INTRODUCTION
Breast milk is a natural emulsion of lipids in water secreted by the mammary glands, providing all of the energy and nutrients to support human growth and development at the beginning of life [1]. Exclusive breastfeeding has been proven to improve health and maintain child survival, starting from the early initiation of breastfeeding, then continuing for the first six months after birth, and with complementary food intake until two years or longer [2]. Globally, only 44% of babies are breastfed immediately after birth, and 40% of those under six months receive exclusive breastfeeding. Regarding exclusive breastfeeding worldwide, the United Nations has set a 50% target for 2025 [3]. If the target is reached, it is estimated to prevent 823,000 annual deaths in children under five years and 20,000 annual deaths from breast cancer in women [4]. In addition, breastfeeding also has the potential to generate economic benefits by reducing diseases and healthcare costs, and increasing productivity [5].
Reproductive hormones, especially prolactin (PRL) and oxytocin (OXT), are responsible for controlling lactogenesis. Prolactin is produced and secreted by the anterior pituitary gland or adenohypophysis after parturition to increase milk production in the alveoli of mammary glands, while OXT is produced by the hypothalamus and released into the circulatory system through the posterior pituitary gland or neurohypophysis to stimulate contraction of myoepithelial cells around alveoli, encouraging ejection of milk [6,7]. Low milk production during breastfeeding is one of the reasons mothers stop breastfeeding their babies. Some factors affecting breast milk production and composition include genetic and physiological factors [8]. Optimizing exclusive breastfeeding for babies and reducing the gap in breastfeeding practices must be continuously pursued by ensuring that every mother can produce sufficient amounts of breast milk [9].
Galactogogues are pharmaceutical agents, foods, or herbal supplements used to initiate, maintain, and increase milk production in clinical conditions such as agalactia and hypogalactia [10,11]. Drugs are often used outside of the approved indications as “off-label” galactogogues, include antiemetics (metoclopramide and domperidone) and antipsychotics (sulpiride and chlorpromazine). Previous studies reveal phytomedicines such as Trigonella foenum-graecum, Foeniculum vulgare, Pimpinella anisum, Galega offinalis, Asparagus racemosus, and Silybum marianum could stimulate lactogenesis [11]. The pharmacological activity of many natural ingredients occurs at the transcriptional level by binding phyto-estrogenic compounds similar to 17β-estradiol (E2) with oestrogen receptors (ERs) or their bioactive compounds that act as dopamine antagonists in the central nervous system. This mechanism will induce the expression of PRL, PRL receptor (PRLR), OXT, and OXT receptor (OXTR) genes in the pituitary and mammary glands directly or indirectly after herbal galactogogue supplementation [11,12]
Pluchea indica (P. indica) is a plant widespread in tropical and subtropical regions. Various pharmacological studies have demonstrated its anti-inflammatory, anticancer, antioxidant, and antimicrobial activities from the main bioactive compound quercetin-3-potassium bisulfate [13,14]. Studies on the galactogogue effects of P. indica leaves are inadequate. However, previous studies have shown that P. indica leaf extract (EPI) can increase milk production in lactating rats based on the measurements of pups’ weight [15]. The intervention of EPI was also reported to elevate PRL and OXT levels in the serum [16]. The Sauropus androgynus (S. androgynus) leaves are widely known in most Southeast Asian countries, such as Indonesia, Thailand, and Malaysia, to increase milk production during lactation. The galactogogue effect of S. androgynus leaves is mediated by kaempferol-3-O-glucosyl-7-O-ramnoside, a phytoestrogen that modulates hormones involved in the synthesis and ejection of breast milk, namely PRL and OXT [13,17]. Several studies have identified the galactogogue abilities of the S. androgynus leaf extract (ESA) alone and in polyherbal formulation with other plants. In vivo studies reveal that ESA can induce the expression of PRL and OXT genes [18]. Combining two or more medicinal plants is a common practice used to prevent and/or treat diseases due to the presence of secondary metabolites in the plants that can generate additive or synergistic effects through various mechanisms of action. This is useful for preserving raw materials, reducing the dosage of each component, and minimizing the toxic effects of some metabolites.
Kaempferol and quercetin are bioactive compounds associated with lactogenesis [19]. Kaempferol, the marker compound in the leaves of S. androgynus, has been widely reported as playing a vital role in PRL signaling pathways, while quercetin, the marker compound for P. indica leaves, releases OXT from the originating organs [20]. Studies on PRLR and OXTR also confirm the plants’ ability to increase hormone-receptor binding and maximize the expected pharmacological effects. In this study, we hypothesized that the EPI-ESA mixture could have possible synergistic effects in increasing milk production and ejection, but the mechanism of action at the molecular level is unknown. Accordingly, this study focused on the standardization and evaluation of the activity of the EPI-ESA mixture on the expressions of PRL, PRLR, OXT, and OXTR genes in the pituitary and mammary glands of lactating rats.
MATERIAL AND METHODS
Collection and authentication of plants material
The leaves of P. indica were collected from Ngaglik, Sleman, Yogyakarta, Indonesia, and identified in the Pharmacognosy and Phytochemistry Laboratory, Department of Pharmaceutical Biology, Faculty of Pharmacy, Universitas Gadjah Mada (No. 12.18.08/UN1/FFA/BF/PT/2020). S. androgynus leaves were supplied and authenticated by the Center for Research and Development of Medicinal Plants and Traditional Medicines, Tawangmangu, Karanganyar, Central Java (No. KM.04.01/2/555/2022).
Preparation of extracts
The leaves of the two plants were dried separately at 50°C in an oven Memmert 854 Schwabach (Memmert GmbH + Co. KG) and crushed with a grinder Cosmos CB-172P (PT. Star Cosmos) into a fine powder. Dry powder of P. indica leaves (200 g) and S. androgynus leaves (1 kg) were macerated separately with 70% ethanol (Merck & Co.) for 24 hours at room temperature. Extraction was repeated three times or done till a pale color of extract was observed, which signifies complete extraction of phytoconstituents. Extracts were filtered and concentrated. The yield of the extracts was calculated [21]. In vivo treatment was prepared by mixing different proportions of EPI and ESA in CMC 0.5% (Merck & Co.).
