INTRODUCTION
Depression is the leading cause of worldwide disability (7.5% of all years lived with disability in 2015) and a key risk factor for suicide, with about 800,000 cases per year, according to the World Health Organization [1]. Females suffer from depression at a higher rate (5.1%) than males (3.6%). In terms of health loss, the effects of these illnesses are enormous. Most of the disease burden was concentrated in low- and middle-income countries [1]. Elderly patients with major depressive disorder (MDD) have a poorer prognosis, are less responsive to treatment, and show a more significant functional decline compared to younger patients, highlighting the need for effective treatment in this population group [1].
Ketamine (or RS-ketamine) is a racemic combination of R-ketamine (or arketamine) and S-ketamine (or esketamine) in equal parts. Ketamine was first produced in 1962 as a replacement for the anesthetic medication phencyclidine [2]. Ketamine and its enantiomers have recently been highlighted as one of the most effective therapeutic options in treatment-resistant depression (TRD). In 2000, it was first discovered that an intravenous (IV) sub-anesthetic ketamine dose resulted in a rapid and sustained decrease of MDD symptoms that lasted for 72 hours [3]. The United States Food and Drug Administration finally approved intranasal esketamine in conjunction with an oral antidepressant for TRD in 2019, more than 50 years after it was approved as an IV anesthetic [4]. Unlike typical antidepressants, ketamine has a quick (within 2 hours) and long-lasting (up to 7 days) antidepressant effect, as well as having considerable antisuicidal benefits [5].
As interest in ketamine increases and more research is conducted on the topic, a few bibliometric studies related to ketamine research were published recently [6,7]. These studies demonstrated the accumulative increase in ketamine research from year to year up until the year 2020, and that the antidepressant effect of ketamine has been a research hotspot for the last 20 years [6,7]. Thus, we present a bibliometric analysis of the literature on ketamine and depression, examining the development of themes and publication trends in this field. Our analysis sheds light on the current state of research on ketamine and depression that may inform future research efforts and clinical practice.
MATERIALS AND METHODS
Search strategy
On March 21, 2023, the literature was searched and retrieved from the Scopus database. In the article’s title, the following search query was used: *ketamine” AND “depress*. We included original and review articles that study ketamine for depression (Fig. 1). All retrieved documents were downloaded in the CSV and RIS format for further analysis.
Data analysis
Publish or Perish by Harzing software was used to conduct citation analysis on data downloaded in RIS format [8] while the author’s keywords were mapped using VOSviewer (1.6.17) software on data downloaded in CSV format [9]. The VOSviewer is an established instrument for generating keyword co-occurrence maps [10]. This strategy is especially valuable for this study because it permits the identification of significant keywords and major clusters in ketamine and depression research. Thesaurus file was used to clean the data by combining multiple variants of phrases (e.g., (s)-ketamine, s-ketamine, esketamine, biomarker, and biomarkers) to improve VOSviewer analysis precision [9].
RESULTS
The trend in publication and citations
There were 994 documents retrieved, consisting of original articles (829, 83.4%) and review articles (165, 16.6%). Other documents such as letters, notes, editorials, brief surveys, books, book chapters, commentaries, and conference papers were excluded. Between 1971 and 2011, the document number consisted of a single digit except for the year 2010. From 2012 to 2019, the number of documents reached two digits, and from 2019 onward, it reached three digits. The number of citations peaked in the years 2000, 2013, and 2018 (Fig. 2).
