INTRODUCTION
Scrophularia buergeriana Miq., belonging to the genus Scrophularia (Scrophulariaceae), is also known as figwort, bei xuan shen, hac sam (black ginseng), or nguyen sam because of its bitter taste as ginseng and black color [1]. This species is mainly found in Northern China, Korea, Manchuria, and Japan. It is noted as a strongly fragrant perennial or biennial plant that plays an important role in traditional medicine in several Eastern country cultures because of its diverse biological activities [2]. The flowers of S. buergeriana Miq. are pale yellow–white in color, which is different from S. ningpoensis Hemsl., whose flowers grow in umbels and are purple. While extensive research has been conducted on S. ningpoensis Hemsl., studies on S. buergeriana Miq. are still in their early stages. Scrophularia buergeriana Miq. is known for its slightly cold taste and has been utilized as an anti-inflammatory agent in the treatment of tonsillitis, pharyngitis, swelling, and laryngitis. In addition, it exhibits antibiotic properties against various strains of skin bacteria and is applied for the alleviation of constipation [1,3]. Besides, it also helps cure fever, lower blood sugar, neuritis, and reduce beta-amyloid-induced neurotoxicity to improve memory impairment and dilate blood vessels [4].
Counterfeit products and poor-quality pharmaceuticals have taken advantage of and caused negative effects on consumers both in terms of health and finance. To prevent the manufacture and trade of counterfeit products and low-quality medicinal materials, it is necessary to conduct inspections and quality control on medicinal herbs and herbal preparations. One of the most accurate and effective methods currently used to control the quality of medicinal herbs is the use of reference standards, both isolated from natural sources and synthesized in laboratories. They have played critical roles in the quality system and trace analysis of products containing medicinal plants. Harpagide and harpagoside are well-known as reference standards for S. buergeriana. However, they are purchased from global companies (such as International Pharmacopeia, United States Pharmacopeia, European Pharmacopeia, British Pharmacopoeia, Sigma, Chromadex reference standards, and so on) at high costs with delivery time lasting weeks or months. In addition, there is also the risk of not finding suitable reference standards, potentially causing delays and errors in the analysis and testing of medicinal materials. Remarkably, we identified a significant proportion of trans-cinnamic acid in this species which could be established as the reference standard.
In this study, we described the isolation and structural elucidation of trans-cinnamic acid from the roots of S. buergeriana. The purity of the compound was determined by spectroscopic and high-performance liquid chromatography (HPLC) methods. trans-Cinnamic acid was subsequently established as the reference standard to control the quality of medicinal herbs and pharmaceuticals following standard references [5,6]. The homogeneity and assigned values were also evaluated.
MATERIALS AND METHODS
General experimental procedures
Nuclear magnetic resonance (NMR) was recorded on a Bruker Avance III 500 [500 MHz (1H) and 125 MHz (13C)] with CDCl3 as the solvent. The HPLC method was developed on Shimadzu CBM20A (Japan). The IR and Ultraviolet spectrum (UV) spectra were performed by using iS50FT-IR and Shimadzu UV-2700, respectively. The melting point was defined by Wagner and Munz Polytherm A.
Thin layer chromatography (TLC) was performed on silica gel F254 (250 µm, Merck) or Rp-18 F254s (Merck). Components on TLC were detected using an ultraviolet lamp (wavelength 254/365 nm, CN-15-LC-Vilber Loumart. Column chromatography (CC) was conducted by using silica gel 60 (40-63 μm, Merck) and silica gel Rp-18 (40-63 μm, Merck).
n-Hexane, acetone, and ethyl acetate were provided by J. T. Baker, ethanol was obtained from Merck. Methanol for HPLC standards was purchased from Supelco.
Plant material
Dried roots of S. buergeriana Miq. were purchased from OPC Pharmaceutical Joint Stock Company with a net weight of 10 kg. The medicinal sample was tested according to Vietnamese Pharmacopoeia V standards [7].
Extraction and isolation
The ground-dried roots of S. buergeriana (10 kg) were homogenized in 30 l of ethanol at room temperature. The solution was then filtered and evaporated under reduced pressure to give an ethanolic crude extract. The crude extract was suspended in water and extracted with ethyl acetate using a liquid–liquid extraction method, followed by rotary evaporation to yield an ethyl acetate extract (154.7 g).