Non-specific parameters standardization
Evaluations of the water content, total ash, and acid-insoluble ash were performed using gravimetric methods according to the Indonesian Herbal Pharmacopoeia [13].
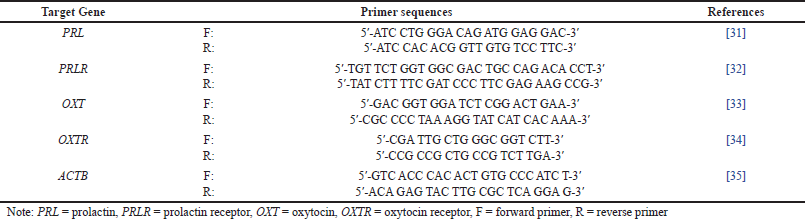 | Table 1. Primer sequences used for RT-qPCR. [Click here to view] |
Water content
About 10 g of extract was placed in a tared evaporating dish, dried at 105°C for 3 hours, and weighed. Drying and weighing were continued at 1 hour intervals until the difference between two successive weights was ≤0.25%. Water content was measured and presented in percentage v/w.
Total ash
About 2–3 g extract was placed in a furnace at 600°C for 8 hours. Furnace was ignited slowly until the organic portion of the sample was burnt. After cooling the residue, the ash content was weighed. Total ash content was calculated from the amount of ash obtained by dividing the test sample and expressed as a percentage w/w.
Acid-insoluble ash
The total ash obtained was boiled with 25 ml of 0.2 N HCl solution (Merck & Co.) for 5 minutes and filtered through Whatman® filter paper (Merck & Co.). The insoluble portion was heated in a crucible at 600°C to constant weight. The mass of acid-insoluble ash was compared to the test sample and calculated as a percentage w/w.
Estimation of total flavonoid content
Total flavonoid content was estimated using spectrophotometric analysis based on the Indonesian Herbal Pharmacopoeia [13] with slight modification following previous studies on measuring total flavonoids in medicinal plants [22].
Reference solution was prepared by weighing approximately 10 mg of quercetin (Sigma-Aldrich) in a volumetric flask, mixed with 0.3 ml of 5% NaNO2 (Merck & Co.), at 25°C for 5 minutes. 0.6 ml of 10% Al(NO3)3 (Merck & Co.) was added to the mixture and incubated at 25°C for 5 minutes. 2 ml of 1 M NaOH (Merck & Co.) was added to the mixture and aquabides were used to make up the volume to 10 ml. Different concentrations of the reference solution were made, including 0.5, 1, 2, 5, 10, 25, 50, 75, and 100 ppm.
The test solution was prepared by weighing 100 mg of extract in an Erlenmeyer flask, mixed with 0.3 ml of 5% NaNO2 (Merck & Co.), at 25°C for 5 minutes. 0.6 ml of 10% Al(NO3)3 (Merck & Co.) was added to the mixture and incubated at 25°C for 5 minutes. 2 ml of 1 M NaOH was added. Sonication was performed to ensure complete dissolution of the extract. The extract was filtered into a 10 ml volumetric flask, and the volume was adjusted with aquabides.
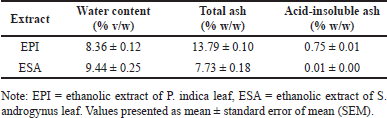 | Table 2. Non-specific parameters standardization of EPI and ESA. [Click here to view] |
Reference and test solutions were homogenized and incubated for 30 minutes at room temperature. Absorbance was measured at a wavelength of 510 nm using a Shimadzu UV-1800 spectrophotometer (Shimadzu Corporation). Absorbance was measured in triplicate. The standard curve equation for reference solution measurements is y = 0.00311337x – 0.00174070 with a correlation coefficient R2 = 0.99944. The standard curve serves as a comparison so that the total flavonoid content of the test sample can be determined based on the absorbance value at the maximum wavelength.
Experimental animals
Lactating Wistar strain rats, aged 6–8 weeks and weighing 200–250 g were selected as the experimental animals in this study. The experimental animals were obtained from the Integrated Research and Testing Laboratory, Universitas Gadjah Mada. Before the study, the experimental animals were kept in the animal house of the Department of Pharmacology and Therapy to acclimatize. The animal house was maintained in an aseptic condition at a temperature of 25°C ± 2°C, relative humidity of 65% ± 5%, and a lighting cycle of 12 hours light and 12 hours dark [23,24]. Experimental animals were placed in plastic cages (2–3 rats/cage). On the 15th day of gestation, rats were separated into 1 rat/cage with 6 pups until the end of the study. Pellets were given twice daily, and drinking water ad libitum. The study protocol was approved by the Medical and Health Research Ethics Committee, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia (No. KE/FK/0265/EC/2022).
