INTRODUCTION
Stroke is a catastrophic disease for humans caused by hemorrhage and thrombus. The thrombus is becoming a primary etiology in about 91% of global stroke cases [1]. The thrombus leads to ischemic stroke with a significant decrease in human quality of life, including disability and death. Ischemic stroke is also becoming a financial burden for individuals, societies, and governments worldwide [2]. Ischemic stroke management involves a multifaceted approach, such as stroke management, post-stroke treatment, recovery program, and rehabilitation [3]. One of those essential approaches in ensuring patients’ proper healing is post-stroke treatment. Post-stroke treatment is commonly conducted using drugs, either chemical or herbal-derived agents.
Calabash fruit (Crescentia cujete L.) which has various bioactive compounds is one of the alternative treatments that can be utilized. Fermented calabash fruit has been proven to contain choline [4]. Choline is a neurotransmitter and acts potentially as a brain supplementation. The previous study by Hidayah et al. [5] found that fermented calabash fruit-derived choline (FC-C) has the potential for brain histopathology after artificially induced ischemic stroke in rats.
Therefore, it is necessary to elucidate the role of FC-C, which uses other parameters to measure the success of ischemic stroke treatment. The success of ischemic stroke treatment in humans is commonly monitored using the patient’s clinical responses and neuro-deficit scores. At the same time, an ischemic stroke involves intricate pathogenesis, including changes in blood, serum, and the immunological expression of the brain tissue. A previous study by Prakoso et al. [6] reported that ischemic stroke significantly increases the ratio of neutrophil/lymphocyte (N/LR), platelet-to-white blood cell ratio (PWR), and C-reactive protein (C-RP) from 24 hours until 7 days after induction. Moreover, the study by Navarro-Sobrino et al. [7] added that neuronal necrosis inside a brain tissue reduces the immune expression of granulocyte-macrophage colony-stimulating factors (GM-CSFs), which is essential during brain trauma in protecting a brain from more severe damage. Besides GM-CSF, there is an immune-expression affected by ischemic stroke, such as vascular endothelial growth factor (VEGF). VEGF is essential during angiogenesis and increases the oxygenation within ischemic brain tissue [8].
Hence, this study aimed to analyse the efficacy of FC-C as a complementary treatment against N/LR, PWR, histopathology, GM-CSF, and VEGF in artificially induced ischemic stroke in rats.
MATERIAL AND METHODS
Ethic approval
This study has been approved, monitored, and evaluated by the ethical clearance committee from the Faculty of Veterinary Medicine, University of Wijaya Kusuma Surabaya, Research Ethical Clearance Commission. The study has been monitored and registered with the approval number 108-KKE/2023. This study was conducted from March to December 2023 in the Department of Pharmacology, FVM, University of Wijaya Kusuma Surabaya, Indonesia.
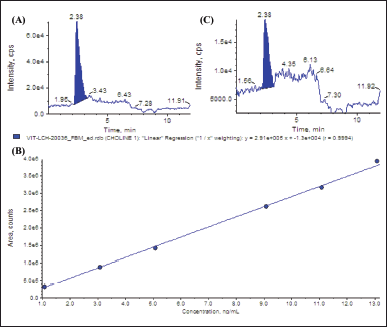 | Figure 1. Chromatogram and a calibration curve of choline using LC-MS/MS. Standard chromatogram of choline (A), calibration curve of choline (B), and chromatogram of choline from FC-C (C). [Click here to view] |
Calabash fruit species determination
The calabash was collected from the Botanical Garden at the University of Wijaya Kusuma Surabaya, Indonesia. The species of this herb was then determined at the Centre for Research and Development of Medicinal Plants and Traditional Medicine, Indonesia, with herbarium number KM.04.02/2/1248/2022.