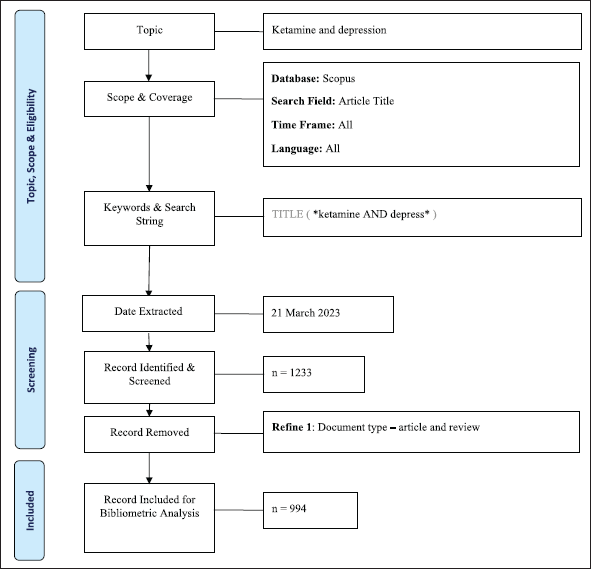 | Figure 1. Search strategy. [Click here to view] |
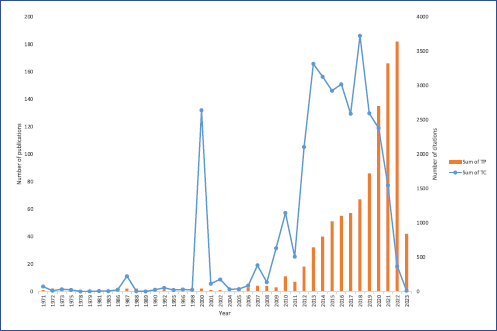 | Figure 2. Trends in the number of publications and citations from 1971 to 2023. [Click here to view] |
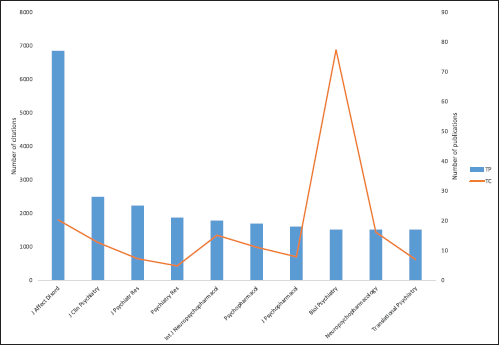 | Figure 3. Distribution of topmost productive journals on ketamine and depression research. [Click here to view] |
Top 10 most productive journals on ketamine and depression research
The distribution of the top 10 journals publishing studies related to ketamine and depression research is shown in Figure 3. In terms of the number of articles published, the journal of affective disorder ranked first, followed by frontier in psychiatry, journal of clinical psychiatry, and international journal of neuropsychopharmacology. In terms of the number of citations, however, biology of psychiatry ranked first, followed by journal of affective disorder, neuropsychopharmacology, international journal of neuropsychopharmacology, and journal of clinical psychiatry.
Visualization analysis of dynamic changes in co-occurrence of author’s keywords
Visualization co-occurrence keyword analysis is an effective instrument that provides insight into the most prevalent topics in publications in a particular research field and how their frequency changes over time [11]. The size of nodes in Figure 4 indicates their occurrence frequency, while the lines connecting the nodes indicate their co-occurrence in the same publication. The greater the co-occurrence of two keywords, the shorter the distance between two nodes. According to the results, there are 77 out of the 1255 author’s keywords satisfy the minimum threshold of five occurrences (Fig. 4a). These keywords can be divided into four main clusters. The red cluster is the largest (24 keywords) and their keywords represent the cognitive effects of ketamine. The keywords in the second largest cluster (green, 22 keywords) represent mechanisms underlying the antidepressant effects of ketamine. The keywords in the third (blue, 20 keywords) and fourth (yellow, 11 keywords) clusters represent safety and tolerability, and suicide associated with TRD, respectively.