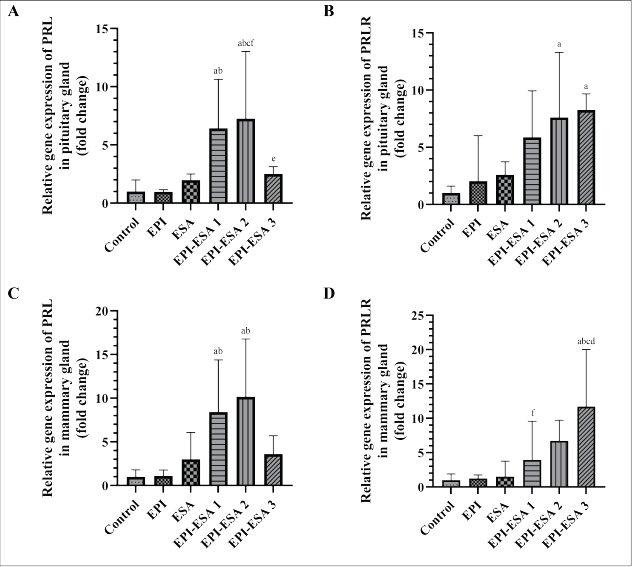 | Figure 1. Relative gene expression of PRL in pituitary gland (A), PRLR in pituitary gland (B), PRL in mammary gland (C), and PRLR in mammary gland (D). Data were statistically analysed using One Way ANOVA followed by Tukey’s post hoc multiple comparisons test, and presented as geometric mean ± SD. PRL = prolactin, PRLR = prolactin receptor, control = CMC 0.5% 1 ml/200 gBW, EPI = ethanolic extract of P. indica leaf 500 mg/kgBW, ESA = ethanolic extract of S. androgynus leaf 125 mg/kgBW, EPI-ESA 1, 2, 3, respectively, were mixtures of EPI and ESA with a fixed ratio (4:1) and dose levels of 125+31.25 mg/kgBW, 250+62.5, and 500+125 mg/kgBW. Intervention was administered orally, once a day, from the second until the fifteenth day after parturition. ap<0.05 compared with control, bp<0.05 compared with EPI, cp<0.05 compared with ESA, dp<0.05 compared with EPI-ESA 1, ep<0.05 compared with EPI-ESA 2, and fp<0.05 compared with EPI-ESA 3. [Click here to view] |
Animal grouping and treatment
A total of twenty-four lactating rats were randomly divided into six groups (n = 4). The number of experimental animals for each group was calculated according to Federer’s formula and resource equation method [25]. Several dosage levels were made based on the drug combinations principle [26].Group I: CMC 0.5% 1 ml/200 gBW as control, group II: EPI 500 mg/kgBW, group III: ESA 125 mg/kgBW, and groups IV–VI were given a mixture of EPI and ESA doses of 125+31.25 mg/kgBW (EPI-ESA 1), 250+62.5 (EPI-ESA 2), and 500+125 mg/kgBW (EPI-ESA 3), respectively. Treatment was given orally, once a day, from the second day until the fifteenth day after parturition [27]. On the sixteenth day, experimental animals were anesthetized intraperitoneally with an anesthesia cocktail containing ketamine 100 mg/mL, xylazine 100 mg/ml, acepromazine 10 mg/ml, and 0.9% NaCl. After losing response and consciousness, the abdominal area was cleaned using 70% ethanol. The skin of the abdomen between the two hind legs was pulled, and an incision ±1 cm was made using forceps and surgical scissors to remove the mammary glands [28]. The pituitary gland was surgically removed by decapitating and cutting skull bones [29]. The collected pituitary and mammary glands were soaked in FavorPrep™ RNA Stabilization Solution (Favorgen Biotech Corporation) to prevent ribonucleic acid (RNA) degradation by ribonuclease and extend the shelf life of samples.
Gene expression studies
RNA isolation
The RNA isolation process was conducted following the protocol of FavorPrep™ Tissue Total RNA Mini Kit (Favorgen Biotech Corporation). The concentration and purity of isolated RNA were tested with NanoVue Plus™ Micro-Volume UV-Vis Spectrophotometer 28-9569-66 (Biochrom). Contamination of phenols and other compounds with similar characteristics was measured at 230 nm, nucleic acids were measured at 260 nm, while proteins and other contaminants were measured at 280 nm. The value of ratio A260/A230 ranges from 1.8 to 2.2, and the A260/A280 ratio ~ 2.0 indicates a pure RNA sample and can be used for the next step [30].
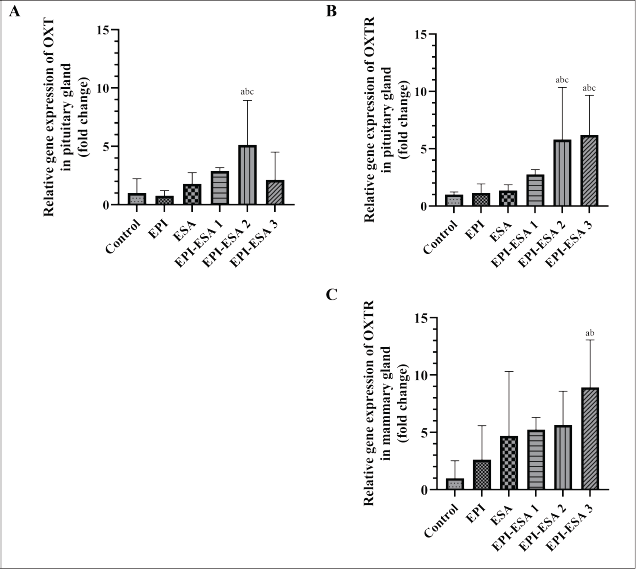 | Figure 2. Relative gene expression of OXT in pituitary gland (A), OXTR in pituitary gland (B), and OXTR in mammary gland (C). Data were statistically analysed using One Way ANOVA followed by Tukey’s post hoc multiple comparisons test, and presented as geometric mean ± SD. OXT = oxytocin, OXTR = oxytocin receptor, control = CMC 0.5% 1 ml/200 gBW, EPI = ethanolic extract of P. indica leaf 500 mg/kgBW, ESA = ethanolic extract of S. androgynus leaf 125 mg/kgBW, EPI-ESA 1, 2, 3, respectively, were mixtures of EPI and ESA with a fixed ratio (4:1) and dose levels of 125+31.25 mg/kgBW, 250+62.5 mg/kgBW, and 500+125 mg/kgBW. Intervention was administered orally, once a day, from the second until the fifteenth day after parturition. ap<0.05 compared with control, bp<0.05 compared with EPI, cp<0.05 compared with ESA, dp<0.05 compared with EPI-ESA 1, ep<0.05 compared with EPI-ESA 2, and fp<0.05 compared with EPI-ESA 3. [Click here to view] |
Complementary DNA (cDNA) synthesis
The cDNA synthesis procedure refers to ExcelRT™ Reverse Transcription Kit II (Smobio Technology Inc.) user guide. Synthesis products can be used directly for real-time reverse transcription-quantitative polymerase chain reaction (RT-qPCR) or stored at −20°C for analysis at different times.
