INTRODUCTION
Parkinson’s disease (PD) is a degenerative brain disorder that displays both motor and non-motor symptoms, such as bradykinesia, rigidity, resting tremor, postural instability, autonomic dysfunction, depression, and sleep disorders [1]. As these symptoms and problems worsen, there is a substantial reduction in functioning and quality of life, as well as high rates of disability and care needs [2]. The disability and death rates of PD are increasing faster than those of other neurological disorders [3]. The pathological characteristic of PD is the progressive loss of dopaminergic neurons in the substantia nigra [4]. The precise mechanism of neuronal loss is still a mystery. Among the suggested mechanisms, increased oxidative stress and malfunction of the mitochondria are significant [5].
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a widely recognized dopaminergic neurotoxin, has the ability to produce clinical symptoms in a number of animal species that are similar to those of human parkinsonism [6]. PD in mice is frequently induced using this technique. The mechanism for PD development in this model has been previously outlined. Specifically, monoamine oxidase B converts MPTP to MPP+ in astrocytes. MPP+ preferentially enters dopaminergic neurons via the DA transporter, inhibiting mitochondrial respiration complex I, depleting ATP, and generating reactive oxygen species (ROS) [7]. Due to the high sensitivity of the brain to ROS, oxidative stress causes damage to dopaminergic neurons, thereby contributing to the development of PD [8]. Although many Parkinson’s medications can alleviate motor symptoms, PD can be prevented or delayed by inhibiting dopaminergic neuron death. Thus, there is significant interest in exploring treatment options for PD that involve neuroprotective substances capable of slowing or halting the neurodegenerative process.
Tiliacora triandra (Colebr) Diels (TT), also known as Ya-nang in Thai, belongs to the Menispermaceae family. It is a Southeast Asian native plant that grows organically and is commonly utilized in northeastern Thai cooking. This plant has been used in Thai traditional medicine for treating a variety of illnesses, including fever and malaria [9,10]. The TT aqueous extract contains phenolic components such as quercetin, cyanidin, and gallic acid [11]. Furthermore, TT has a high concentration of beta-carotene, tannin, triterpene, flavonoids, and saponins [12].
TT leaves revealed a number of pharmacological actions, including antimycobacterial [13], anti-inflammatory [14], hypoglycemic [15,16], anticancer [17], and antioxidant [18]. In mice given a high-fat diet, TT leaf water extract reduced hyperglycemia, hyperlipidemia, inflammation, and oxidative stress [19]. Ethanolic TT leaf microemulsion showed the benefit of anti-grey hair treatment via antioxidant and melanogenesis stimulating activities [20]. Furthermore, neuroprotective characteristics were reported in models of brain ischemia/reperfusion injury [21], ethanol dependence [11], and anticancer agent-induced neurotoxicity in both central and peripheral nervous systems [22,23]. However, research on the neuroprotective potential of TT leaf aqueous extract in the MPTP-induced PD mice model has not yet been conducted.
MATERIALS AND METHODS
Plant material and extract preparation
Fresh TT leaves were harvested from Maha Sarakham province in Thailand between August and September 2022. The leaves were carefully washed and dried in a hot air oven set to 60°C for 72 hours. After drying, they were blended into a powder. The leaf powder was boiled with distilled water at a 1:5 ratio for 10 minutes. The mixture was extracted using a rotary evaporator. Ultimately, a lyophilizer was used to freeze-dry the TT extract (TT), which was then kept at −20°C until used. The percentage extraction yield was 16.67%.
