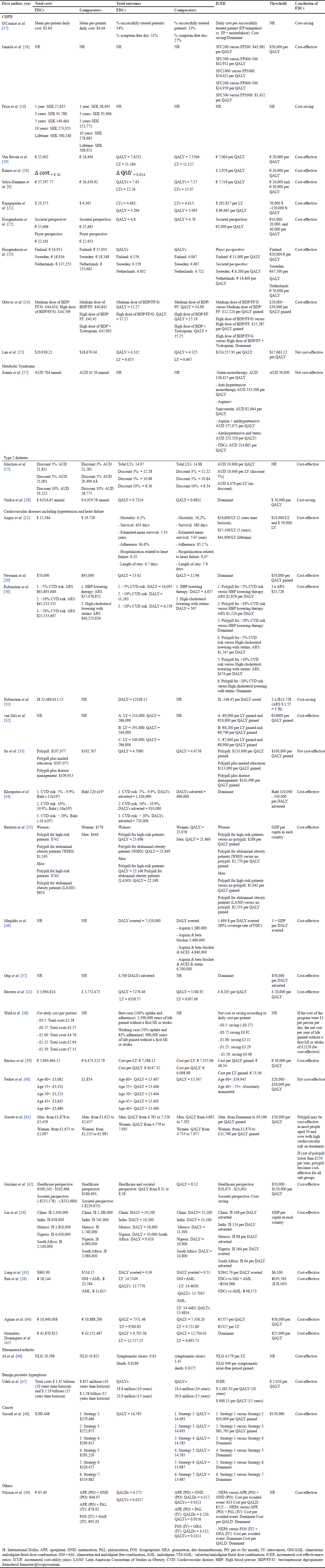 | Table 3. The cost-effectiveness results of fixed-dose combination drugs. [Click here to view] |
DISCUSSION
This systematic review aimed to review the methods and summarize key assumptions of previous economic evaluations of FDC drugs. A total of 39 studies across different health topics were included in the review. Economic evaluations of FDCs were relatively more common for the treatment of NCDs such as COPD or cardiovascular diseases. Two studies [28,49] were assessed to have moderate quality, while the remaining studies had high quality.
Most studies show that the FDC was cost-effective except for four studies [25,27,33,40]. All four studies concluded that FDCs could achieve cost-effectiveness with lower prices. Lan et al. [25] reported that both FDC and comparator prices were based on average bidding prices, with the FDC’s price higher than other LABAs/LAMAs. Ito et al. used the price of the most expensive brand-name drug as the FDC’s price, while comparator prices were sourced from generic drugs that had gone off-patent [33]. Ferket et al. [40] did not explicitly state prices, providing only annual costs, and notably, the FDC’s annual cost (£382.64) was considerably higher than individual components. In Zomer et al. [27] study, they assumed the FDC cost was 25% less than the combined costs of its components. However, they expressed their concern about the WTP threshold in Australia, which was below the WHO’s recommended threshold (A$ 92,123). However, even with an increased WTP of A$ 92,123, the FDC remained noncost effective, with an ICER of A$ 214,865 compared to no treatment [27]. Even though most studies suggest FDCs are cost-effective, there was substantial variation among the studies regarding methodological choices and assumptions applied.
Among included studies, the FDCs could be compared in one or more of three groups: (1) regimen of multiple separate components that belong to FDCs, (2) mono-component that belongs to FDCs with or without other drugs, and (3) no treatment/placebo/usual care. Only five studies explicitly indicated the selection of comparators in accordance with current treatment guidelines [18,20,40,41,49]. The selection of a comparator could be based on treatment guidelines such as the first-line treatment, second-line treatment, or combination therapy such as mono-therapy, dual- and triple therapy in some diseases such as diabetes. However, the rationale for comparator selection was not well reported in most of the included studies (n = 34). For best practices, the comparators for FDCs should consist of regimens with multiple separate components, similar to those included in the FDC as FDCs have demonstrated their ability to enhance adherence rates compared to multiple separate components [51,52].