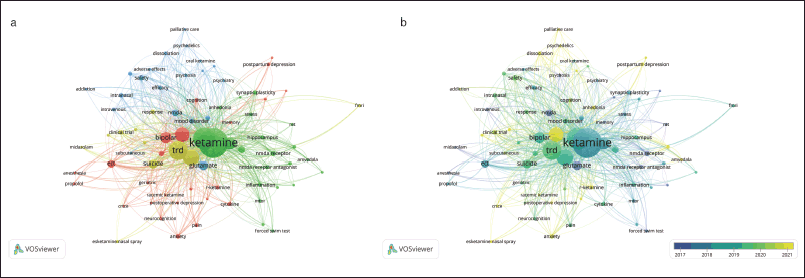 | Figure 4. Co-occurrence of author keywords showing (a) network visualization and (b) overlay visualization. [Click here to view] |
As presented in Figure 4b overlay visualization, we classify the specified network connections from 2017 to 2021 using overlay visualization. The purple nodes represent older keywords while the yellow nodes represent recent keywords. Before 2017, the keywords anesthesia and memory occurred more frequently together in ketamine and depression-related research. During 2017–2018, keywords such as bipolar, ect, animal models, propofol, and psychopharmacology appeared more frequently. Finally, from 2019 to 2021, the terms esketamine, meta-analysis, anxiety, cytokine, r-ketamine, cognition/neurocognition, pain, postpartum depression, and treatment response received more attention from researchers. The observed shifts in the co-occurrence of the author’s keywords over the years likely reflect the evolving trends and research priorities within the field of ketamine and depression.
DISCUSSION
Our results indicate a clear trend of growth in the number of publications on the topic of ketamine and depression over time. From 1971 to 2011, the number of publications remained low but began to increase in 2012 and has continued to increase ever since. The increase in the number of publications on ketamine and depression research reflects the growing interest in this topic among researchers and clinicians. The significant increase in the number of documents published from 2012 onward may be due in part to the growing recognition of ketamine as a potential TRD and other mood disorders, which has led to increased funding for research in this area [7,12]. Another possible explanation is partly due to the approval of esketamine for the management of TRD in adults in 2018 and the treatment of MDD with acute suicidal ideation or behavior in adults in 2020 [13]. We anticipate a sustained upward trajectory in the research and publication of ketamine in the context of depression. This trend is expected to persist in the coming years as researchers aim to gain a more comprehensive understanding of its molecular mechanisms related to antidepressant and anti-suicidal effects [14]. In addition, there is a growing interest in exploring the impact of long-term and repeated ketamine use on cognitive functions [15]. Notably, preclinical and clinical data suggest that arketamine exerts a more potent and longer lasting antidepressant effect, with fewer side effects [16]. This emerging aspect adds a significant dimension to the evolving landscape of ketamine research in depression.
This emerging aspect adds a significant dimension to the evolving landscape of ketamine research in depression.?The increasing trend is reflected in the number of citations, which also increased significantly over time, with peaks in 2000, 2013, and 2018. The peak in the number of citations in 2000 may be due to the publication of seminal studies on the use of ketamine for depression [3], while the peaks in 2013 and 2018 may reflect the publication of randomized clinical trials [17,18] or a model to explain rapid onset of ketamine’s antidepressant [19], which tend to be highly cited in the literature. It is also possible that the peaks in citations may be due to the increased visibility of ketamine as a potential treatment for depression, leading to greater interest and scrutiny of the research in this field.
There is a discrepancy in the ranking of journals when considering the number of articles published versus the number of citations received for studies related to ketamine and depression research. While the journal of affective disorders was the most productive in terms of the number of published articles, biology of psychiatry received the most citations, suggesting that the latter journal publishes higher impact research. For instance, biology of psychiatry published ground-breaking and randomized controlled trials related to ketamine and depression received very high citations from other researchers in the field [3,17,20–23]. On the other hand, the journal of affective disorders publishes a greater volume of clinical research related to ketamine and depression [24,25]. Overall, these findings have important implications for researchers and practitioners interested in ketamine and depression research. It is important to consider both the quantity and quality of publications when evaluating the research literature on this topic and to be aware of the potential limitations of different journals in terms of the types of studies they publish.