Animals and experimental protocols
A total of 40 male C57BL/6 mice weighing 25–30 g and aged 9 weeks were obtained from Nomura Siam International Co. Ltd. (Bangkok, Thailand). Mice were housed in controlled environments with free access to food and water, a 12-hour light/dark cycle, and a temperature of 25°C. The mice were divided into five groups (n = 8): (1) control group; mice received no treatment, (2) vehicle plus MPTP group; mice received distilled water plus MPTP injection, (3) TT 100 mg/kg BW plus MPTP injection, (4) TT 200 mg/kg BW plus MPTP injection, and (5) TT 400 mg/kg BW plus MPTP injection. This study used the acute intoxication model of PD as previously done by Jackson-Lewis and Przedborski [24]. Mice in groups (2)–(5) were administered with either TT or distilled water (vehicle) for a week (days 1–7) before and 7 days after receiving MPTP injection. MPTP was intraperitonially injected into mice 4 times every 2 hours at a dose of 20 mg/kg BW. On the seventh day following the MPTP injection, mice were examined for motor coordination in the beam and pole tests. The following day, all mice were sacrificed, and their right cerebral hemispheres were removed for immunohistochemical analysis to determine the dopaminergic neurons in the substantia nigra par compacta (SNpc). The striatum from the left hemisphere of the brain was isolated and homogenized, and the supernatant was collected. This supernatant was used to evaluate the expression of DA, the oxidative stress marker malondialdehyde (MDA), and antioxidant enzymes comprising reduced glutathione (GSH), catalase (CAT), and superoxide dismutase (SOD).
Assessments of motor function
Pole test
The wooden pole stands 50 cm tall and has a diameter of 8 mm. Each mouse was positioned with its head facing upwards. The duration of time each mouse spent turning its head downward (turning time) and the time taken to descend from the pole to the floor (descent time) were recorded [25]. Each mouse underwent the pole test 5 times. Impairment in coordination is suggested by the longer turning and descent times.
Beam walking test
The beam walking test was used to measure motor coordination in mice [26]. The wooden beams utilized in this study had diameters of 12 and 6 mm, and they extended 100 cm in length. The beam was positioned 50 cm above the floor atop two stands. Each mouse underwent two tests on the beam, positioned at 12 and 6 mm, respectively. The time taken by the mouse to successfully traverse the beam was recorded as the travel time. If the mouse fell off, the test was stopped and then restarted. Longer travel times to cross the beam indicated poorer motor coordination.
Measurement of oxidative stress marker and antioxidant activities
Malondialdehyde
Following sacrifice, the striatum of each group was collected and homogenized in 0.1 M phosphate-buffered saline (PBS) (pH 7.4). The samples were then centrifuged at 4°C at 15,000 × g for 15 minutes, and the supernatant was harvested for the thiobarbituric acid reaction [27]. The mixture consisted of 75 µl of supernatant sample or standard (1,1,3,3-tetraethoxypropane) (Cat. No. 108383, Merck KGaA), 10% TCA (Cat. No. 100807, Merck KGaA), 5 mM EDTA (Cat. No. AR1240, RCI Labscan Ltd.), 8% SDS (Cat. No. S/5200/53, Thermo Fisher Scientific, Inc.), and 0.5 µg/ml BHT (Cat. No. 02381, LOBA Chemie Pvt. Ltd.). After an additional 10 minutes of incubation at room temperature, 250 µl of 0.6% TBA (Cat. No. 108180, Merck KGaA) was added to the mixture and thoroughly mixed. After 30 minutes of heating in a water bath, the mixture was allowed to cool before being centrifuged at 10,000 × g for 5 minutes at 4°C. The absorbance was determined at 532 nm using a microplate reader (Synergy H1, BioTek Instruments, Inc.). MDA concentrations are given as µmol/mg protein.
Reduced glutathione
The oxidative stress response in the striatum supernatant was assessed by measuring GSH, which was done using the technique previously conducted by Polycarp et al. [28]. In brief, 0.1 N HCl was used to dilute 1 mM GSH (Cat. No. 70-18-8, Sigma-Aldrich, USA). Subsequently, 0.2, 0.4, 0.6, and 0.8 mM GSH were serially diluted to produce a standard curve. A phosphate buffer (pH 7.6) containing 1 mM 5,5'-dithio-bis-(2-nitrobenzoic acid) (Cat. No. 69-78-3, Merck, USA) was then added to each serial dilution or striatum supernatant. After 5 minutes of dark incubation at room temperature, the absorbance of the mixture was measured using a microplate reader set at 412 nm. The GSH values are shown as U/mg protein.