Fermented calabash fruit preparation and choline standardisation
The fruit was fermented, and the choline determination was conducted following the procedure that Wilujeng et al. demonstrated [4]. Liquid chromatography-tandem mass spectrometry (LC-MS/MS, SCIEX Triple Quad 5500+, SCIEX, Framingham, MA) was used to evaluate the amount of choline present. The chromatogram of the choline standard was shown in Figure 1A, and the retention time was indicated at 2.38 minutes. The equation for the calibration curve for choline separation was y = 2.91e + 5x + -1.3e + 4, with R2 = 0.999 (Fig. 1B). The analyte peak area for choline was 3.08e + 005, and the peak height was 1.02e + 004. The LC-MS/MS was performed fivefold, and the results indicated that the fermented calabash contained choline at the level of 112.40 ± 3.13 mg/kg. The chromatogram of choline from fermented calabash fruit is shown in Figure 1C. This product was then called FC-C.
Animal model and research design
This study used 40 male Sprague Dawley rats, six months old and weighing 251.35 ± 1.12 g, from the Department of Pharmacology, FVM, University of Wijaya Kusuma Surabaya. The rats were kept in metabolic cages (measuring 40 × 30 × 40 cm) and the oven husk was used as the bedding. The rats were maintained at 12/12 hours (light/dark), and the room temperature was set at 25°C with 60% humidity using an air conditioner. The rats were provided with Rat Bio® (Citra Feed, Indonesia) as their diet and water were given ad libitum.
Before the experiment, the rats were acclimated for seven days. They were then divided into four groups: CO group (sham-operated group); CU group (artificially induced ischemic stroke without treatment); FC group (artificially induced ischemic stroke + 2.94 mg/kg BW FC-C) [5]; and PR group (artificially induced ischemic stroke + 496 mg/kg BW piracetam (Piracetam®, Hexpharm Jaya, Indonesia)). The therapy was conducted 24 hours after induction and performed once daily for 14 days.
 | Table 1. Score of neuro-deficit analysis. [Click here to view] |
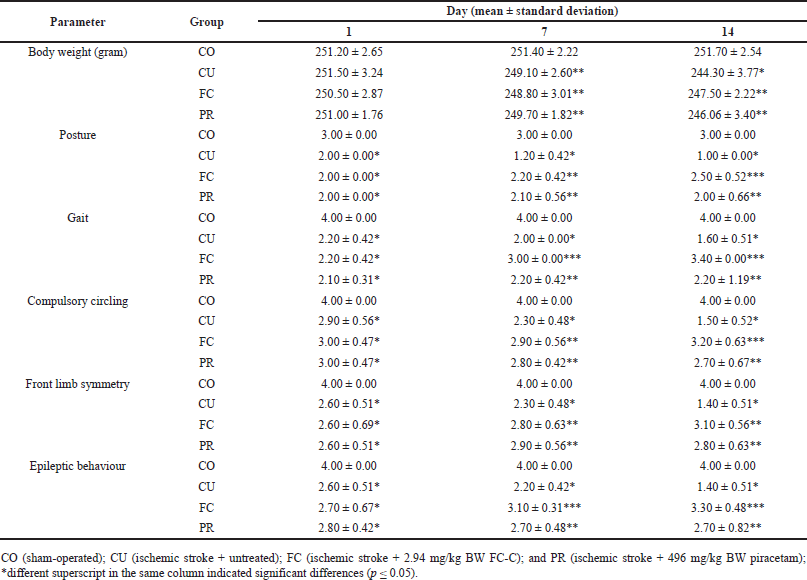 | Table 2. Body weight and score of neuro-deficit of artificial induced ischemic stroke in rat model. [Click here to view] |
Artificially induced ischemic stroke in rat models
Ischemic stroke induction was conducted following a previous study [6]. Before the induction, the rat was anaesthetised using 50 mg/kg BW ketamine (Agrovet Market, Peru) and 4 mg/kg BW xylazine (Interchemie, Holland). The rat’s neck hair was shaved and disinfected using isopropyl alcohol. The neck skin was incised, and the artery was ligated using a 4-0 monofilament suture (Ethilon®, Ethicon, US). After 4 hours, the artery was reperfused by cutting the knotted suture, and the incision was then closed using a simple interrupted suture. The rat was injected using penicillin G (Meiji, Indonesia) to prevent infection.