The ethyl acetate extract was subjected to silica gel CC using gradient elution with n-hexane-acetone (0%–100%) to give 12 fractions (RSE1-12). Fraction RSE5 (10.05 g) was chromatographed on silica gel with n-hexane-acetone (0%–100%) as eluent to obtain 13 fractions (RSE5.1-13). By recrystallization technique, fraction RSE5.5 (7.29 g) was dissolved in acetone to yield solution RSE 5.5.1 (1.61 g) and RSE5.5.2 (5.76 g) precipitated in acetone. Fraction RSE5.5.2 was recrystallized and further purified by CC using RP-18 with acetone-isopropanol-MeOH-H2O (1:1:3:5) to obtain trans-cinnamic acid (1.196 g).
Method validation and establishment of reference substance
The purity of trans-cinnamic acid was verified by TLC with three different mobile phases on silica gel F254 and Rp-18 plates. The compound is pure when the plates appear only in a single spot.
The compound was tested for its purity by the HPLC technique which was determined based on the % peak area of the analyte obtained in the chromatography. Mobile phase: phosphoric acid solution 0.1%—acetonitrile (linear gradient from 0% to 90% acetonitrile in 60 minutes), Gemini column C18 (250 × 4.6 mm, 5 µm), Photodiode array detection detector with detection wavelength set up at 272 nm, flow rate: 1.0 ml/minute, and injection volume: 20 µl, analysis time: 70 minutes. The test solution was prepared by dissolving 5 mg of the examination sample with 25 ml of methanol (MeOH) in a 50 ml volumetric flask, sonicating the sample completely, adding MeOH to the volumetric mark, and shaking to obtain a solution which the corresponding concentration was 0.1 mg/ml. Blank solution: MeOH solvent which was filtered through a 0.45 µm filtration membrane.
The test and blank solution sequentially were injected into the chromatography system and recorded by the chromatogram. Ignore all secondary peaks in the test solution with retention times corresponding to the peaks in the blank solution and peaks with areas smaller than the limit of quantity (LOQ) in the method validation when viewing the chromatogram at the detection wavelength of refined products. A compound was considered pure when the ratio of the main peak area of the test solution in the chromatogram reached > 95%.
Check the quality of raw materials according to established standards
Conduct experiments on sensory properties, measuring IR, UV, NMR spectrum, moisture, sulfate ash, and determining percent (%) purity HPLC calculated on anhydrous products.
Packing
Distribute a certain amount of sample into brown glass vials in an inert gas environment (nitrogen gas) with humidity < 30%RH using a Glove-box device, then seal the lid.
Determine the homogeneity of the vial filling process (ISO 17034) [5]
Samples were randomly taken from 10 to 30 units using Excel software. Determination of the purity of the substances was carried out according to the developed and validated HPLC method, each vial was determined twice. The homogeneity of the vial filling process was confirmed based on ISO 13528 guidance [6].
The homogeneity value of interlaboratory consistency
This experiment was performed by one-factor analysis of variance ANOVA (p = 0.95). Test samples that fulfill requirements from the above process randomly and conduct HPLC purity determination at three independent laboratories conformed to GLP (good laboratory practice) or ISO/IEC 17025 main principles [6].
The assigned value and measurement uncertainty
Follow the instructions of ISO 13528:2005 based on the valuation of laboratory results [6].
RESULTS AND DISCUSSION
Structure elucidation of trans-cinnamic acid
From the ethyl acetate of the roots of S. buergeriana, a purified compound was isolated as a white flake crystal. Mp. 124°C–128°C. The UV spectrum (in MeOH) showed maxima at λmax 274, 215, and 204 nm. The infrared spectrum (IR) exhibited characteristic absorption at νmax 3064 (O-H), 2525 and 2343 (C-H), 1693 (C = O), and 1622 (C = C) cm-1. The 1H NMR spectrum (Table 1) revealed signals corresponding to two trans-olefinic protons [δH 7.81 (1H, d, J= 16.0 Hz, H-3) and 6.47 (1H, d, J= 16.0 Hz, H-2)] and a monosubstituted benzene ring [δH 7.56 (2H, m, H-2′, H-6′) and 7.41 (3H, m, H-3′, H-4′, H-5′)]. The 13C NMR spectrum (Table 1) showed resonances for a carbonyl carbon [δC 172.3 (s, C-1)], two olefinic carbons [(δC 147.1 (d, C-3) and 117.3 (d, C-2)] and six aromatic carbons [δC 134.1 (s, C-1′), 130.8 (d, C-4′), 129.0 (d, C-3′, C-5′) and 128.4 (d, C-2′, C-6′)]. Based on the elucidation of the spectral data and comparison with relevant literature [8], the isolated compound was identified as trans-cinnamic acid (also called 3-phenylacrylic acid) (Fig. 1).