Catalase
In this study, the activity of CAT was measured using the method previously reported [29]. Briefly, 20 µl of striatum supernatant and 100 µl of 6 mM hydrogen peroxide (H2O2) were mixed for the reaction. After one minute of incubation, the reaction was stopped by adding 100 µl of 32.4 mM ammonium molybdate. The absorbance at 405 nm was then measured using a microplate reader. The CAT activity of the striatum supernatant is expressed as U/mg protein.
Superoxide dismutase
The striatum supernatant’s SOD activity was determined using a test kit (Cat. No. S19160, Sigma-Aldrich; Merck KGaA). The procedures were carried out in compliance with the manufacturer’s instructions. The inhibition rate (%) was used to display the SOD activity.
Measurement of DA content
An enzyme-linked immunosorbent assay kit (Cat. No. E-EL-0046, Elabscience Biotechnology Co. Ltd; Texas, USA) was employed to quantify the amount of DA in the striatum supernatant. The assay procedures were conducted according to the manufacturer’s instructions. The DA content is expressed as pg/mg protein.
Immunohistochemistry of the dopaminergic neurons in the SNpc
Mice were anesthetized with an intraperitoneal dose of sodium thiopental (60 mg/kg BW), then perfused with a cool 0.9% normal saline solution, and their brains were promptly removed. The right hemispheres of the brain were fixed in 4% paraformaldehyde in 0.1 M PBS and then immersed for 48 hours at 4°C in a 30% sucrose solution. The brains were then sectioned into 30 μm coronal sections using a cryostat microtome (CM1950; Leica, Nussloch, Germany). Following a 10-minute treatment with 0.3% hydrogen peroxide, selected brain slices were rinsed in PBS and then incubated overnight at 4°C with anti-TH (anti-Tyrosine hydroxylase (TH); 1:500; Sigma-Aldrich, CA, USA) primary antibodies in PBS containing 0.3% Triton X-100 and 0.5% bovine serum albumin. The sections were washed with PBS and treated for 2 hours at room temperature with anti-rabbit biotinylated secondary antibody (1:500; Jackson Immunoresearch, CA, USA) in PBS containing 0.3% Triton X-100. Subsequently, the avidin-biotin-horseradish peroxidase complex solution (Sigma-Aldrich, CA, USA) was incubated for 1 hour at room temperature. After that, the sections were stained with 3,3′-diaminobenzidine tetrahydrochloride. After staining, the sections were mounted on positively charged slides, dehydrated with ethanol, cleared with xylene, and cover-slipped with permount (Cat. No. SP15-500, Fisher Scientific, Leicestershire, UK). The TH-positive (TH+) neurons in each group were examined under a light microscope, and pictures of TH+ neurons were taken at 4X magnification. After that, the number of TH+ neurons was determined using Image J.
Statistical analysis
Results were shown as means ± standard error of the mean (SEM). To assess the significance of differences between groups, a one-way analysis of variance with Tukey’s post hoc test was used in the statistical analysis, which was carried out using GraphPad Prism 9 (GraphPad Software, Inc.). A p-value of less than 0.05 denotes a significant difference.
RESULTS
Effect of TT on motor function tests
The results showed that MPTP significantly increased the travel time on the 12 mm beam in all MPTP-injected groups compared to the control group. The TT treatment with all dosages reversed this motor impairment (Fig. 1A). The results from the 6 mm beam test demonstrated that the vehicle-treated group spent more time traveling across the beam compared to the control group. All dosages of TT significantly decreased the travel time compared to the vehicle-treated group (Fig. 1B). In the pole test, MPTP administration significantly increased the turning time and descent time in the vehicle-treated group compared to the control group (p < 0.01 and p < 0.05). The TT treatment at doses of 200 and 400 mg/kg BW significantly reduced descent time (p < 0.05 and p < 0.01) (Fig. 2B). However, there was no significant difference between the TT-treated groups compared to the vehicle-treated group in the turning time (Fig. 1A).