Body weight measurement and neuro-deficit analysis
The rat’s body weight and neuro-deficit were measured on days 1, 7, and 14. The neuro-deficit was scored following the scoring system demonstrated by Bertrand et al. [9]. The neuro-deficit parameters are embedded in Table 1.
Hematology
The blood was collected on days 1, 7, and 14. The blood was collected via plexus retro-orbital under general anesthesia using a combination of ketamine and xylazine. The blood was then stored in an ethylenediaminetetraacetic acid tube at 4°C. The blood was tested against N/LR and PWR following the demonstrated procedure by Sharma et al. [10] and Amalia and Dalimonthe [11], concomitantly.
Brain tissue collection, infarct measurement, and tissue preparation
The rat was euthanised using 150 mg/kg BW ketamine on day 14, and the brain tissue was collected. Before cutting, the brain was preserved at −20°C for 10 minutes. The brain was then cut into three pieces for infarct area measurement, histopathology, and enzyme-linked immunosorbent assay (ELISA). The first piece was measured regarding its infarct area using 2% 2,3,5-triphenyl tetrazolium chloride (Cat No. T8877, Sigma-Aldrich, Indonesia). The infarct area appeared white, while the typical brain was stained deep red. Furthermore, the percentage of infarct area was measured using ImageJ software. The second piece was fixed using 10% neutral buffer formalin (NBF), and the third was stored at −20°C for ELISA until being used.
 | Figure 2. Level of PWR and N/LR after treatment from artificially induced ischemic stroke in rat models. CO (sham-operated); CU (ischemic stroke + untreated); FC (ischemic stroke + 2.94 mg/kg BW FC-C); and PR (ischemic stroke + 496 mg/kg BW piracetam); *different superscript in the same column indicated significant differences (p ≤ 0.05). [Click here to view] |
Histopathology and immunohistochemistry (IHC)
After 24 hours of fixation using 10% NBF, the brain was dehydrated using graded alcohol and xylene and embedded using liquid paraffin. The block was then cut using a microtome at 5 µm of thickness. The slide was then processed to routine histopathology using hematoxylin and eosin (H&E) staining (Leica Biosystems, USA) and IHC using several biomarkers. The IHC biomarkers included GM-CSF (sc32-753, Santa Cruz Biotechnology Inc, USA) and VEGF (sc-7269, Santa Cruz Biotechnology Inc, USA). The IHC staining procedure was performed following a previous study by Prakoso et al. [12]. The semi-quantitative analysis of histopathology and IHC slides was conducted using a grading system, including 1 (no histopathological changes/ no expression), 2 (mild), 3 (moderate), and 4 (severe/intense).
ELISA
The collected rat brains were weighed 0.5 g and homogenised using a buffer solution. The mixture was homogenised, and the supernatant was collected. The ELISA was performed against several biomarkers, including GM-CSF and VEGF. The ELISA kit for GM-CSF (ERCSF2) and VEGF (ERVEGFA) from Invitrogen, USA. The ELISA was performed following the standard guideline from the manufacturer.