We used co-occurrence keywords analysis to identify themes based on the highest co-occurrence. The results imply that the cognitive effects of ketamine, mechanisms underlying ketamine’s antidepressant effects, safety, and tolerability of ketamine, and suicide associated with TRD were the current research themes. The subsequent discussion will focus on the recent issues related to these topics.
Cognitive effects of esketamine
Findings on the cognitive effects of ketamine are controversial because long-term use of ketamine at high doses and high frequencies may cause cognitive impairment even though low and single doses of ketamine infusion have been shown to favor cognitive performance in the short term [26].
Lee et al. [27] in a review suggested that IV acute low-dose ketamine improved visual memory, simple working memory, and complex working memory in individuals with TRD, proposing that the precognitive effects of ketamine work via its targeting intracellular proteins such as brain-derived neurotrophic factor (BDNF) and mammalian target of rapamycin 1 (mTORC1). The investigators also suggested that the acute cognition effects of ketamine may mediate the acute decrease of depressive symptoms and suicidal ideation. Similarly, in a recent review by Gill et al. [28], they suggested that IV ketamine may improve visual and working memory [29], anxious depression, and anhedonia [30]. However, due to the limited number of clinical studies that have examined the effects of IV ketamine on cognition in a clinically representative sample of patients with depression, future studies should examine the effects of IV ketamine on the identified cognitive areas and use the research domain criteria to evaluate cognition transdiagnostically.
Mechanism underlying ketamine’s antidepressant
The exact mechanism underlying the rapid antidepressant effects of ketamine is still not fully understood [14]. However, several mechanisms have been proposed which include N-methyl-D-aspartate (NMDA) receptor antagonism, increased BDNF expression, anti-inflammatory effects, and modulation of the default mode network (DMN).
Ketamine is an antagonist of the NMDA receptor, a glutamate receptor that plays a crucial role in synaptogenesis and neuroplasticity [31] by partially reversing glutamatergic and gamma-aminobutyric acid-ergic dysfunction in depression [32]. Ketamine has been shown to have a unique ability to increase the AMPA-NMDA receptor throughput by directly blocking NMDA receptors and indirectly enhancing AMPAR density and/or function [33]. This, in turn, activates downstream synaptogenic signaling pathways such as BDNF, mTOR, and eukaryotic elongation factor 2, which restore synaptic strength and connectivity in the hippocampus and prefrontal cortex [31,34–37].
Ketamine’s antidepressive effects have been linked to the modulation of glutamate transmission in the brain via metabotropic glutamate receptor, and opioidergic signaling pathways. Ketamine induces long-term synaptic depression by replacing calcium-permeable AMPARs with calcium-impermeable AMPARs in synapses. These alterations at glutamatergic synapses in the ventral tegmental area dopaminergic neurons restore the balance of dopamine signaling in the brain which may be responsible for ketamine’s antidepressant effect [38,39]. µ-opioid receptor and NMDAR colocalize and communicate with one another to act as complementary regulators in the brain [40–42]. It has been hypothesized that ketamine can restore opioid receptor activity and its signaling, aiding in the alleviation of depression.
Ketamine has been reported to have anti-inflammatory effects, including decreasing the production of pro-inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6, and tumor necrosis factor-alpha, which have all been repeatedly found to be associated with depressive illness [43–46]. These three cytokines are known to increase the activity of the enzyme indoleamine 2,3-dioxygenase, which transforms tryptophan into kynurenine [47]. IL-1 also increases the production of the enzyme 3-monooxygenase, which further breaks down kynurenine into its neurotoxic metabolites [48]. Along with their impact on tryptophan metabolism, pro-inflammatory cytokines also affect monoamine metabolism, the hypothalamic-pituitary-adrenal axis, and glutamate signaling, which results in glutamate excitotoxicity and decreased BDNF [49–51].