Effect of TT on MDA levels and antioxidant enzymes
Mice administered with the vehicle and injected with MPTP exhibited significantly higher levels of MDA compared to the control group (p < 0.001). Conversely, mice receiving TT at doses of 200 and 400 mg/kg BW demonstrated decreased MDA levels compared to the vehicle-treated mice plus MPTP (p < 0.01) (Fig. 3A). The MPTP injection significantly reduced the activities of GSH, CAT, and SOD (p < 0.001) in the vehicle-treated group as compared to the control group (Fig. 3B–D). However, TT treatment attenuated the decrease in antioxidant enzymes. As compared to the vehicle-treated group plus MPTP, treatment with TT at a dose of 100 mg/kg BW significantly increased the activity of GSH (p < 0.05), whereas the medium dose (200 mg/kg BW) increased the activities of GSH and SOD (p < 0.05 and p < 0.001). Additionally, only the high dose of TT (400 mg/kg BW) significantly increased the activities of GSH, CAT, and SOD compared to the vehicle-treated group plus MPTP.
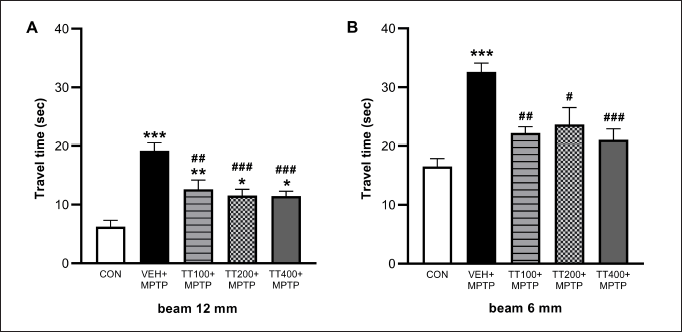 | Figure 1. MPTP-induced increased travel times both in 12 mm (A) and 6 mm (B) of beam test. The motor functions were observed on the 7th day after the MPTP injection. TT-treated groups exhibited less travel time in the beam test as compared to the vehicle-treated + MPTP group. Results were expressed as mean ± SEM (n = 8), *** p < 0.001, ** p < 0.01, * p < 0.05 compared to the control group; ### p < 0.001, ## p < 0.01, and # p < 0.05 compared to the vehicle-treated + MPTP group. [Click here to view] |
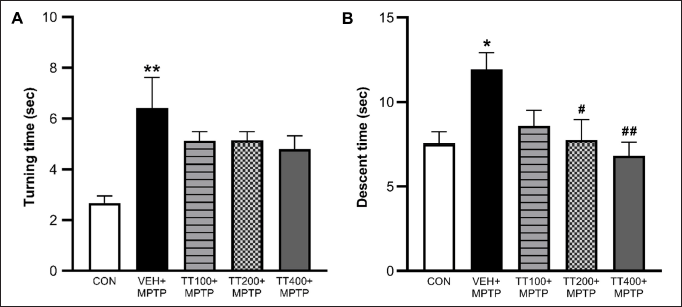 | Figure 2. MPTP-induced increased turning time (A) and descent time (B) in the pole test. The motor functions were observed on the 7th day after MPTP injection. TT-treated groups exhibited less descent time in the pole test as compared to the vehicle-treated + MPTP group. Results were expressed as mean ± SEM (n = 8), ** p < 0.01, * p < 0.05 compared to the control group; ## p < 0.01, # p < 0.05 compared to the vehicle-treated + MPTP group. [Click here to view] |
Effect of TT on TH-positive neurons
Figure 4 demonstrates the TH-positive neuron counted at the SNpc. In all MPTP-injected groups, TH-positive neurons in the SNpc are significantly reduced by MPTP (p < 0.001) as compared to the control group. However, a high dose of TT (400 mg/kg BW) significantly reduced neuronal loss, resulting in more TH-positive neurons compared to the vehicle-treated group with MPTP (p < 0.05).