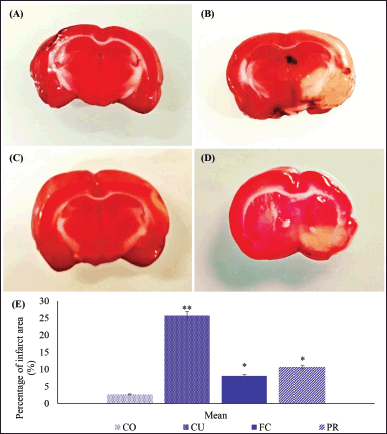 | Figure 3. Infarct area in the brain from artificially induced ischemic stroke in rat models. The normal brain was stained deep red from CO group (A), while the infarct area from groups CU, FC, and PR was white (B–D); compared to the percentage infarct area (E). CO (sham-operated); CU (ischemic stroke + untreated); FC (ischemic stroke + 2.94 mg/kg BW FC-C); and PR (ischemic stroke + 496 mg/kg BW piracetam); *different superscript indicated significant differences (p ≤ 0.05). [Click here to view] |
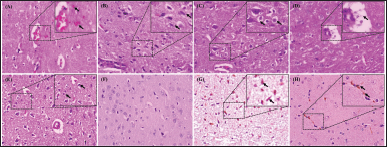 | Figure 4. Histopathological changes and IHC of the brain from artificially induced ischemic stroke in rat models after treatment. The secondary hemorrhage of the brain marked by the extravasation of erythrocytes into the Virchow Robin’s chamber and parenchyma (arrow) (A); increased number of microglia that indicated to microgliosis (arrow) (B); neuronal vacuolisation (arrow) (C); the dilatation of Virchow Robin chamber filled by neutrophil and homogenous eosinophilic mass (arrow) (D); pycnotic neuron (arrow) (E); immunohistochemical staining of brain tissue without labeling showed no immunoreactivity (F); immune-expression of GM-CSF (arrow) expressed as the brown color within the parenchyma (G); and VEGF (arrow) expressed on the new capillaries within the brain parenchyma (H). H&E staining, 400× (A–E), IHC without labeling, 400× (F), IHC antibody anti-GM-CSF, 400× (G), IHC antibody anti-VEGF, 400× (H). [Click here to view] |
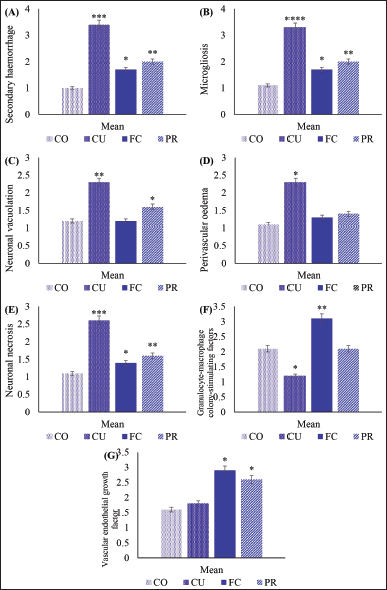 | Figure 5. Histopathology and immune-expression of GM-CSF and VEGF of the brain from artificially induced ischemic stroke in rat models after treatment. A score of secondary hemorrhage (A); microgliosis (B); neuronal vacuolation (C); perivascular oedema (D); neuronal necrosis (E); immune-expression of GM-CSF (F); and VEGF (G). [Click here to view] |
Analysis data
The data was analysed using SPSS version 26. Homogeneous and normally distributed data were analysed using analysis of variance (ANOVA). The Bonferroni test confirmed the analysis if significant differences between groups were found. On the other hand, non-homogeneous and abnormal data were analysed using Kruskal-Wallis’s test and confirmed by the Mann-Whitney U test. The study used a significance value of p ≤ 0.5.
RESULTS
Body weight and neuro-deficit score
The study revealed a decrease in body weight after artificial ischemic stroke induction in a rat model. The highest decrease in body weight was observed in Group CU compared to the other groups (p ≤ 0.05), and this decrease was observed on days 7 and 14 after induction. Among all treated groups, the FC and PR group showed the slightest decrease in body weight after induction compared to the group CU (p ≤ 0.05); however, it was still different from the sham-operated group (p ≤ 0.05) (Table 2). Additionally, the posture of rats changed from day 1 until 14 after induction. The CU group displayed the poorest body posture compared to the other groups. However, there was a concurrent increase in the body posture score in PR and FC. The FC group showed a significant increase in the score of body posture, while the CU and PR groups showed a decrease (p ≤ 0.05) (Table 2).