Method validation and establishment of trans-cinnamic acid as reference standard
Purity determination
Thin-layer chromatography: Each test sample in three different conditions gives exactly one single spot at a wavelength of 254 nm on the TLC. It can be concluded the purity of trans-cinnamic acid sample on TLC (Fig. 2).
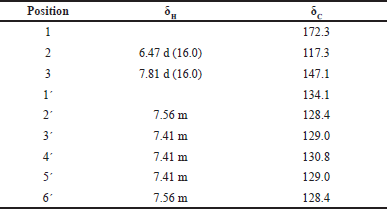 | Table 1. 1H and 13C NMR (CDCl3) data of trans-cinnamic acid. [Click here to view] |
Condition 1: Silica gel, chloroform – EtOAc (8:2), Rf = 0.25 (Fig. 2a);
Condition 2: Silica gel, n-hexane – acetone (7:3), Rf = 0.5 (Fig. 2b);
Condition 3: Silica gel Rp-18, acetone – H2O (8:2), Rf = 0.75 (Fig. 2c).
HPLC method: trans-Cinnamic acid achieved the standard requirement of purity standard more than 95%, according to ASEAN standards, so it was chosen as the material to establish the reference standard (Fig. 3).
System suitability
Verified by six repeated injections of a standard solution with a concentration of approximately 0.1 mg/ml. The results showed the suitability for analyzing trans-cinnamic acid compounds (Table 2).
Selectivity
Determined by comparing the chromatogram of the blank sample, the test sample, along with the 10 ml test samples separately added with 1 ml NaOH 1N, HCl 1N, and H2O2 10 % solutions, and the test sample was incubated at 70°C for 24 hours. The chromatogram of the blank did not show a peak with a retention time corresponding to the analytical peak. Based on the chromatogram superposition results, the trans-cinnamic acid peak is completely separated from other impurity peaks that were tested under four different stress conditions. This procedure achieved the requirements of specificity. The results of spectral superposition are shown in Figure 4.
 | Figure 1. The chemical structure of trans-cinnamic acid. [Click here to view] |
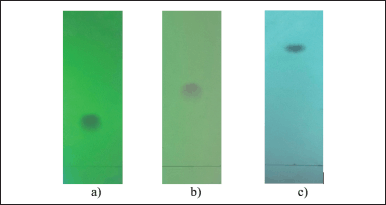 | Figure 2. TLC of trans-cinnamic acid sample in three different conditions. [Click here to view] |
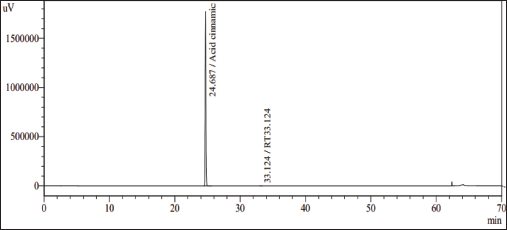 | Figure 3. HPLC chromatography of trans-cinnamic acid. [Click here to view] |
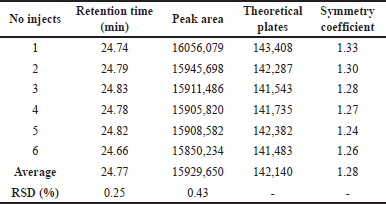 | Table 2. The parameters of system suitability testing. [Click here to view] |
Linearity
Investigating the correlation between x (concentration) and y (peak area) using the least squares method, the results showed a close correlation between concentration and peak area (R is in the range of 0.998—1,000). Based on the calibration curve, determine the regression equation y = 159685292 × + 221886 (R2 = 0.9995), the limit of detection and LOQ values of the method were 0.005 mg/ml and 0.014 mg/ml, respectively (Fig. 5).