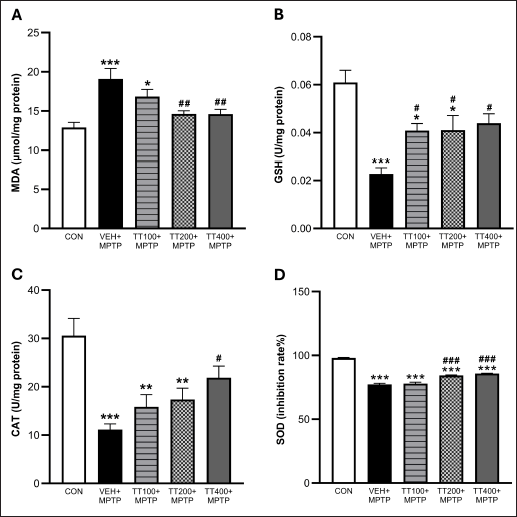 | Figure 3. TT treatment attenuated lipid peroxidation as demonstrated by decreased MDA (A). TT treatment attenuated MPTP-induced oxidative stress by increasing GSH, CAT, and SOD activities (B–D). Results were expressed as mean ± SEM (n = 8), *** p < 0.001, ** p < 0.01, * p < 0.05 compared to the control group; ### p < 0.001, ## p < 0.01, # p < 0.05 compared to the vehicle-treated + MPTP group. [Click here to view] |
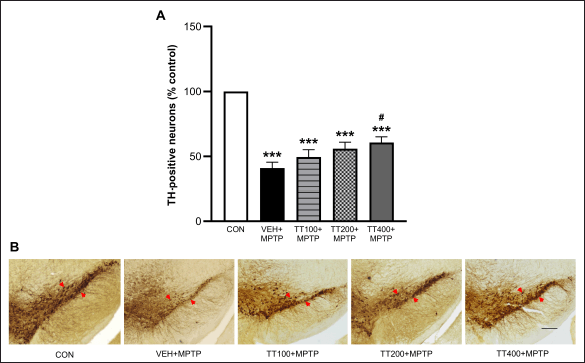 | Figure 4. TH staining of SNpc presented as a number of neurons in the percentage of control (A). Results were expressed as mean ± SEM (n = 8), *** p < 0.001 compared to the control group; # p < 0.05 compared to the vehicle-treated + MPTP group. Representative photomicrographs of TH-positive neurons in the SNpc at 4X magnification, the scale bar is 200 μm, and the arrows indicate the TH-positive neurons (B). [Click here to view] |
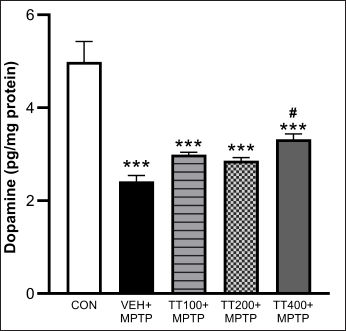 | Figure 5. TT on DA expression in the striatum homogenate. Results were expressed as mean ± SEM (n = 8), *** p < 0.001 compared to the control group; ## p < 0.05 compared to the vehicle-treated + MPTP group. [Click here to view] |
Effect of TT on DA levels
The striatum’s DA levels were considerably lowered by MPTP injection, as demonstrated in Figure 5. The vehicle-treated group with MPTP had lower levels of DA compared to the control group (p < 0.001). Mice treated with TT at 400 mg/kg BW had significantly greater levels of DA than the vehicle-treated group (p < 0.05).
DISCUSSION
The effects of TT on motor functions and dopaminergic neurons were investigated in C57BL/6 mice treated with MPTP. The findings revealed that TT significantly reduced motor deficit and dopaminergic neuron degeneration by lowering MDA levels, boosting scavenging enzymes, and elevating DA levels.