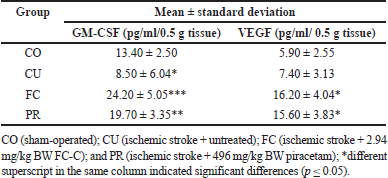 | Table 3. Level of GM-CSF and VEGF of the brain of artificially induced ischemic stroke in rat models after treatment. [Click here to view] |
According to the study, the treatment groups significantly impacted the neuro-deficit score. The group that received treatment showed a decrease in neuro-deficit score on day 1 after the induction of ischemic stroke compared to the healthy group (p ≤ 0.05). On days 7 and 14, the FC group had better scores for gait, compulsory circling, and epileptic behavior than the other treatment groups (p ≥ 0.05). However, the front limb asymmetry did not show any differences between the FC and PR groups, as per Table 2.
Level of PWR and N/LR
Results showed a significant increase in N/LR and PWR levels on day 1 after induction. Group CU showed a constant increase in these levels on days 7 and 14 compared to the other groups (p ≤ 0.05). However, FC and PR groups showed a dramatic decrease in N/LR during the observation period, similar to the sham-operated group (p ≥ 0.05). All the treated groups showed a similar level of PWR compared to the sham-operated group (p ≥ 0.05) (Fig. 2).
Percentage of infarct area
The result showed that the group that did not receive treatment had the largest infarct area on day 14, as evidenced by the white tissue (Fig. 3). In contrast, the groups that received treatment using FC-C and piracetam had significantly reduced infarct areas compared to the untreated group, concomitantly (p ≤ 0.05), as per Figure 3C–D. However, the similar percentage of infarcts as the sham-operated group was not observed in any treated groups (Fig. 3E).
Histopathology and immune-expression of GM-CSF and VEGF
During the pathogenesis of stroke, the rats experienced an increase in secondary hemorrhage, microgliosis, neuronal vacuolation, perivascular oedema, neuronal necrosis, and changes of immune-expression of GM-CSF and VEGF (Fig. 4). The results of the study showed that the rat model with ischemic stroke experienced an increase in secondary hemorrhage, microgliosis, neuronal vacuolation, perivascular oedema, neuronal necrosis compared to the sham-operated group (p ≤ 0.05). However, treatment with FC-C significantly reduced the occurrence of secondary hemorrhage, similar to the PR group (p ≥ 0.05). The FC group had better histopathology scores regarding microgliosis, neuronal vacuolation, and perivascular oedema than the other treatment groups (p ≤ 0.05). Furthermore, the score of neuronal vacuolation and perivascular oedema of the FC group was similar to the CO group (p ≥ 0.05). FC and PR groups were not different from the CO group regarding the neuronal necrosis (p ≥ 0.05) (Fig. 5).