For studies that compared FDCs to placebo/usual care or FDCs versus single component drugs, the efficacy of drugs could be predictable, i.e., FDC had higher efficacy while reducing adverse events [9,15,21,22]. The costs of FDCs were generally higher when compared to a placebo or single-component drug [9,11,15,19,21,23,25–27,35,45–47].
In their study, Khonputsa et al. [34] utilized an FDC comprising four blood pressure-lowering drugs, each administered at half the standard dose. They presumed that the efficacy of individual components at this reduced dosage in the FDC was 20% lower than that at the standard doses [34]. This assumption was derived from an analysis of 354 clinical trials that assessed the effectiveness of blood pressure-lowering drugs at half the standard dose compared to standard doses [53]. Another clinical trial consistently supported these findings when comparing two types of FDCs—one in the standard dose and the other in the half-standard dose. The results indicated that the standard dose FDC exhibited comparable tolerance but delivered a 25%–30% higher efficacy compared to the half-standard dose FDC [54]. For FDC’s effect, Khonputsa et al. [34] assumed that it was equal to the multiplication of the individual components’ effects. This assumption was different from other approaches in the included studies and may result in an overestimation of the effects of FDCs compared to multiple separate components.
For economic evaluations that compared FDCs to regimens of separate components of the FDC, there were three main groups of assumptions applied. The first assumption related to the costs of FDC as compared to the comparator while the second assumption was applied to the efficacy of FDCs, and the third assumption was about treatment adherence.
Some studies have assumed the costs of FDC were lower than the corresponding costs of comparators [9,27,35,36,41]. The costs of FDC were assumed to be generally lower [36] or 25% lower [27] than the additive cost of each single drug in the free combination. This assumption was made due to the unavailability of FDC’s price in the countries where studies were conducted [9,41]. While the cost of FDC might be lower than the summation costs of multiple monotherapies in some situations, this depends on factors such as generic availability, pricing, and negotiation policies in a given country. In their study, Hong et al. [55] showed that the monthly cost of FDCs for antihypertensive drugs in the U.S. was higher than that of the separate components when generic FDCs were not available. In other words, the assumption of the cost advantage of FDCs over separate components might not be true when the separate components are generically available, particularly when the FDC is branded. Furthermore, a US-based study reported that FDC antihypertensive drugs had higher out-of-pocket costs than did the sum of their components [56] but the total costs were lower for FDC drugs. This reflects an opportunity for a better value-based insurance design that reduces out-of-pocket costs for patients for higher value therapies. This also suggested that choosing analysis perspectives other than the third-party payer perspective such as the societal perspective would affect the conclusion of the cost-effectiveness of FDC.
Future guidance should be issued regarding the cost of intervention when the intervention is not marketed yet. This need is also applicable to the guidance of early health technology assessment which is conducted at earlier stages of the development of healthcare technology [57]. Guidance is crucial for successful health technology development and an efficient research and development system. This ensures innovations meet market demand, remain accessible to the target population, and contribute significantly to improving overall population health [58].
For the drug’s efficacy, the assumption of equal efficacy between FDCs and the free combination was applied in a study by Price et al. [10], Ito et al. [33], and Lin et al. [16]. The adherence improvement of FDCs when compared to several monotherapies was highlighted in previous meta-analyses [59,60]. This is an important factor that contributes to the overall effectiveness of medical treatment, especially in chronic diseases. However, among the included studies, the adherence rate was not well reported and different assumptions were made about the relative adherence of FDCs versus alternatives. Most studies (n = 22) did not report the adherence rate. In model-based economic evaluations where adherence rate parameters were derived, the adherence rates were higher in FDCs as compared to free combination drugs [11,26,39]. Meanwhile, two trial-based economic evaluations [12,15] reported a lower adherence rate in the FDCs group compared to that of the placebo. Failure to consider adherence rate in economic evaluations of FDCs could result in the underestimation of the cost-effectiveness of FDCs. Furthermore, for some conditions such as asthma, ignoring adherence advantages with FDCs could lead to inaccurate conclusions regarding whether FDCs meet cost-effectiveness thresholds, with consequences for coverage and access. A systematic review by Chongmelaxme et al. [61] showed that few economic evaluations of asthma incorporated adherence in the analysis. Chongmelaxme et al. [61] also identified one method of incorporating adherence, which involved adjusting treatment effectiveness based on adherence levels. Moreover, further economic evaluations based on long-term clinical trials with larger populations are necessary. Future guidance is necessary to establish best practices on how to incorporate adherence into the economic evaluation of health technology, especially model-based economic evaluations.