Dysfunction within neural networks and disruptions in the functional connectivity between brain regions have been implicated in the manifestation of MDD symptoms [52]. The DMN is a group of high-order brain regions, so-called for its decreased activation during tasks of high attentional demand, relative to the high baseline activation of the DMN at rest [53]. It is associated with processes such as introspective thoughts, mind-wandering, and self-referential thinking [54]. In the context of depression, aberrant connectivity patterns of the DMN with other large-scale neural networks have been identified [55]. Ketamine has been shown to restore the dysfunctional connectivity of the DMN with key regions such as the dorsal nexus, pregenual anterior cingulate cortex, and medial prefrontal cortex, with the posterior cingulate cortex acting as its representative hub [56]. These regions play a pivotal role in alleviating depression symptoms, presenting a potential target for interventions in affective disorders [57–59].
Safety and tolerability of ketamine
Ketamine is generally safe and well-tolerated. The majority of adverse drug reactions are mild and tend to mostly disappear within 30 minutes to 2 hours of administration [60,61]. Ketamine’s most typical adverse effects include nausea and vomiting, but occasionally result in more severe side effects such as delirium, and accelerated blood pressure [62]. The major concerns with the use of ketamine are the psychomimetic effects such as hallucinations and dissociation [63], abuse, and dependence potential, especially with repeated or prolonged use [64,65].
In general, esketamine has a better antidepressant effect and safety profile than racemic ketamine, but there is currently insufficient data to support this. More research on both the short- and long-term effectiveness and safety of various ketamine formulations is needed to better understand its safety and tolerability, and to establish best clinical practices for its use [61].
Suicide associated with TRD
About 90% of people who commit suicide have a mental illness, with MDD accounting for a substantial portion of those [66]. Suicidality, however, affects people with TRD more severely than those with MDD who respond to treatment [67].
Ketamine has garnered increasing attention as a rapid-acting treatment for depression and suicidal ideation over the past decade [25]. In a few meta-analyses, ketamine had been shown to possess antidepressant and antisuicidal effects [68–70]. A moderate reduction in suicidal ideation has been shown to occur 4 hours after ketamine infusion and the effect lasts for 24 hours [70], or 72 hours [69]. The different protocols and comparator treatments in each trial make the comparisons across trials not feasible. Further studies are needed to confirm the potential therapeutic option for ketamine’s anti-suicidal effect, especially for TRD.
Limitations
This study has a few limitations. First, even though this research concentrates on the Scopus-indexed journal sources, some journals that are not listed in this database might be excluded. Second, editorials, book and book chapters, conference papers, short communication, and letters to editors were not included in the listed literature to keep its formality and comprehensiveness, which may have resulted in the exclusion of some representative works. Third, only high-frequency author keywords were chosen, some important keywords with low frequency may be ignored.
CONCLUSION
Our bibliometric analysis shows that ketamine and depression research trends and themes are rapidly growing and likely to continue. The journal of affective disorders publishes more articles related to ketamine and depression research, while a few higher impact research were published in biology of psychiatry, giving it the highest citations. The research themes include the cognitive effects of ketamine, mechanisms underlying ketamine’s antidepressant effects, its safety and tolerability, and its anti-suicidal effect in TRD. We expect future research in ketamine and depression to focus on these areas.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. World Health Organization. Depression and other common mental disorders: global health estimates. Geneva, Switzerland: World Health Organization; 2017.
2. Vuyk J, Sitsen E, Reekers M. Intravenous anesthetics. In: Miller R, editor. Anesthesia. 8th ed. Philadelphia, PA: Elsevier Saunders; 2015. p. 858.
3. Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–4. CrossRef
4. Salahudeen MS, Wright CM, Peterson GM. Esketamine: new hope for the treatment of treatment-resistant depression? a narrative review. Ther Adv Drug Saf. 2020;11:2042098620937899. CrossRef
5. Matveychuk D, Thomas RK, Swainson J, Khullar A, MacKay MA, Baker GB, et al. Ketamine as an antidepressant: overview of its mechanisms of action and potential predictive biomarkers. Ther Adv Psychopharmacol. 2020;10:2045125320916657. CrossRef
6. Li X, Xiang P, Liang J, Deng Y, Du J. Global trends and hotspots in esketamine research: a bibliometric analysis of past and estimation of future trends. Drug Des Devel Ther. 2022;16:1131–42. CrossRef
7. Miao H, Yu K, Gao D, Lin X, Cao Y, Liu X, et al. A bibliometric analysis of research on ketamine from 2001 to 2020. Front Mol Neurosci. 2022;15:839198. CrossRef
8. Harzing AW. The publish or perish book. Melbourne, Australia: Tarma Software Research Pty Limited; 2010.
9. Van Eck NV, Waltman L. VOSviewer manual-Manual for VOSviewer version 1.6. 17. Leiden, Netherlands: Universiteit Leiden; 2021.
10. Zakaria WNA, Sasongko TH, Al-Rahbi B, Al-Sowayan N, Ahmad AH, Zakaria R, et al. Gene and schizophrenia in the pregenome and postgenome-wide association studies era: a bibliometric analysis and network visualization. Psychiatr Genet. 2023;33(2):37–49. CrossRef
11. Zakaria R, Ahmi A, Ahmad AH, Othman Z, Azman KF, Ab Aziz CB, et al. Visualising and mapping a decade of literature on honey research: a bibliometric analysis from 2011 to 2020. J Apic Res. 2021;60(3):359–68. CrossRef
12. Duan L, Gao Y, Shao X, Tian C, Fu C, Zhu G. Research on the development of theme trends and changes of knowledge structures of drug therapy studies on major depressive disorder since the 21st century: a bibliometric analysis. Front Psychiatry. 2020;11:647. CrossRef
13. Mischel NA, Balon R. Esketamine: a drug to treat resistant depression that brings more questions than answers. J Clin Psychopharmacol. 2021;41(3):233. CrossRef
14. Yavi M, Lee H, Henter ID, Park LT, Zarate Jr CA. Ketamine treatment for depression: a review. Discov Ment Health. 2022;2(1):9. CrossRef
15. Goldberg JF. Ketamine and cognitive function in depression: detrimental, neutral, or advantageous? J Clin Psychiatry. 2022;83(1):38970. CrossRef
16. Zhang JC, Yao W, Hashimoto K. Arketamine, a new rapid-acting antidepressant: a historical review and future directions. Neuropharmacology. 2022;218:109219. CrossRef
17. Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, Aan het Rot M, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74(4):250–6. CrossRef
18. Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139–48. CrossRef
19. Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554(7692):317–22. CrossRef
20. Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66(5):522–6. CrossRef
21. Aan Het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67(2):139–45. CrossRef
22. Zarate Jr CA, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71(11):939–46. CrossRef
23. Lapidus KA, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014;76(12):970–6. CrossRef
24. Shiroma PR, Johns B, Kuskowski M, Wels J, Thuras P, Albott CS, et al. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord. 2014;155:123–9. CrossRef
25. Bahji A, Vazquez GH, CA Zarate Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542–55. CrossRef
26. Bartoli F, Wlkinson ST. Ketamine and esketamine for suicidal ideation: recent progress and practical issues. Aust NZJ Psychiatry. 2020;54(2):206–7. CrossRef
27. Lee Y, Syeda K, Maruschak NA, Cha DS, Mansur RB, Wium-Andersen IK, et al. A new perspective on the anti-suicide effects with ketamine treatment: a procognitive effect. J Clin Psychopharmacol. 2016;36(1):50–6. CrossRef