Quantification of gene expression
Quantification of gene expression was performed using qPCR 7500 Fast Real Time PCR System (Applied Biosystems) instrument. Specific primers for PRL, PRLR, OXT, and OXTR genes were designed by using sequences from previous studies [31–35] (Table 1). In this study, β-actin (ACTB) was used as a reference gene to normalize data [36]. The configuration of the RT-qPCR instrument was set according to the recommendations of SensiFAST™ SYBR® Lo-ROX Kit (Meridian Bioscience), with reverse transcription at 42°C for 5 minutes, followed by enzymatic activation at 95°C for 2 minutes, denaturation for 15 seconds at 95°C, and elongation for 30 seconds at 60°C. Relative gene expression was calculated based on fold change in the cycle threshold (Ct) value using 2(-ΔΔCt) method. ΔCt is the result of Ct target gene – Ct reference gene, then ΔΔCt is the difference between ΔCt treatment group – ΔCt control group [37].
 | Figure 3. The classic mechanism of phyto-estrogenic compounds and ER-mediated transcription regulatory. Phyto-estrogenic compounds enter the cells and bind to intracellular ERs. In the inactive form, ER binds to the chaperone protein complex, and then the receptor structure will dimerize. ER dimers bind to response elements in the promoter region of target genes. A series of phosphorylation cascades activate response element binding proteins and recruit the attachment of coactivators, basal transcription factors, and RNA polymerase II. The formation of the transcription machinery can induce mRNA synthesis, and increasing protein products that provide biological activity. [Click here to view] |
Statistical analysis
Relative gene expression data were analyzed using GraphPad Prism version 9.3.1 for Windows operating system. Data were tested with One Way ANOVA at 95% confidence level (CI) followed by Tukey’s post hoc multiple comparisons test and presented as geometric mean ± standard deviation (SD). A value of p<0.05 was considered statistically significant [38].
RESULTS
Based on previous studies, the crude extract of P. indica leaf is required to have an extraction yield of not less than 8.3%, while the crude extract of S. androgynus leaf should be not less than 7.6% [13]. In this study, the extraction yields of EPI and ESA exceeded the minimum standards, with 20.00% w/w (2.41 times higher than the reference value) and 25.77% w/w (3.39 times higher than the reference value), respectively.
Standardization of extracts was achieved by examining several non-specific parameters, as listed in Table 2. Each parameter was replicated three times to ensure data reproducibility. According to standard limits [13], crude extract of P. indica leaves was characterized by water content ≤9.6%, total ash ≤8.1%, and acid-insoluble ash ≤1.6%. Crude extract of S. androgynus leaves must have water content ≤10%, total ash ≤0.4%, and acid-insoluble ash ≤0.1%. Test results showed that the parameters of water content and acid-insoluble ash of EPI and ESA met the established criteria, but the total ash parameter did not meet the requirements because the values obtained were higher than the reference values.
According to the Indonesian Herbal Pharmacopoeia criteria, crude extract of P. indica leaf contains ≥1.50% total flavonoid, and crude extract of S. androgynus leaf contains ≥1.96% total flavonoid. Spectrophotometric measurements estimated the total flavonoid content of EPI 7.33 ± 0.00% w/w (4.89 times higher than the reference value) and ESA 3.84 ± 0.01% w/w (1.96 times higher than the reference value).
Relative gene expression of PRL in the pituitary glands (Fig. 1A) and mammary glands (Fig. 1C) showed a significant induction compared to the control (p<0.05) after EPI-ESA 1 and EPI-ESA 2 treatment. The PRLR relative gene expression in the pituitary glands (Fig. 1B) experienced a significant induction over the control (p<0.05) after receiving EPI-ESA 2 and EPI-ESA 3 interventions, while relative gene expression of PRLR in the mammary glands (Fig. 1D) was significantly increased compared to the control (p<0.05) in the EPI-ESA 3 group.
The OXT-relative gene expression was observed in the pituitary glands (Fig. 2A), and the increase in its expression induced by EPI-ESA 2 administration was significant compared to the control (p<0.05). The relative gene expression of OXTR gene in the pituitary glands (Fig. 2B) was significantly different from the control (p<0.05) as found in the EPI-ESA 2 and EPI-ESA 3 groups, and the relative gene expression of OXTR in the mammary glands (Fig. 2C) increased significantly compared to the control (p<0.05) in the EPI-ESA 3 group.
The analysis provided fluctuating data among all study groups. The data indicated that the EPI-ESA 2 group had the highest fold change in relative gene expressions of PRL and OXT. However, the EPI-ESA 3 group had the highest fold change in relative gene expressions of PRLR and OXTR. Induction in hormones and receptors gene expression reached a balance after treatment with EPI-ESA 2 at 250+62.5 mg/kgBW.
DISCUSSION
Quality control testing of EPI and ESA, such as calculation of extraction yield, standardization of non-specific parameters, and determination of total flavonoids, aim to ensure that the extracts meet minimum quality requirements according to their intended use. Extraction yield can illustrate solvent efficiency in diffusing secondary metabolites out of the plant matrices based on the degree of polarity [39,40]. In this study, a polar solvent, 70% ethanol, was used during the maceration process. The results prove that 70% ethanol can extract bioactive compounds in P. indica and S. androgynus leaves, dominated by flavonoid class components with polar properties, especially quercetin and kaempferol [14,17]. Water content needs to be limited, because in high quantities it will become a growth medium for microbes, fungi, and insects, and trigger damage mediated by hydrolysis reactions, so its presence has the potential to degrade the quality and reduce the shelf life of an extract [41].