According to the findings from the included studies, FDCs were deemed cost-saving [10,17,28,31] and cost-effective when compared to their comparators. These results provide substantial support for the integration and utilization of FDCs in clinical practice. Recent studies have demonstrated a high prevalence of FDC prescriptions in both primary and secondary healthcare settings, underscoring the proven efficiency of FDCs [62–64]. Improved adherence to treatment is a critical factor contributing to treatment effectiveness. Simplifying medication regimens by reducing the number of pills can enhance both uptake and adherence rates, particularly in chronic diseases that require lifelong medications. Nevertheless, the utilization of FDCs warrants careful consideration, especially in light of the observed high prevalence of irrational prescribing associated with FDCs [62,64,65]. This highlights the importance of involving pharmacists, who possess the most comprehensive knowledge of available dosage forms, to potentially enhance the prevalence of FDCs while mitigating the risk of irrational prescribing of FDCs.
Our study has limitations to consider. First, heterogeneity between studies including across clinical conditions and methodology made the pooling of data implausible. Second, publications in languages other than English or without full text were not included in this review.
CONCLUSION
In prior studies, FDCs were sometimes found to be cost-effective compared to regimens of separate components of the FDC. Whether an FDC was deemed cost-effective depended on the characteristics of the disease state, drugs under study, study design choices, and assumptions made in the economic evaluation. Variations among previous studies regarding methodological patterns and assumptions highlight an opportunity for guidance to promote the harmonization of methods. Future economic evaluations should comprehensively capture and report the costs and effectiveness of FDCs and justify the choice of comparators. In particular, the advantages of FDCs for enhancing adherence should be captured appropriately in future studies.
LIST OF ABBREVIATIONS
CBA, Cost-benefit analysis; CEA, Cost-effectiveness analysis; CHEERS, Consolidated Health Economic Evaluation Reporting Standards; CMA, Cost-minimization analysis; COPD, Chronic obstructive pulmonary disease; CUA, Cost-utility analysis; DALY, Disability-adjusted life year; FDCs, Fixed-dose drug combinations; INAHTA, International Network of Agencies for Health Technology Assessment; ICER, Incremental cost-effectiveness ratio; LY, Life-year; NCDs, Non-communicable diseases; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; QALY, Quality-adjusted life year.
AUTHOR CONTRIBUTION
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The publication fees of this study were funded by the Fogarty International Center of the U.S. National Institutes of Health [D43 TW011394-01]. Dr. TLP received funding for the post-doctoral fellow, supported by the Fogarty International Center of the U.S. National Institutes of Health [D43 TW011394-01].
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Gautam CS, Saha L. Fixed dose drug combinations (FDCs): rational or irrational: a view point. Br J Clin Pharmacol. 2008;65(5):795–6. CrossRef
2. Sreedhar D, Subramanian G, Udupa N. Combination drugs: are they rational. Curr Sci. 2006;91:406.
3. European Medicines Agency. Guideline on Clinical Development of Fixed Combination Medicinal Products. Amsterdam, The Netherlands: European Medicines Agency; 2017.