28. Gill H, Gill B, Rodrigues NB, Lipsitz O, Rosenblat JD, El-Halabi S, et al. The effects of ketamine on cognition in treatment-resistant depression: a systematic review and priority avenues for future research. Neurosci Biobehav Rev. 2021;120:78–85. CrossRef
29. Shiroma PR, Albott CS, Johns B, Thuras P, Wels J, Lim KO. Neurocognitive performance and serial intravenous subanesthetic ketamine in treatment-resistant depression. Int J Neuropsychopharmacol. 2014;17(11):1805–13. CrossRef
30. Liu W, Zhou Y, Zheng W, Wang C, Zhan Y, Lan X, et al. Repeated intravenous infusions of ketamine: neurocognition in patients with anxious and nonanxious treatment-resistant depression. J Affect Disord. 2019;259:1–6. CrossRef
31. Zanos P, Gould T. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801–11. CrossRef
32. Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, et al. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol Psychiatry. 2017;81(10):88697. CrossRef
33. Aleksandrova LR, Phillips AG, Wang YT. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J Psychiatry Neurosci. 2017;42(4):222–9. CrossRef
34. Abdallah CG, Adams TG, Kelmendi B, Esterlis I, Sanacora G, Krystal JH. Ketamine’s mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety. 2016;33(8):689–97. CrossRef
35. Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry. 2014;29(7):419–23. CrossRef
36. Du J, Machado-Vieira R, Maeng S, Martinowich K, Manji HK, Zarate Jr CA. Enhancing AMPA to NMDA throughput as a convergent mechanism for antidepressant action. Drug Discov. Today Ther Strateg. 2006;3(4):519–26. CrossRef
37. Maeng S, Zarate Jr CA, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63(4):349–52. CrossRef
38. Yao N, Skiteva O, Zhang X, Svenningsson P, Chergui K. Ketamine and its metabolite (2R, 6R)-hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Mol Psychiatry. 2018;23(10):2066–77. CrossRef
39. Skiteva O, Yao N, Chergui K. Ketamine induces opposite changes in AMPA receptor calcium permeability in the ventral tegmental area and nucleus accumbens. Transl Psychiatry. 2021;11(1):530. CrossRef
40. Trujillo KA. The neurobiology of opiate tolerance, dependence and sensitization: mechanisms of NMDA receptor-dependent synaptic plasticity. Neurotox Res. 2002;4(4):373–91. CrossRef
41. Glass MJ, Vanyo L, Quimson L, Pickel VM. Ultrastructural relationship between N-methyl-D-aspartate-NR1 receptor subunit and mu-opioid receptor in the mouse central nucleus of the amygdala. Neuroscience. 2009;163(3):857–67. CrossRef
42. Chartoff EH, Connery HS. It’s MORe exciting than mu: crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system. Front Pharmacol. 2014;5:116. CrossRef
43. Borsini A, Di Benedetto MG, Giacobbe J, Pariante CM. Pro-And anti-inflammatory properties of interleukin in vitro: relevance for major depression and human hippocampal neurogenesis. Int J Neuropsychopharmacol. 2020;23(11):738–50. CrossRef
44. Osimo EF, Pillinger T, Rodriguez IM, Khandaker GM, Pariante CM, Howes OD. Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun. 2020;87:901–9. CrossRef
45. Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. CrossRef
46. Rosenblat JD, ChaDS, Mansur RB, McIntyre RS. Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuro-Psychopharmacol Biol Psychiatry.2014;53:23–34. CrossRef
47. Maes M, Mihaylova I, Ruyter MD, Kubera M, Bosmans E. The immune effects of TRYCATs (tryptophan catabolites along the IDO pathway): relevance for depression--And other conditions characterized by tryptophan depletion induced by inflammation. Neuro Endocrinol Lett. 2007;28(6):826–31.
48. Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81(4):247–65. CrossRef
49. Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. CrossRef
50. Nikkheslat N, McLaughlin AP, Hastings C, Zajkowska Z, Nettis MA, Mariani N, et al. Childhood trauma, HPA axis activity and antidepressant response in patients with depression. Brain Behav Immun. 2020;87:229–37. CrossRef
51. Nikkheslat N, Pariante CM, Zunszain PA. Neuroendocrine abnormalities in major depression: an insight into glucocorticoids, cytokines, and the kynurenine pathway. In: Baune, BT, editor. Inflammation and immunity in depression. Cambridge, MA: Academic Press; 2018. pp. 45–60. CrossRef
52. Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72(6):603–11. CrossRef
53. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. CrossRef
54. Buckner RL, DiNicola LM. The brain’s default network: updated anatomy, physiology and evolving insights. Nat Rev Neurosci. 2019;20(10):593–608. CrossRef
55. Scalabrini A, Vai B, Poletti S, Damiani S, Mucci C, Colombo C, et al. All roads lead to the default-mode network-global source of DMN abnormalities in major depressive disorder. Neuropsychopharmacology. 2020;45(12):2058–69. CrossRef
56. Scheidegger M, Walter M, Lehmann M, Metzge, C, Grimm S, Boeker H, et al. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One. 2012;7(9):e44799. CrossRef
57. Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA. 2010;107(24):11020–5. CrossRef
58. Li J, Chen J, Kong W, Li X, Hu B. Abnormal core functional connectivity on the pathology of MDD and antidepressant treatment: a systematic review. J Affect Disord. 2022;296:622–34. CrossRef
59. Krug S, Müller T, Kayali Ö, Leichter E, Peschel SKV, Jahn N, et al. Altered functional connectivity in common resting-state networks in patients with major depressive disorder: a resting-state functional connectivity study. J Psychiatr Res. 2022;155:33–41. CrossRef
60. W?odarczyk A, Cuba?a WJ. Safety and tolerability of ketamine use in treatment-resistant bipolar depression patients with regard to central nervous system symptomatology: literature review and analysis. Medicina. 2020;56(2):67. CrossRef
61. Smith-Apeldoorn SY, Vischjager M, Veraart JK, Kamphuis J, Aan Het Rot M, Schoevers, RA. The antidepressant effect and safety of non-intranasal esketamine: a systematic review. J Psychopharmacol. 2022;36(5):531–44. CrossRef
62. Dilip TS, Chandy G.M, Hazra D, Selvan J, Ganesan P. The adverse effects of ketamine on procedural sedation and analgesia (PSA) in the emergency department. J Family Med Prim Care. 2021;10(6):2279. CrossRef
63. D’Souza DC, Ahn K, Bhakta S, Elander J, Singh N, Nadim H, et al. Nicotine fails to attenuate ketamine-induced cognitive deficits and negative and positive symptoms in humans: implications for schizophrenia. Biol Psychiatry. 2012;72(9):785–94. CrossRef
64. Drug Enforcement Administration (DEA). Drug fact sheet: ketamine. 2020. Available from https://www.dea.gov/sites/default/files/2020-06/Ketamine-2020.pdf
65. Kleczkowska P, Zaremba M. An update of ketamine illicit use. IntechOpen; 2022. CrossRef
66. Mann JJ, Apter A, Bertolote J, Beautrais A, Currier D, Haas A, et al. Suicide prevention strategies: a systematic review. JAMA. 2005;294(16):2064–74. CrossRef
67. Bergfeld IO, Mantione M, Figee M, Schuurman PR, Lok A, Denys D. Treatment-resistant depression and suicidality. J Affect Disord. 2018;235:362–7. CrossRef
68. Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150–8. CrossRef
69. Witt K, Potts J, Hubers A, Grunebaum MF, Murrough JW, Loo C, et al. Ketamine for suicidal ideation in adults with psychiatric disorders: a systematic review and meta-analysis of treatment trials. Aust NZJ Psychiatry. 2020;54(1):29–45. CrossRef
70. Chen CC, Zhou N, Hu N, Feng JG, Wang XB. Acute effects of intravenous sub-anesthetic doses of ketamine and intranasal inhaled esketamine on suicidal ideation: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2023;9:587–99. CrossRef