Systemic administration, particularly intraperitoneal injection of MPTP, is a reliable and reproducible method for inducing PD [24]. Oxidative stress, generated by MPP+-induced mitochondrial dysfunction, has been connected to the underlying mechanisms of MPTP-induced degeneration of dopaminergic neurons in the nigrostriatal pathway [30]. The findings of the current study are in line with previous investigations. MPTP raises MDA levels while lowering the activity of scavenging enzymes. TT treatment can reduce MDA levels and increase scavenging enzyme activities. It was reported that the degeneration of dopaminergic neurons lowers DA expression [31]. DA is an important neurotransmitter that controls motor function particularly DA in the nigrostriatal pathway [32]. The decrease of DA in the SNpc is a pathogenic feature identified in PD brains [33]. Previous studies linked decreased antioxidant enzyme levels and increased oxidative stress to the degenerated dopaminergic neurons in the SNpc [34,35]. Hence, the present study investigated DA expression and TH immunohistochemistry. The findings show that MPTP significantly lowered the amount of DA in the striatum and the number of TH+ neurons in the SNpc. TT treatment mitigated MPTP-induced dopaminergic neuronal death, as evidenced by an increase in the number of TH+ neurons in the SNpc, resulting in enhanced DA levels in the striatum. Therefore, higher DA levels in the nigrostriatal pathway were related to improved motor performance after MPTP-induced PD.
Motor dysfunction can arise from dopaminergic neuron degeneration. The longer travel time in the beam test and the longer descent time in the pole test indicated that MPTP injection resulted in delayed movement and decreased motor coordination. These deficits were significantly lessened with the TT treatment. This study found that TT decreased the oxidative stress produced by MPTP injection, which in turn decreased deaths of dopaminergic neurons in the substantia nigra and increased DA levels. Consequently, the increased availability of DA in the nigrostriatal pathway enhanced motor performance.
Our study found that TT has neuroprotective properties, which are consistent with previous findings. Phunchago et al. [11] showed that TT ameliorates ethanol-induced neurotoxicity. They proposed that TT’s putative neuroprotective benefits could be attributed to the extract’s high concentration of gallic acid, cyanidin, and quercetin [11]. Furthermore, Thong-asa and Bullangpoti [21] found that TT exhibits neuroprotective properties against neuronal death through its antioxidant action in the cerebral ischemia-reperfusion mice model. Huang et al. [22] revealed that TT can ameliorate cisplatin-induced neurotoxicity by restoring redox balance and anti-inflammatory characteristics of the TT hydro-ethanolic extract. Recently, our TT exhibited the neuroprotective effect in the scopolamine-induced memory deficit by increasing antioxidant enzyme activities and inhibiting acetylcholinesterase [36]. According to liquid chromatography electrospray ionization quadrupole time-of-flight mass spectrometry (LC-ESI-QTOF-MS/MS) analysis, our TT comprises polyphenols such as verbasoside, caffeic acid, catechin, piperine, and 6-gingerol, along with essential and nonessential amino acids including l-arginine, l-histidine, l-valine, l-leucine, d-tryptophan, l-tyrosine, l-glutamine, and choline [36]. Several studies have examined these active components in the context of PD animal models, using a variety of approaches. It was demonstrated that piperine treatment attenuated MPTP-induced deficits in motor coordination and cognitive function [37]. The neuroprotective effect of (±)-catechin against the toxicity of MPTP was also reported [38]. Furthermore, caffeic acid exhibited a neuroprotective effect on the striatum of MPTP-treated mice by inhibiting the inflammatory injury [39]. Thus, these components may be responsible for the neuroprotective effect of the TT leaf extract. Nevertheless, there is insufficient data to identify the precise bioactive ingredient in the TT that provides neuroprotection.
CONCLUSION
The findings of this study demonstrated that TT had neuroprotective benefits by lowering MDA levels, increasing scavenging enzymes, and elevating DA levels, resulting in improved motor performance in the C57BL/6 mice. Consequently, TT may be considered for medicinal use in treating PD through a neuroprotective approach. Nevertheless, before beginning pre-clinical and clinical trials involving individuals with PD, additional investigation into the safety of the extract needs to be conducted.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research project was supported by the University of Phayao and Thailand Science Research and Innovation Fund (Grant No. FF66-RIM064).
CONFLICTS OF INTEREST
The authors declare no financial or other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Laboratory Animal Research Center of the University of Phayao, Thailand (Approval No.1-021-65).