The total ash and acid-insoluble ash are essential indicators to evaluate the purity of medicinal plant materials during extraction and their digestibility in the digestive system. Total ash determination is designed to measure the total amount of material remaining after igniting at 600oC, including physiological ash derived from the plant tissue itself, and non-physiological ash, which is the residue of extraneous matter (e.g. sand and soil) adhering to plant surfaces. Total ash content independently is not enough to reflect the quality of medicinal plant materials, so it is necessary to measure the acid-insoluble ash, which can detect the number of silica minerals after boiling the total ash with dilute hydrochloric acid, then burning the remaining insoluble material [41,42]. A comparison between test results and standard literature [13] shows that the total ash of EPI and ESA exceeds the reference value thresholds. The high total ash content is influenced by several factors including differences in cultivation and equipment used for extraction, so it also impacts the intrinsic and extrinsic mineral profiles of medicinal plant materials and extracts. Although the total ash content does not meet the requirements, referring to the same guidelines, the test results of acid-insoluble ash comply with the established criteria. This condition explains that EPI and ESA contain massive minerals but can be dissolved entirely in the presence of a strong acid solution and re-ignited. The minimum acid insoluble ash value means less contamination with siliceous matter. Therefore, EPI and ESA can be safely administered to the experimental animals because both extracts are well-digested in an acidic environment and do not have residue in the gastrointestinal tract.
Estimation of the total flavonoid content serves to quantify bioactive compounds so that extracts are standardized according to applicable regulations and can be referenced by other researchers who want to replicate or continue similar research. Although the crude extracts consist of various secondary metabolites, flavonoids were chosen as indicators because they act as marker compounds for both EPI and ESA. The flavonoid levels obtained from this study are much higher than the reference values [13]. Fluctuations in total flavonoid content between studies occur due to differences in nutrient elements at cultivation sites, which play an important role in plants forming secondary metabolites. Fusing several types of flavonoids is expected to have a synergistic effect that can optimize their pharmacological action. However, it also does not rule out the emergence of antagonistic effects because high doses can interfere with signaling pathways, even triggering cell death [43,44].
Research parameters include relative gene expressions of PRL, PRLR, OXT, and OXTR, which are directly involved in lactogenesis. PRL, PRLR, and OXTR gene expressions were observed in the pituitary and mammary glands, whereas OXT gene expression was observed only in the pituitary gland. Tissue samples in pituitary and mammary glands of lactating rats were selected based on the site of synthesis and action of the target genes which is used to identify the transcription process. Transcription is a process of copying genetic information from deoxyribonucleic acid (DNA) template to messenger RNA (mRNA). Transcription begins with the initiation stage when RNA polymerase II through various transcription factors, binds to double-stranded DNA mainly in the promoter area containing specific sequences known as TATA box, to form a transcription initiation complex. The DNA strands break down into leading and lagging strands. RNA polymerase II continues the transcription process to the elongation stage. Synthesis and elongation of the mRNA precursor chain occur from the 5’ to the 3’ direction by pairing complementary nucleotides. Elongation continues until RNA polymerase II encounters a hairpin loop structure, which causes the enzymes to detach from binding to the DNA template and finally enter the termination stage [45,46].
All cells can undergo gene expression changes in response to environmental conditions. Many steps are involved during gene expression, and initiation of mRNA transcription is the most essential control point. One of the controllers of the transcription process is the transcription regulatory protein. Transcription regulators recognize and bind to specific DNA sequences about 5–12 nucleotide pairs long and located on the same chromosome. Each transcriptional regulator has distinctive features, but most bind to the DNA strand in a homodimer or heterodimer structure. The binding triggers a series of reactions determining the target gene to be transcribed and its transcription rate [47].
The increased relative expression of PRL, PRLR, OXT, and OXTR genes identified through this study is thought to arise from the diversity of secondary metabolites from the two medicinal plants, P. indica and S. androgynus. The marker compound quercetin-3-potassium bisulfate in P. indica leaves [13,14], and kaempferol-3-O-glucosyl-7-O-ramnoside in S. androgynus leaves [13,17] are examples of the many bioactive compounds contained therein. Both belong to the flavonol class of flavonoid derivatives, in the main phytoestrogens group with biological activity similar to E2 [48].
As part of the nuclear receptor superfamily that contributes significantly to the endocrine system, ER has a DNA-binding domain that allows it to control gene expression events and a ligand-binding domain that makes it responsive to the activating ligand, E2 [49,50]. In the genomic domain, E2 works following one or several pathways, including ligand-receptor binding, activation of coregulators and promoters, production of mRNA from target genes, protein synthesis, and control of cell growth and mortality [51]. Various ER-mediated transcription regulatory mechanisms are as follows [52].
- The classic mechanism occurs when the E2-ER bond forms a dimer with assistance from the chaperon protein, then the dimer is translocated to the nucleus and directly attaches to the oestrogen response element (ERE) in the promoter of gene targets as transcription factors (Fig. 3).
- The ERE-independent mechanism occurs when the E2-ER complex does not have an affinity for the ERE sequence, but promotes protein–protein interactions with other transcription factor complexes present in the promoter of target genes.
- The ligand-independent mechanism occurs when ER does not bind E2, but growth factor stimulation provides a signal to initiate a protein kinase cascade leading to phosphorylation and activation of ER dimers in the ERE promoter of target genes.
- The nongenomic mechanism occurs when E2 binds to ER in the cell membrane and stimulates the activation of protein kinase cascades, thereby affecting the function of proteins in the cytoplasm including the phosphorylation of transcription factors.