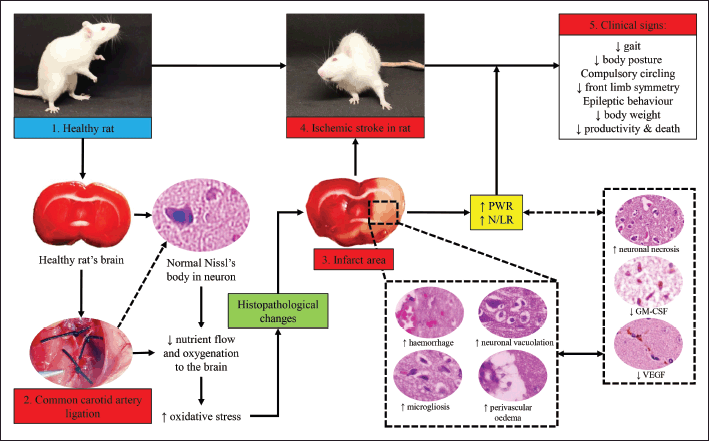 | Figure 6. Pathogenesis of ischemic stroke in rat models in this study. [Click here to view] |
 | Figure 7. Mechanism of action of FC-C against artificial ischemic stroke in rat models in this study. [Click here to view] |
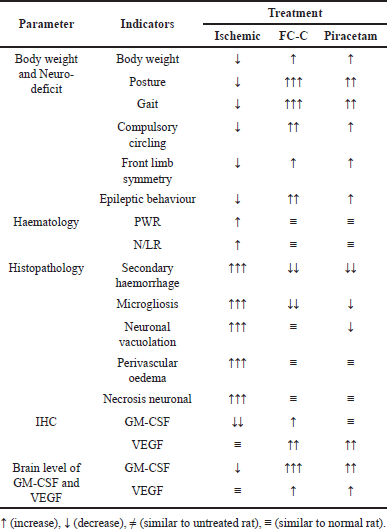 | Table 4. Comparison between effects of FC-C and piracetam in artificially induced ischemic stroke in rat models. [Click here to view] |
Additionally, the study observed a decrease in GM-CSF in the CU group compared to the CO group (p ≤ 0.05). This study found a significant increase in the immune expression of GM-CSF in the FC group compared to the others (p ≤ 0.05), showing the potential effects of FC-C in increasing GM-CSF in ischemic stroke. The ischemic stroke evidence in the CU group did not influence the score of VEGF immune expression compared to the CO group. The FC group showed an increase in immune expression of VEGF compared to the CO group (p ≤ 0.05). Meanwhile, the PR group showed a pattern similar statistically to the FC group regarding the increased score of VEGF, although not as good as the FC group’s (Fig. 5).
Level of GM-CSF, VEGF, and COX-2 from brain tissue
The level of GM-CSF in the brain from the untreated group indicated a significant decrease compared to the sham-operated group (p ≤ 0.05); however, they were not different regarding VEGF level (p ≥ 0.05). Furthermore, the FC group had higher levels of GM-CSF compared to the other groups, and it was concomitantly followed by the PR group (p ≤ 0.05). FC and PR groups were not different regarding VEGF level (p ≥ 0.05) (Table 3).
DISCUSSIONS
Stroke is a catastrophic disease, and one of the causative mechanisms is the occurrence of a thrombus. Thrombus can be established through endothelial damage that slowly promotes the accumulation of platelets and fibrin. The thrombus in advances traps more severe fibrin accumulation, neutrophils, von Willebrand factors, and erythrocytes [13]. The pathogenesis becomes more severe when the thrombus occurs within the cerebral artery and/or arteries supply blood to the brain, and it causes brain infarction. Brain infarction caused by a thrombus is known as ischemic stroke [14]. In this study, the cellular mechanisms involved in ischemic stroke are an increase in secondary hemorrhage, microgliosis, perivascular oedema, and neuronal necrosis. A previous study by Spronk et al. [15] described that the secondary hemorrhage within the ischemic stroke is a common complication in patients marked by erythrocyte extravasation. The erythrocyte extravasation occurs post-stroke through the disrupted blood-brain barrier, and it causes blood penetration into the parenchyma. The main factor of secondary hemorrhage is a change in blood pressure. Fischer et al. [16] elucidated that ischemic stroke triggers systolic blood pressure changes among patients for several days after stroke and becomes premorbid. Moreover, the blood pressure in ischemic stroke generates dilatation of Virchow Robin space, perivascular cuffing and oedema [17]. In advances, a vascular change increases neuronal vacuolation and death [18]. It is related to this study that neuronal death gets worse on day 14 after a stroke in an untreated group.