Precision (including repeatability and intermediate precision)
The method achieves repeatability relative standard deviation (RSD) (%) = 0.03% ≤ 2% with n = 6 (day 1) and intermediate precision RSD (%) = 0.05% ≤ 2% with n = 12 (for 2 days) (Table 3).
Accuracy
Accuracy demonstrates the recovery rate of the values measured based on the regression equation compared to the actual value of isolated trans-cinnamic acid at the corresponding investigation. The results showed that at all three different concentrations investigated, the recovery efficiency was in the range of 98%—102%. The method indicated the accuracy requirements (Table 4).
Establishment of trans-cinnamic acid as reference standard
Material evaluation
The isolated trans-cinnamic acid achieves the reference material requirements to establish a standard substance according to WHO TRS 943 (Table 5).
Homogeneity and interlaboratory comparison
Proceed packing 150 vials, each vial contains 5 mg. Take 10 random vials to check homogeneity to ensure the results are the same between vials. Each vial is analyzed twice to determine purity. Trial the homogeneity between vials using the statistical method of one-factor ANOVA analysis. Evaluating the homogeneity between vials according to the statistical method of one-factor ANOVA analysis gives the result Fexperimental = 0.13 < Ftheoretical = 4.41. Therefore, it is concluded that there is homogeneity between vials (Table 6).
Randomly take 6 other vials and send them to 3 independent analytical laboratories to analyze trans-cinnamic acid. The assessment of vial homogeneity among the 3 laboratories was performed through quantitative results between the three laboratories. The homogeneity was tested by the statistical method of one-factor ANOVA analysis. Based on the results of the one-factor ANOVA statistical analysis method, a comparison between the two values showed that Fexperimental = 0.798 < Ftheoretical = 3.682, proving the quantitative results between the 3 other laboratories. The difference is not statistically significant. The procedure for analyzing trans-cinnamic acid content is highly repeatable, the analyte content does not depend on the laboratory participating in the evaluation (Table 7).
Assigned value
The value is determined from the percentage purity results of 3 laboratories. Conformed to ISO 13528 statistics, xaverage, s* values, and calculation of the assigned value of trans-cinnamic acid content (n = 18) are confirmed by statistical processing. Trans-cinnamic acid is eligible to register as a national standard with the assigned value of 99.60% on anhydrous substance (n = 18, s = 0.06) and the measurement uncertainty is 0.13%.
 | Figure 4. HPLC chromatogram of trans-cinnamic acid. Blank, 2, 3, 4, 5, 6. Test solution treated with H2O2, HCl, NaOH, and high temperature, respectively. [Click here to view] |
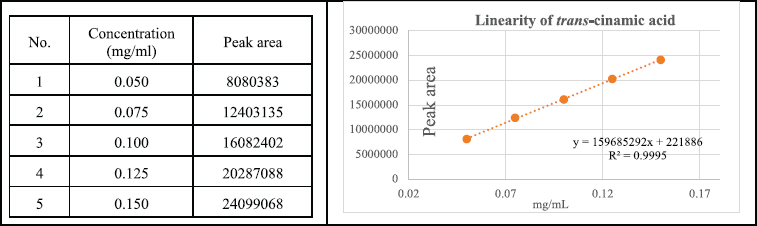 | Figure 5. Calibration curve of trans-cinnamic acid. [Click here to view] |
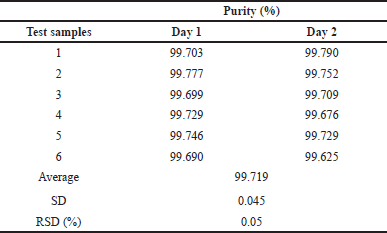 | Table 3. Comparison results of repeatability and intermediate precision. [Click here to view] |
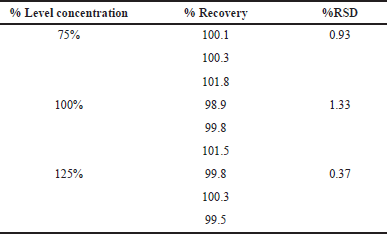 | Table 4. Results of the percentage recovery efficiency. [Click here to view] |
 | Table 5. Results of materials evaluation according to reference standards. [Click here to view] |
 | Table 6. Measurement results for homogeneity test. [Click here to view] |