Phyto-estrogenic agents with a structure similar to E2 can stimulate lactotroph cell proliferation and induce the production of PRL and its receptors by upregulating ER alpha (ERα) as a transcription factor in the promoter area [53–55]. Synergistic activity between ERα and other transcription factors, such as pituitary-specific positive transcription factor 1 (Pit-1), Sp1 transcription factor (Sp1), and cytosine-cytosine-adenosine-adenosine-thymidine (CCAAT) enhancer binding protein beta (C/EBPβ), also contribute to supporting the synthesis of PRL mRNA, and PRLR mRNA in the pituitary gland [56,57]. The cyclic adenosine monophosphate (cAMP) molecule induces PRL at many extra pituitary sites by activating protein kinase A (PKA) that migrates to the nucleus and phosphorylates transcription factors such as cAMP response element binding protein (CREB). Several active cAMP ligands, such as isoproterenol, β-adrenergic receptor agonist, and pituitary adenylate cyclase-activating peptide (PACAP), increase PRL gene expression in mammary gland preadipocytes through various signaling pathways [58]. Another mechanism of herbal galactogogue is antagonism to dopamine, which induces PRL gene expression, increases PRL levels in the blood, accelerates the rate of protein synthesis in breast milk, and stimulates the proliferation of mammary gland myoepithelial cells [11]. Phyto-estrogenic compounds are also known to compete strongly with E2 to occupy ER. Reducing the amount of endogenous oestrogen reaching its receptors may provide positive feedback on PRL synthesis [59].
Phytoestrogens related to the expression of the OXT and OXTR genes focus on activating the promoter for initiating transcription [12]. In addition, E2 and other exogenous molecules that have similar chemical structures can modulate OXT mRNA expression in the hypothalamus and posterior pituitary gland through regulation of ER beta (ERβ) dimers, which are widely distributed in supraoptic nucleus (SON) and paraventricular nucleus (PVN) neurons [60,61]. Phytoestrogen-induced phosphorylation cascades of transcription factors Pit-1 and C/EBPβ are also capable of driving their attachment to the promoter sequence of the OXT gene and activation of transcriptional machinery [62]. The OXTR gene expression is controlled through the same pathway, and the ERβ dimer is involved as a transcription factor in the pituitary gland, while the ERα dimer controls the transcription process more in the mammary glands. Phytoestrogens that bind to ER can strengthen the formation of transcription initiation complexes in the ERE area of the OXT and OXTR gene promoters [12,63].
The pharmacological assay of the EPI-ESA mixture on gene expression of hormones and their receptors associated with lactation is novel and has not been studied before. Relative gene expressions of PRL in both glands and OXT in the pituitary gland did not show a dose-response relationship between treatment groups, where the EPI-ESA 3 group experienced a significant decrease when compared to the EPI-ESA 2 and EPI-ESA 1 groups. A progressive increase in oxidative stress due to a high concentration of secondary metabolites of the flavonoid group, which are converted to pro-oxidants, is thought to disrupt oxidation-reduction homeostasis. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are signaling molecules for the inflammatory process, both of which are often associated with oxidative stress conditions that can change gene expression patterns through transcriptional regulators sensitive to ROS or RNS [64]. Parameters of receptor gene expression, PRLR and OXTR in both glands, showed linearity of increasing relative gene expression from the lowest to the highest dose of mixed extract. The differences in dose-response relationships observed in hormone genes and receptor genes are also determined by the capacity and sensitivity of each gene and the various transcription machinery activating the proteins involved. It is projected that high expression of the PRL and OXT genes, followed by adequate expression of the PRLR and OXTR genes in EPI-ESA 2 create a proportional genetic environment to the production and ejection of breast milk.
CONCLUSION
This study investigated the effects of different combinations of the EPI and ESA on hormones and receptors implicated in lactogenesis, which originate from the pituitary and mammary glands. The results from the standardization of non-specific parameters and determination of total flavonoids revealed that the parameters evaluated fall within the reference criteria. In general, this study shows that the relative gene expressions of PRL, PRLR, OXT, and OXTR in lactating rats are achieved when the experimental animals received EPI-ESA 2 at 250+62.5 mg/kgBW. This treatment has the potential to support lactogenesis by stimulating the transcription process. Further studies on the toxicity profile might be necessary to facilitate the development of the formulation into a phytomedicine.
ACKNOWLEDGMENTS
This study was supported by funding from the Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada with contract number 345/UN1/FKKMK/PPKE/PT/2022. The authors thank all laboratory staff in the related laboratories for technical assistance.
AUTHOR CONTRIBUTIONS
EDW designed the phytochemical study, conducted the literature search, performed the experiments, extracted and analyzed data, and drafted the manuscript. RAS designed the In vivo study, obtained funding, analyzed data, contributed to the critical revisions of the manuscript, supervision, and is the corresponding author. DES designed the in vivo study, obtained funding, analyzed data, contributed to the critical revisions of the manuscript, and provided supervision. SP and ENS contributed to the critical revisions of the manuscript and provided supervision. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Medical and Health Research Ethics Committee, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia (Approval No. KE/FK/0265/EC/2022).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Sánchez C, Franco L, Regal P, Lamas A, Cepeda A, Fente C. Breast milk: a source of functional compounds with potential application in nutrition and therapy. Nutrients 2021;13(3):1–34. CrossRef
2. Scherbaum V, Srour ML. The role of breastfeeding in the prevention of childhood malnutrition. World Rev Nutr Diet. 2016;115:82–97. CrossRef
3. Vitalis D, Witten C, Pérez-Escamilla R. Gearing up to improve exclusive breastfeeding practices in South Africa. PLoS One 2022;17(3):1–12. CrossRef
4. Victora CG, Bahl R, Barros AJD, França GVA, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016; 387(10017):475–90. CrossRef
5. Rollins NC, Bhandari N, Hajeebhoy N, Horton S, Lutter CK, Martines JC, et al. Why invest, and what it will take to improve breastfeeding practices? Lancet 2016; 387(10071):491–504. CrossRef
6. Kennett JE, Mckee DT. Oxytocin: an emerging regulator of prolactin secretion in the female rat. J Neuroendocrinol. 2012;24(3):403–12. CrossRef
7. Grzeskowiak LE, Wlodek ME, Geddes DT. What evidence do we have for pharmaceutical galactagogues in the treatment of lactation insufficiency?—a narrative review. Nutrients 2019;11(5):1–21. CrossRef
8. Golan Y, Assaraf YG. Genetic and physiological factors affecting human milk production and composition. Nutrients 2020;12(5):1–20. CrossRef
9. Matare CR, Craig HC, Martin SL, Kayanda RA, Chapleau GM, Kerr RB, et al. Barriers and opportunities for improved exclusive breast-feeding practices in Tanzania : household trials with mothers and fathers. Food Nutr Bull. 2019;40(3):308–25. CrossRef
10. Zapantis A, Steinberg JG, Schilit L. Use of herbals as galactagogues. J Pharm Pract. 2012;25(2):222–31. CrossRef
11. Tabares FP, Jaramillo JVB, Ruiz-Cortés ZT. Pharmacological overview of galactogogues. Vet Med Int. 2014;2014:1–20. CrossRef
12. Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev. 2001;81(2):629–83. CrossRef
13. Ministry of Health of the Republic of Indonesia. Indonesian Herbal Pharmacopeia. 2nd ed. Jakarta, Indonesia: Ministry of Health of the Republic of Indonesia; 2017.