4. Auwal F, Dahiru MN, Abdu-Aguye SN. Availability and rationality of fixed dose combinations available in Kaduna, Nigeria. Pharm Pract (Granada). 2019;17(2):1470. CrossRef
5. Sawicki-Wrzask D, Thomsen M, Bjerrum OJ. An analysis of the fixed-dose combinations authorized by the European Union, 2009-2014: a focus on benefit-risk and clinical development conditions. Ther Innov Regul Sci. 2015;49(4):553–9. CrossRef
6. Duconge J, Ruaño G. Fixed-dose combination products and unintended drug interactions: urgent need for pharmacogenetic evaluation. Pharmacogenomics. 2015;16(15):1685–8. CrossRef
7. Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120(8):713–9. CrossRef
8. Godman B, McCabe H, D Leong T, Mueller D, Martin AP, Hoxha I, et al. Fixed dose drug combinations—are they pharmacoeconomically sound? findings and implications especially for lower- and middle-income countries. Expert Rev Pharmacoecon Outcomes Res. 2020;20(1):1–26. CrossRef
9. Selya-Hammer C, Gonzalez-Rojas Guix N, Baldwin M, Ternouth A, Miravitlles M, Rutten-Van Mölken M, et al. Development of an enhanced health-economic model and cost-effectiveness analysis of tiotropium + olodaterol Respimat® fixed-dose combination for chronic obstructive pulmonary disease patients in Italy. Ther Adv Respir Dis. 2016;10(5):391–401. CrossRef
10. Price D, Keininger D, Costa-Scharplatz M, Mezzi K, Dimova M, Asukai Y, et al. Cost-effectiveness of the LABA/LAMA dual bronchodilator indacaterol/glycopyrronium in a Swedish healthcare setting. Respir Med. 2014;108(12):1786–93. CrossRef
11. Becerra V, Gracia A, Desai K, Abogunrin S, Brand S, Chapman R, et al. Cost-effectiveness and public health benefit of secondary cardiovascular disease prevention from improved adherence using a polypill in the UK. BMJ Open. 2015;5(5):e007111. CrossRef
12. Angus DC, Linde-Zwirble WT, Tam SW, Ghali JK, Sabolinski ML, Villagra VG, et al. Cost-effectiveness of fixed-dose combination of isosorbide dinitrate and hydralazine therapy for blacks with heart failure. Circulation. 2005;112(24):3745–53. CrossRef
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. CrossRef
14. Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ. 2022;376:e067975. CrossRef
15. Glasziou PP, Clarke P, Alexander J, Rajmokan M, Beller E, Woodward M, et al. Cost-effectiveness of lowering blood pressure with a fixed combination of perindopril and indapamide in type 2 diabetes mellitus: an advance trial-based analysis. Med J Aust. 2010;193(6):320–4. CrossRef
16. Lin JK, Moran AE, Bibbins-Domingo K, Falase B, Pedroza Tobias A, Mandke CN, et al. Cost-effectiveness of a fixed-dose combination pill for secondary prevention of cardiovascular disease in China, India, Mexico, Nigeria, and South Africa: a modelling study. Lancet Glob Health. 2019;7(10):e1346–e58. CrossRef
17. O’Connor RD, Nelson H, Borker R, Emmett A, Jhingran P, Rickard K, et al. Cost effectiveness of fluticasone propionate plus salmeterol versus fluticasone propionate plus montelukast in the treatment of persistent asthma. Pharmacoeconomics. 2004;22(12):815–25. CrossRef
18. Ismaila AS, Risebrough N, Li C, Corriveau D, Hawkins N, FitzGerald JM, et al. Cost-effectiveness of salmeterol/fluticasone propionate combination (Advair(®)) in uncontrolled asthma in Canada. Respir Med. 2014;108(9):1292–302. CrossRef
19. Van Boven JFM, Kocks JWH, Postma MJ. Cost-effectiveness and budget impact of the fixed-dose dual bronchodilator combination tiotropium–olodaterol for patients with COPD in the Netherlands. Int J Chron Obstruct Pulmon Dis. 2016;11(1):2191–201. CrossRef
20. Ramos M, Haughney J, Henry N, Lindner L, Lamotte M. Cost versus utility of aclidinium bromide 400 µg plus formoterol fumarate dihydrate 12 µg compared to aclidinium bromide 400 µg alone in the management of moderate-to-severe COPD. Clinicoecon Outcomes Res. 2016;8:445–56. CrossRef