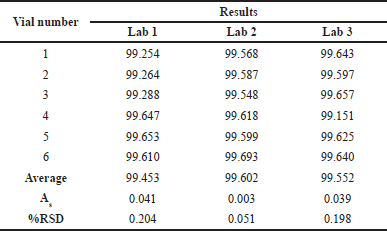 | Table 7. Results of inter-laboratory vial homogeneity assessment. [Click here to view] |
CONCLUSION
From the ethyl acetate extract of the roots of S. buergeriana Miq., a pure compound was isolated and determined to be trans-cinnamic acid. The compound was analyzed by the HPLC method with purity > 95% and chosen to establish the reference standard. The construction process achieves the requirements for system compatibility, linearity, specificity, precision, repeatability, and intermediate precision. The trans-cinnamic acid standard derived from the medicinal herb S. buergeriana with a spectral dataset including UV, IR, NMR spectra, and basic standards that attain the requirements of national standards, has been established to contribute to the list of standard substances of the Institute of Drug Quality control of Ho Chi Minh City, Vietnam. In conclusion, the study presents the successful establishment of the chemical reference compound, trans-cinnamic acid, to serve the quality testing of medicinal products containing roots of S. buergeriana Miq. circulated. The discovery has shown a significant contribution to the quality control of medicinal herbs and their products on the market.
LIST OF ABBREVIATIONS
d Doublet
HPLC High-performance liquid chromatography
IR Infrared spectrum
LOD Limit of detection
LOQ Limit of quantity
NMR Nuclear magnetic resonance
PDA Photodiode array detection
RSD Relative standard deviation
s Singlet
TLC Thin-layer chromatography
UV Ultraviolet spectrum
AUTHOR CONTRIBUTIONS
Ngan Nguyen Kim Luu: Isolated the compound, conducted experiments, and wrote the manuscript.
Ngan My Tran: Conducted experiments and acquired data.
Phuong Thu Tran: Organized research and revised the manuscript.
Duong Hoang Trinh: Designed experiments, acquired and interpreted data, and completed the manuscript.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Do TL. Medicinal plants and herbs of Vietnam. Hanoi, Vietnam: Medical Publisher; 2004. p 820−1.
2. Park SU, Chae YA, Facchini RJ. Genetic transformation of the figwort, Scrophularia buergeriana Miq., an oriental medicinal plant. Plant Cell Rep. 2003;21:1194−8. CrossRef
3. Pham HH. An illustrated floral of Vietnam. Band 2. Ho Chi Minh City, Vietnam: Youth Publishing House; 2003. p 927−8.
4. Lee HJ, Lee DY, Kim HL, Yang SH. Scrophularia buergeriana extract improves memory impairment via inhibition of the apoptosis pathway in the mouse hippocampus. Appl Sci. 2020;10(22):7987−8000. CrossRef
5. Vietnam Standard and Quality. ISO/IEC 17034: General requirements for the competence of reference material producers [Internet]. 2017. Available from: https://caselaw.vn/van-ban-phap-luat/345574-tieu-chuan-quoc-gia-tcvn-iso-17034-2017-iso-17034-2016-ve-yeu-cau-chung-ve-nang-luc-cua-nha-san-xuat-mau-chuan-nam-2017?vanbangoc=true
6. International Organization for Standardization. ISO13528: Statistical methods for use in proficiency testing by interlaboratory comparison [Internet]. 2015. Available from: https://www.iso.org/standard/56125.html
7. Vietnamese pharmacopoeia V. Band 2. Hanoi, Vietnam: Medical Publishing House; 2018. p 1199−201.
8. Pham TV, Hoang HNT, Nguyen HT, Nguyen HM, Huynh CT, Vu TY, et al. Anti-inflammatory and antimicrobial activities of compounds isolated from Distichochlamys benenica. Biomed Res Int. 2021:1−10. CrossRef