14. Ruan J, Li Z, Yan J, Huang P, Yu H, Han L, et al. Bioactive constituents from the aerial parts of pluchea indica less. Molecules 2018;23(9):1–11. CrossRef
15. Syarif RA, Anggorowati N, Munawaroh M, Wahyuningsih MSH. Ethanolic extract of pluchea indica less leaf increases serum growth hormone in lactating rats. Trad Med J. 2021;26(2):111–6. CrossRef
16. Syarif RA, Anggorowati N, Munawaroh M, Adyaksa DNM, Wahyuningsih MSH. Lactogenic activity of ethanolic extract of Pluchea indica Less leaf in lactating rats. J Herbmed Pharmacol. 2023;12(3):380–7. CrossRef
17. Zhang B dou, Cheng J xin, Zhang C feng, Bai Y dan, Liu W yuan, Li W, et al. Sauropus androgynus L. Merr.-A phytochemical, pharmacological and toxicological review. J Ethnopharmacol. 2020;257:1–13. CrossRef
18. Soka S, Alam H, Boenjamin N, Agustina TW, Suhartono MT. Effect of Sauropus androgynus leaf extracts on the expression of prolactin and oxytocin genes in lactating BALB/C Mice. J Nutri Nutrigenom 2010;3(1):31–6. CrossRef
19. Zhong J, Liang Y, Chen Y, Zhang J, Zou X, Deng J, et al. Study and experimental validation of the functional components and mechanisms of Hemerocallis citrina Baroni in the treatment of lactation deficiency. Foods 2021;10(8):1–14. CrossRef
20. Tarko A, Štochmal’Ová A, Jedli?ková K, Hrabovszká S, Vachanová A, Halim Harrath A, et al. Effects of benzene, quercetin, and their combination on porcine ovarian cell proliferation, apoptosis, and hormone release. Arch Anim Breed. 2019;62(1):345–51. CrossRef
21. Huang X, Ai C, Xiao J, Xiang C. Guideline for the extraction, isolation, purification, and structural characterization of polysaccharides from natural resources. EFood 2022; 3(e37):1–16. CrossRef
22. Sulaiman CT, Balachandran I. Total phenolics and total flavonoids in selected Indian medicinal plants. Indian J Pharm Sci 2012;74(3):258–60. CrossRef
23. Garber JC, Barbee RW, Bielitzki JT, Clayton LA, Donovan JC, Hendriksen CFM, et al. Guide for the care and use of laboratory animals. 8th ed. Washington, DC: The National Academies Press; 2011.
24. Mustofa, Yuliani FS, Purwono S, Sadewa AH, Damayanti E, Heriyanto DS. Polyherbal formula (ASILACT®) induces Milk production in lactating rats through upregulation of α-Lactalbumin and aquaporin expression. BMC Complement Med Ther. 2020;20(1):1–8. CrossRef
25. Charan J, Kantharia N. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4(4):303–6. CrossRef
26. Chou TC. Preclinical versus clinical drug combination studies. Leuk Lymphoma 2008;49(11):2059–80. CrossRef
27. Hosseinzadeh H, Tafaghodi M, Mosavi MJ, Taghiabadi E. Effect of aqueous and ethanolic extracts of Nigella sativa seeds on milk production in rats. JAMS J Acupunct Meridian Stud. 2013;6(1):18–23. CrossRef
28. Honvo-Houéto E, Truchet S. Indirect immunofluorescence on frozen sections of mouse mammary gland. J Vis Exp. 2015; 106:1–24. CrossRef
29. Cao D, Ma X, Zhang WJ, Xie Z. Dissection and coronal slice preparation of developing mouse pituitary gland. J Vis Exp. 2017; 129:1–5. CrossRef
30. Unger C, Lokmer N, Lehmann D, Axmann IM. Detection of phenol contamination in RNA samples and its impact on qRT-PCR results. Anal Biochem. 2019; 571:49–52. CrossRef
31. Ochoa A, Montes de Oca P, Rivera JC, Dueñas Z, Nava G, Martínez de la Escalera G, et al. Expression of prolactin gene and secretion of prolactin by rat retinal capillary endothelial cells. Investig Ophthalmol Vis Sci. 2001;42(7):1639–45.