21. Rajagopalan K, Bloudek L, Marvel J, Dembek C, Kavati A. Cost-effectiveness of twice-daily indacaterol/glycopyrrolate inhalation powder for the treatment of moderate to severe COPD in the US. Int J Chron Obstruct Pulmon Dis. 2018;13:3867–77. CrossRef
22. Hoogendoorn M, Ramos IC, Baldwin M, Luciani L, Fabron C, Detournay B, et al. Long-term cost-effectiveness of the fixed-dose combination of tiotropium plus olodaterol based on the DYNAGITO trial results. Int J Chron Obstruct Pulmon Dis. 2019;14:447–56. CrossRef
23. Hoogendoorn M, Ramos IC, Soulard S, Cook J, Soini E, Paulsson E, et al. Cost-effectiveness of the fixed-dose combination tiotropium/olodaterol versus tiotropium monotherapy or a fixed-dose combination of long-acting β2-agonist/inhaled corticosteroid for COPD in Finland, Sweden and the Netherlands: a model-based study. BMJ Open. 2021;11(8):e049675. CrossRef
24. Orlovic M, Magni T, Lukyanov V, Guerra I, Madoni A. Cost-effectiveness of single-inhaler extrafine beclometasone dipropionate/formoterol fumarate/glycopyrronium in patients with uncontrolled asthma in England. Respir Med. 2022;201:106934. CrossRef
25. Lan Y, Yang N, Wang Y, Yang Y, Xu M, He Q. Cost-effectiveness analysis of fixed-dose tiotropium/Olodaterol versus tiotropium for COPD patients in China. Int J Chron Obstruct Pulmon Dis. 2023;18:2093–103. CrossRef
26. Ren M, Xuan D, Lu Y, Fu Y, Xuan J. Economic evaluation of olmesartan/amlodipine fixed-dose combination for hypertension treatment in China. J Med Econ. 2020;23(4):394–400. CrossRef
27. Zomer E, Owen A, Magliano DJ, Ademi Z, Reid CM, Liew D. Predicting the impact of polypill use in a metabolic syndrome population: an effectiveness and cost-effectiveness analysis. Am J Cardiovasc Drugs. 2013;13(2):121–8. CrossRef
28. Vaidya V, Anupindi VR, Pinto S, Kaun M. Cost utility analysis of fixed-dose and free-dose combinations of oral medications in type 2 diabetes patients. J Pharm Health Serv Res. 2016;7(3):181–7. CrossRef
29. Newman J, Grobman WA, Greenland P. Combination polypharmacy for cardiovascular disease prevention in men: a decision analysis and cost-effectiveness model. Prev Cardiol. 2008;11(1):36–41. CrossRef
30. Rubinstein A, García Martí S, Souto A, Ferrante D, Augustovski F. Generalized cost-effectiveness analysis of a package of interventions to reduce cardiovascular disease in Buenos Aires, Argentina. Cost Eff Resour Alloc. 2009;7:10. CrossRef
31. Rubinstein A, Colantonio L, Bardach A, Caporale J, Martí SG, Kopitowski K, et al. Estimation of the burden of cardiovascular disease attributable to modifiable risk factors and cost-effectiveness analysis of preventative interventions to reduce this burden in Argentina. BMC Public Health. 2010;10:627. CrossRef
32. van Gils PF, Over EA, Hamberg-van Reenen HH, de Wit GA, van den Berg M, Schuit AJ, et al. The polypill in the primary prevention of cardiovascular disease: cost-effectiveness in the Dutch population. BMJ Open. 2011;1(2):e000363. CrossRef
33. Ito K, Shrank WH, Avorn J, Patrick AR, Brennan TA, Antman EM, et al. Comparative cost-effectiveness of interventions to improve medication adherence after myocardial infarction. Health Serv Res. 2012;47(6):2097–117. CrossRef
34. Khonputsa P, Veerman LJ, Bertram M, Lim SS, Chaiyakunnaphruk N, Vos T. Generalized cost-effectiveness analysis of pharmaceutical interventions for primary prevention of cardiovascular disease in Thailand. Value Health Reg Issues. 2012;1(1):15–22. CrossRef
35. Bautista LE, Vera-Cala LM, Ferrante D, Herrera VM, Miranda JJ, Pichardo R, et al. A ‘polypill’ aimed at preventing cardiovascular disease could prove highly cost-effective for use in Latin America. Health Aff (Millwood). 2013;32(1):155–64. CrossRef