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Prim. 2017;3(1):17013. CrossRef
2. Shulman LM. Understanding disability in Parkinson’s disease. Mov Disord. 2010;25 Suppl 1:S131–5. CrossRef
3. Feigin VL, Vos T, Nichols E, Owolabi MO, Carroll WM, Dichgans M, et al. The global burden of neurological disorders: translating evidence into policy. Lancet Neurol. 2020;19(3):255–65. CrossRef
4. Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M, et al. Missing pieces in the Parkinson’s disease puzzle. Nat Med. 2010;16(6):653–61. CrossRef
5. Maiti P, Manna J, Dunbar GL. Current understanding of the molecular mechanisms in Parkinson’s disease: targets for potential treatments. Transl Neurodegener. 2017;6:28. CrossRef
6. Chia SJ, Tan EK, Chao YX. Historical perspective: models of Parkinson’s disease. Int J Mol Sci. 2020;21(7):2464. CrossRef
7. Marti Y, Matthaeus F, Lau T, Schloss P. Methyl-4-phenylpyridinium (MPP+) differentially affects monoamine release and re-uptake in murine embryonic stem cell-derived dopaminergic and serotonergic neurons. Mol Cell Neurosci. 2017;83:37–45. CrossRef
8. Mamelak M. Parkinson’s disease, the dopaminergic neuron and gammahydroxybutyrate. Neurol Ther. 2018;7(1):5–11. CrossRef
9. Petrakard P. YA NANG Tiliacora triandra (Colebr.) Diels. J Thai Trad Alt Med. 2016;14(1):73–4.
10. Singthong J, Oonsivilai R, Oonmetta-aree J, Ningsanond S. Bioactive compounds and encapsulation of Yanang (Tiliacora Triandra) leaves. Afr J Tradit Complement Altern Med. 2014;11(3):76–84. CrossRef
11. Phunchago N, Wattanathorn J, Chaisiwamongkol K. Tiliacora triandra, an anti-intoxication plant, improves memory impairment, neurodegeneration, cholinergic function, and oxidative stress in hippocampus of ethanol dependence rats. Oxid Med Cell Longev. 2015;2015:918426. CrossRef
12. Boonsong P, Laohakunjit N, Kerdchoechuen O. Identification of polyphenolic compounds and colorants from Tiliacora triandra (Diels) Leaves. Agric Sci J. 2009;40:13–6.
13. Sureram S, Senadeera SPD, Hongmanee P, Mahidol C, Ruchirawat S, Kittakoop P. Antimycobacterial activity of bisbenzylisoquinoline alkaloids from Tiliacora triandra against multidrug-resistant isolates of Mycobacterium tuberculosis. BMCL. 2012;22(8):2902–5. CrossRef
14. Weerawatanakorn M, Rojsuntornkitti K, Pan M-H, Wongwaiwech D. Some phytochemicals and anti-inflammation effect of juice from Tiliacora triandra leaves. J Food Nutr Res. 2018;6(1):32–8. CrossRef
15. Katisart T, Rattana S. Hypoglycemic activity of leaf extracts from Tiliacora triandra in normal and streptozotocin-induced diabetic rats. Pharmacogn J. 2017;9(5):621–5. CrossRef
16. Makinde EA, Radenahmad N, Adekoya AE, Olatunji OJ. Tiliacora triandra extract possesses antidiabetic effects in high fat diet/streptozotocin-induced diabetes in rats. J Food Biochem. 2020;44(6):e13239. CrossRef
17. Rattana S, Cushnie B, Taepongsorat L, Phadungkit M. Chemical constituents and in vitro anticancer activity of Tiliacora triandra leaves. Pharmacogn J. 2016;8(1):1–3. CrossRef
18. Chaveerach A, Lertsatitthanakorn P, Tanee T, Puangjit N, Patarapadungkit N, Sudmoon R. Chemical constituents, antioxidant property, cytotoxicity and genotoxicity of Tiliacora Triandra. Int J Pharm Phyto Res. 2016;8(5):722–9.
19. Nanna U, Naowaboot J, Chularojmontri L. Effects of Tiliacora triandra leaf water extract in high-fat diet fed mice. J Med Assoc Thai. 2017;100(6):78–84.