32. Clapp C, Torner L, Gutiérrez-Ospina G, Alcántara E, López-Gómez FJ, Nagano M, et al. The prolactin gene is expressed in the hypothalamic-neurohypophyseal system and the protein is processed into a 14-kDa fragment with activity like 16-kDa prolactin. Proc Natl Acad Sci USA. 1994;91(22):10384–8. CrossRef
33. Xi D, Kusano K, Gainer H. Quantitative analysis of oxytocin and vasopressin messenger ribonucleic acids in single magnocellular neurons isolated from supraoptic nucleus of rat hypothalamus. Endocrinology 1999;140(10):4677–82. CrossRef
34. Liu CX, Takahashi S, Murata T, Hashimoto K, Agatsuma T, Matsukawa S, et al. Changes in oxytocin receptor mRNA in the rat uterus measured by competitive reverse transcription-polymerase chain reaction. J Endocrinol. 1996;150(3):479–86. CrossRef
35. Popovics P, Rekasi Z, Stewart AJ, Kovacs M. Regulation of pituitary inhibin/activin subunits and follistatin gene expression by GnRH in female rats. J Endocrinol. 2011;210(1):71–9. CrossRef
36. Svingen T, Letting H, Hadrup N, Hass U, Vinggaard AM. Selection of reference genes for quantitative RT-PCR (RT-qPCR) analysis of rat tissues under physiological and toxicological conditions. PeerJ 2015;3:1–15. CrossRef
37. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001;25(4):402–8. CrossRef
38. Lee S, Lee DK. What is the proper way to apply the multiple comparison test? Korean J Anesthesiol. 2018;71(5):353–60. CrossRef
39. Patil AS. Plant Secondary Metabolites: Isolation, Characterization and Biological Properties. Delhi, India: Studera Press; 2020.
40. Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products : a comprehensive review. Chin Med. 2018;13(20):1–26. CrossRef
41. World Health Organization. Quality control methods for medicinal plant materials. Geneva, Switzerland: World Health Organization; 1998.
42. Rao Y, Xiang B. Determination of total ash and acid-insoluble ash of Chinese herbal medicine Prunellae Spica by near infrared spectroscopy. Yakugaku Zasshi 2009;129(7):881–886. CrossRef
43. Caesar LK, Cech NB. Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat Prod Rep. 2019;36(6):869–88. CrossRef
44. Xi X, Wang J, Qin Y, You Y, Huang W, Zhan J. The Biphasic effect of flavonoids on oxidative stress and cell proliferation in breast cancer cells. Antioxidants 2022;11(4):1–22. CrossRef
45. Liu X, Bushnell DA, Kornberg RD. RNA Polymerase II transcription: structure and mechanism. Biochim Biophys Acta. 2012;1829(1):2–6. doi: 10.1016/j.bbagrm.2012.09.003
46. Mercadante AA, Dimri M, Mohiuddin SS. Biochemistry, replication and transcription internet. Treasure Island, FL: StatPearls Publishing; 2022 cited 2022 Dec 28. Available from: https://www.ncbi.nlm.nih.gov/books/NBK540152/
47. Alberts B, Heald R, Johnson A, Morgan D, Raff M, Roberts K, et al. Molecular biology of the cell. 7th ed. New York, NY: W. W. Norton & Company; 2022.
48. Kiyama R. Estrogenic flavonoids and their molecular mechanisms of action. J Nutr Biochem. 2023;114:1–50. CrossRef
49. Brisken C, Ataca D. Endocrine hormones and local signals during the development of the mouse mammary gland. Wiley Interdiscip Rev Dev Biol. 2015;4(3):181–95. CrossRef
50. Carroll JS. Mechanisms of oestrogen receptor (ER) gene regulation in breast cancer. Eur J Endocrinol. 2016;175(1):41–9. CrossRef
51. Kiyama R. Estrogenic terpenes and terpenoids: pathways, functions and applications. Eur J Pharmacol. 2017;815:405–15. CrossRef
52. Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19(4):833–42. CrossRef
53. Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80(4):1523–631. CrossRef
54. Ye Q, Zhang QY, Zheng CJ, Wang Y, Qin LP. Casticin, a flavonoid isolated from Vitex rotundifolia, inhibits prolactin release in vivo and in vitro. Acta Pharmacol Sin. 2010;31(12):1564–8. CrossRef
55. Zárate S, Seilicovich A. Estrogen receptors and signaling pathways in lactotropes and somatotropes. Neuroendocrinology 2010;92:215–23. CrossRef
56. Featherstone K, White MRH, Davis JRE. The prolactin gene: a paradigm of tissue-specific gene regulation with complex temporal transcription dynamics. J Neuroendocrinol. 2012;24(7):977–90. CrossRef
57. Kavarthapu R, Dufau ML. Essential role of endogenous prolactin and CDK7 in estrogen induced upregulation of the prolactin receptor in breast cancer cells. Oncotarget 2017;8(16):27353–63. CrossRef
58. McFarland-Mancini M, Hugo E, Loftus J, Ben-Jonathan N. Induction of prolactin expression and release in human preadipocytes by cAMP activating ligands. Biochem Biophys Res Commun. 2006;344(1):9–16. CrossRef
59. Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, Van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998;139(10):4252–63. CrossRef
60. Shughrue PJ, Dellovade TL, Merchenthaler I. Estrogen modulates oxytocin gene expression in regions of the rat supraoptic and paraventricular nuclei that contain estrogen receptor-β. Prog Brain Res. 2002;139:15–29. CrossRef
61. Acevedo-Rodriguez A, Mani SK, Handa RJ. Oxytocin and estrogen receptor β in the brain: an overview. Front Endocrinol. 2015;6:1–7. CrossRef
62. Pauciullo A, Ogah DM, Iannaccone M, Erhardt G, Di Stasio L, Cosenza G. Genetic characterization of the oxytocin-neurophysin I gene (OXT) and its regulatory regions analysis in domestic Old and New World camelids. PLoS One 2018;13(4):1–17. CrossRef
63. Drummond AE, Fuller PJ. The importance of ERβ signalling in the ovary. J Endocrinol. 2010;205:15–23. CrossRef
64. Haddad JJ. Antioxidant and prooxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell Signal. 2002; 14:879–97. CrossRef