36. Megiddo I, Chatterjee S, Nandi A, Laxminarayan R. Cost-effectiveness of treatment and secondary prevention of acute myocardial infarction in India: a modeling study. Global Heart. 2014;9(4):391–8.e3. CrossRef
37. Ong KS, Carter R, Vos T, Kelaher M, Anderson I. Cost-effectiveness of interventions to prevent cardiovascular disease in Australia’s indigenous population. Heart Lung Circ. 2014;23(5):414–21. CrossRef
38. Wald NJ, Luteijn JM, Morris JK, Taylor D, Oppenheimer P. Cost-benefit analysis of the polypill in the primary prevention of myocardial infarction and stroke. Eur J Epidemiol. 2016;31(4):415–26. CrossRef
39. Barrios V, Kaskens L, Castellano JM, Cosin-Sales J, Ruiz JE, Zsolt I, et al. Usefulness of a cardiovascular polypill in the treatment of secondary prevention patients in Spain: a cost-effectiveness study. Revista Espanola de Cardiologia. 2017;70(1):42–9. CrossRef
40. Ferket BS, Hunink MG, Khanji M, Agarwal I, Fleischmann KE, Petersen SE. Cost-effectiveness of the polypill versus risk assessment for prevention of cardiovascular disease. Heart. 2017;103(7):483–91. CrossRef
41. Jowett S, Barton P, Roalfe A, Fletcher K, Hobbs FDR, McManus RJ, et al. Cost-effectiveness analysis of use of a polypill versus usual care or best practice for primary prevention in people at high risk of cardiovascular disease. PLoS One. 2017;12(9):e0182625. CrossRef
42. Gaziano TA, Pandya A, Sy S, Jardim TV, Ogden JM, Rodgers A, et al. Modeling the cost effectiveness and budgetary impact of Polypills for secondary prevention of cardiovascular disease in the United States. Am Heart J. 2019;214:77–87. CrossRef
43. Lung T, Jan S, de Silva HA, Guggilla R, Maulik PK, Naik N, et al. Fixed-combination, low-dose, triple-pill antihypertensive medication versus usual care in patients with mild-to-moderate hypertension in Sri Lanka: a within-trial and modelled economic evaluation of the TRIUMPH trial. Lancet Glob Health. 2019;7(10):e1359–e66. CrossRef
44. Aguiar C, Araujo F, Rubio-Mercade G, Carcedo D, Paz S, Castellano JM, et al. Cost-effectiveness of the CNIC-Polypill strategy compared with separate monocomponents in secondary prevention of cardiovascular and cerebrovascular disease in Portugal: the MERCURY Study. J Health Econ Outcomes Res. 2022;9(2):134–46. CrossRef
45. González-Domínguez A, Durán A, Hidalgo-Vega Á, Barrios V. Cost-effectiveness of the CNIC-Polypill versus separate monocomponents in cardiovascular secondary prevention in Spain. Rev Clin Esp (Barc). 2023;223(7):414–22. CrossRef
46. Al MJ, Michel BC, Rutten FFH. The cost effectiveness of diclofenac plus misoprostol compared with diclofenac monotherapy in patients with rheumatoid arthritis. PharmacoEconomics. 1996;10(2):141–51. CrossRef
47. Udeh EI, Ofoha CG, Adewole DA, Nnabugwu II. A cost effective analysis of fixed-dose combination of dutasteride and tamsulosin compared with dutasteride monotherapy for benign prostatic hyperplasia in Nigeria: a middle income perspective; using an interactive Markov model. BMC Cancer. 2016;16(1):405. CrossRef
48. Sussell JA, Roth JA, Meyer CS, Fung A, Hansen SA. Assessment of the cost-effectiveness of HER2-targeted treatment pathways in the neoadjuvant treatment of high-risk HER2-positive early-stage breast cancer. Adv Ther. 2022;39(3):1375–92. CrossRef
49. Nilsson J, Piovesana V, Turini M, Lezzi C, Eriksson J, Aapro M. Cost-effectiveness analysis of NEPA, a fixed-dose combination of netupitant and palonosetron, for the prevention of highly emetogenic chemotherapy-induced nausea and vomiting: an international perspective. Support Care Cancer. 2022;30(11):9307–15. CrossRef