20. Soradech S, Kusolkumbot P, Thubthimthed S. Development and characterization of microemulsions containing Tiliacora triandra diels as an active ingredient for antioxidant and melanogenesis stimulating activities. J App Pharm Sci. 2018;8(3):46–54. CrossRef
21. Thong-asa W, Bullangpoti V. Neuroprotective effects of Tiliacora triandra leaf extract in a mice model of cerebral ischemia reperfusion. Avicenna J Phytomed. 2020;10(2):202–12. CrossRef
22. Huang Y, Liu C, Song X, An M, Liu M, Yao L, et al. Antioxidant and anti-inflammatory properties mediate the neuroprotective effects of hydro-ethanolic extract of Tiliacora triandra against cisplatin-induced neurotoxicity. J Inflamm Res. 2021;14:6735–48. CrossRef
23. Liu C, Ma M, Wen C, Uz Zaman R, Olatunji OJ. Antiallodynic and anti-hyperalgesia effects of Tiliacora triandra against cisplatin-induced peripheral neuropathy. All Life. 2021;14(1):441–9. CrossRef
24. Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2(1):141–51. CrossRef
25. Matsuura K, Kabuto H, Makino H, Ogawa N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods. 1997;73(1):45–8. CrossRef
26. Luong TN, Carlisle HJ, Southwell A, Patterson PH. Assessment of motor balance and coordination in mice using the balance beam. J Vis Exp. 2011;49:2376. CrossRef
27. Nakmareong S, Kukongviriyapan U, Pakdeechote P, Donpunha W, Kukongviriyapan V, Kongyingyoes B, et al. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with L-NAME-induced hypertension. Naunyn Schmiedebergs Arch Pharmacol. 2011;383(5):519–29. CrossRef
28. Polycarp TN, Obukowho BE, Yusoff SM. Changes in haematological parameters and oxidative stress response of goats subjected to road transport stress in a hot humid tropical environment. Comp Clin Pathol. 2015;25(2):285–93. CrossRef
29. Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196(2-3):143–51. CrossRef
30. Chang KH, Chen CM. The role of oxidative stress in Parkinson’s disease. Antioxidants (Basel). 2020;9(7):597. CrossRef
31. Zhou ZD, Yi LX, Wang DQ, Lim TM, Tan EK. Role of dopamine in the pathophysiology of Parkinson’s disease. Transl Neurodegener. 2023;12(1):44. CrossRef
32. Perez-Fernandez J, Barandela M, Jimenez-Lopez C. The dopaminergic control of movement-evolutionary considerations. Int J Mol Sci. 2021;22(20);11284. CrossRef
33. Guatteo E, Berretta N, Monda V, Ledonne A, Mercuri NB. Pathophysiological features of nigral dopaminergic neurons in animal models of Parkinson’s disease. Int J Mol Sci. 2022;23(9):4508. CrossRef
34. Ni A, Ernst C. Evidence that substantia nigra pars compacta dopaminergic neurons are selectively vulnerable to oxidative stress because they are highly metabolically active. Front Cell Neurosci. 2022;16:826193. CrossRef
35. Guo JD, Zhao X, Li Y, Li GR, Liu XL. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease (Review). Int J Mol Med. 2018;41(4):1817–25. CrossRef
36. Hawiset T, Sriraksa N, Kamsrijai U, Praman S, Inkaew P. Neuroprotective effect of Tiliacora triandra (Colebr.) diels leaf extract on scopolamine-induced memory impairment in rats. Heliyon. 2023;9(12):e22545. CrossRef
37. Yang W, Chen YH, Liu H, Qu HD. Neuroprotective effects of piperine on the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease mouse model. Int J Mol Med. 2015;36(5):1369–76. CrossRef
38. Ruan H, Yang Y, Zhu X, Wang X, Chen R. Neuroprotective effects of (+/-)-catechin against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity in mice. Neurosci Lett. 2009;450(2):152–7. CrossRef
39. Tsai SJ, Chao CY, Yin MC. Preventive and therapeutic effects of caffeic acid against inflammatory injury in striatum of MPTP-treated mice. Eur J Pharmacol. 2011;670(2-3):441–7. CrossRef