In this study, the ischemic stroke influences the expression and level of GM-CSF rather than VEGF. The decrease of GM-CSF after artificial induction of ischemic stroke initiated by a large number of parenchymal dead. It is important to note that GM-CSF and its receptor are expressed in the neurons, ependymal cells, and astrocytes [19]. While the ischemia is occurring, the brain parenchyma will lose some of these cells, impacting the level of GM-CSF. Li et al. [20] supported this finding, which reported that GM-CSF significantly decreased in patients with more severe strokes. Therefore, it is essential to utilise a therapy that can beneficially increase the expression of GM-CSF in ischemic stroke and its mechanism to decrease inflammatory responses and neuroprotective activities [21]. Dikmen et al. [22] added that the therapy using GM-CSF can improve the activation of microglia and contributes to inducing a repair of network dysfunction.
While the expression of GM-CSF decreased during the pathogenesis of strokes in the untreated group, the expression of VEGF is not different from the normal brain. This result indicated that brain tissue is trying to respond and regenerate the parenchymal loss in the infarct area by rearranging the neovascularisation via increased VEGF expression. Prodjohardjono et al. [23] found that the level of VEGF significantly increases after stroke, but the infarct area is still growing. However, Setyopranoto et al. [24] stated that during an acute ischemic stroke, the expression of VEGF is lower than in a healthy group. Those two studies show that the VEGF has two different roles during the pathogenesis of stroke.
The dynamic change within brain tissue during the pathogenesis of ischemic stroke promotes significant alteration in systemic circulation. This was proved by an untreated group that showed increasing levels of PWR and N/LR. Amalia and Dalimonthe [11] found that ischemic stroke correlated to the increased value of PWR because of a massive secondary hemorrhage within the brain parenchyma. Moreover, Hu et al. [25] elucidated that the PWR and platelet-to-neutrophil ratio (PNR) consistently increase and correlate to stroke fatality. The inflammatory response after stroke influences the increase of N/LR level in ischemic stroke in this study. After a stroke, brain tissue releases several types of chemokines and cytokines, and both of them recruit the peripheral circulatory leucocytes, including neutrophils and lymphocytes. This result is correlated to Quan et al. [26], who reported that N/LR increased 24 hours after stroke and increased the risk of ischemic stroke, either short- or long-term.
Furthermore, the dynamic changes within brain tissue after ischemic stroke induction aggravate the score of neuro-deficit that can be clinically observed. The neuro-deficit also promotes incoordination of the rat’s body and complicates the rat’s behavior, including grooming, gaiting, and eating. These cause a significant decrease in body weight after stroke [27]. The illustration of the pathogenesis during ischemic stroke in this study is embedded in Figure 6.
This study used several treatments, including FC-C and piracetam. FC-C and piracetam are nootropics due to their direct mechanism on the neuron. This study revealed that choline from calabash fruit has better effects on the percentage of infarct area. The decrease in infarct area is caused by the mechanism of choline that promotes cellular integrity, lipid transport, neurotransmission, and signaling function [28]. During its metabolism, choline is absorbed in the intestine and penetrates the systemic circulation as lysophosphatidylcholine (LysoPC) or persists as choline. Choline can penetrate the neuron due to its high affinity with the Na+/choline transporter [29]. Choline will be converted into acetylcholine before it can be utilised as the neurotransmitter agent. In this stage, the choline reacts with acetylcholinesterase to form the acetate and choline. The acetate can be used as a source of energy, which is similar to the glucose metabolism in neurons [30]. However, the choline itself will be used on presynaptic and postsynaptic terminals in the next choline cycle (Fig. 7). The repeated cycle of choline may support the persistence of neurotransmitters via its ability to accelerate the synthesis and release of acetylcholine [31]. It is correlated to this study and answers why the neuronal repair after artificially induced ischemic stroke using FC-C is better than the untreated group.
Choline, which promotes acetylcholine synthesis acceleration, increases neuronal metabolism, repair, and neuronal integrity after ischemic stroke. Furthermore, choline influences the dilatation of cerebral arterioles and promotes the endothelial cell proliferation that expresses VEGF [32]. Choline also activates α7 non-neuronal nicotinic acetylcholine receptors (α7nAChR) [33]. Moreover, in this case, the endothelial cell proliferation and activation of α7nAChR sequentially reduces tissue hypoxia during ischemic stroke, decreases neuronal damage, tissue infarction, and hemorrhage, and increases the GM-CSF. This study correlates to Zhang et al. [34], who found that choline increases VEGF and α7nAChR and could be a protective agent against hypoxia.