50. EuroQol. EQ-5D [cited 2023 13 November]. Available from: https://euroqol.org/.
51. Memon RA, Raveena Bai B, Simran F, Kumari M, Aisha F, Sai Kiran K, et al. Effect of the Polypill on adherence and prevention of cardiovascular diseases in patients with or at high risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. Cureus. 2023;15(1):e34134. CrossRef
52. Baumgartner A, Drame K, Geutjens S, Airaksinen M. Does the Polypill improve patient adherence compared to its individual formulations? a systematic review. Pharmaceutics. 2020;12(2):190. CrossRef
53. Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326(7404):1427. CrossRef
54. Yusuf S, Pais P, Sigamani A, Xavier D, Afzal R, Gao P, et al. Comparison of risk factor reduction and tolerability of a full-dose Polypill (With Potassium) versus low-dose Polypill (Polycap) in individuals at high risk of cardiovascular diseases. CircCardiovasc Qual Outcomes. 2012;5(4):463–71. CrossRef
55. Hong SH, Wang J, Tang J. Dynamic view on affordability of fixed-dose combination antihypertensive drug therapy. Am J Hypertens. 2013;26(7):879–87. CrossRef
56. Rabbani A, Alexander GC. Out-of-pocket and total costs of fixed-dose combination antihypertensives and their components. Am J Hypertens. 2008;21(5):509–13. CrossRef
57. Ijzerman MJ, Koffijberg H, Fenwick E, Krahn M. Emerging use of early health technology assessment in medical product development: a scoping review of the literature. PharmacoEconomics. 2017;35(7):727–40. CrossRef
58. Wang Y, Rattanavipapong W, Teerawattananon Y. Using health technology assessment to set priority, inform target product profiles, and design clinical study for health innovation. Technol Forecast Soc Change. 2021;172:121000. CrossRef
59. Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010;55(2):399–407. CrossRef
60. Santo K, Kirkendall S, Laba TL, Thakkar J, Webster R, Chalmers J, et al. Interventions to improve medication adherence in coronary disease patients: a systematic review and meta-analysis of randomised controlled trials. Eur J Prev Cardiol. 2020;23(10):1065–76. CrossRef
61. Chongmelaxme B, Chaiyakunapruk N, Dilokthornsakul P. Incorporating adherence in cost-effectiveness analyses of asthma: a systematic review. J Med Econ. 2019;22(6):554–66. CrossRef
62. Pradhan S, Panda A, Sahu S, Behera JP. An evaluation of prevalence and prescribing patterns of rational and irrational fixed dose combinations (FDCs): a hospital based study. Int J Med Public Health. 2017;6:58–62. CrossRef
63. Poudel A, Mohamed Ibrahim MI, Mishra P, Palaian S. Assessment of utilization pattern of fixed dose drug combinations in primary, secondary and tertiary healthcare centers in Nepal: a cross-sectional study. BMC Pharmacol Toxicol. 2017;18:69. CrossRef
64. Balat JD, Gandhi AM, Patel PP, Dikshit RK. A study of use of fixed dose combinations in Ahmedabad, India. Indian J Pharmacol. 2014;46(5):503–9. CrossRef
65. Nigam MP, Fernandes VLG, Rataboli PV. Fixed dose combinations- to prescribe or not to prescribe: a dilemma of medical profession. Int J Basic Clin Pharmacol. 2014;3:105–13. CrossRef