As previously described, GM-CSF is naturally expressed in the neurons, ependymal cells, and astrocytes. During ischemic stroke, the expression and level of GM-CSF decrease because stroke induces immunodepression and parenchymal death [35]. In contrast, the GM-CSF expression and level increase after treatment using FC-C. The increase in both expression and level of GM-CSF is influenced by the parenchymal repair that increases brain oxygenation. The increase of GM-CSF after treatment using FC-C is able to act as a neuro-protectant to counteract neuro-apoptosis, increase blood vessel diameter during arteriogenesis, activate microglia, and improve the quality of Nissl’s body [36]. It is correlated to this study that found an improvement in neuronal necrosis score on day 14.
Furthermore, Dames et al. [37] described that GM-CSF potentially increased tissue oxygenation after stroke and induced immunoreactivity, especially for leucocyte recruitment and cytokine production. This study is supported by Zhang et al. [38], who proved the role of choline in attenuating thermal and mechanic sensitivity rather than decreasing pro-inflammatory cytokines during the pathogenesis and treatment of chronic constriction nerve injury (CCI). A better tissue repair after FC treatment decreases the infarct area, N/LR, maintains the body weight, and improves the neuro-deficit that can be clinically observed (Fig. 7).
The other therapy against artificially induced ischemic stroke in this study used a piracetam. Piracetam is a nootropic drug that acts directly on the neuron membrane [39]. Even though piracetam is a nootropic, it has also been reported to act on the platelet. Piracetam has a high affinity to some molecules present on the neuron membrane rather than having neurotransmitter-like activity [40]. Therefore, this is what makes the difference between piracetam and FC-C. Piracetam also induces the molecule influx in the neuron, potentially promoting neuronal resistance against noxious stimuli, such as hypoxia, during an ischemic stroke. Furthermore, piracetam increases membrane fluidity and reduces adenosine diphosphate, preventing platelet aggregation [41]. Piracetam directly affects the endothelial cells to stimulate the synthesis of prostacyclin, which is essential in preventing the release of Willebrand factor [42].
In this study, the potency of piracetam has been proved by the elevated histopathological score of the brain after treatment, especially in the decrease of secondary hemorrhage and perivascular oedema score, which is similar to the FC-C treatment. Nevertheless, the potential effects of piracetam are less sound than FC-C regarding microgliosis, neuronal vacuolation, and neuronal necrosis. Because piracetam has no neurotransmitter potency, it cannot increase the immune expression and GM-CSF and FC-C, but its profile is conformable to sham-operated. The potency of piracetam in preventing hypoxia impacts the brain parenchyma, proven by the increase of VEGF [43]. Moreover, piracetam has effects similar to the FC-C treatment on the immune expression and level of COX-2 but is not identical. The related mechanism between FC-C and piracetam as a nootropic drives there are no differences in the several biomarkers, including PWR and N/LR [44]. Mani et al. [45] described that the potency of piracetam in repairing neuro-deficit score in artificially induced ischemic stroke in rat models was influenced by its mechanism to reduce pro-apoptosis protein (Caspase-3 and Bax), malondialdehyde, and inflammatory receptors, including Nf-kB and PGE2. Furthermore, the comparison between all treatment effects in artificially induced ischemic stroke in this study can be found in Table 4.
CONCLUSION
It can be concluded that FC-C has potential effects as the complementary therapy for artificial ischemic stroke in rat models via its ability to directly affect the neuron and its mechanism to sustain choline repeat cycling on pre- and postsynaptic terminals. However, the advanced study regarding the safety of this complementary medicine must be elucidated to support the current study’s findings.
ACKNOWLEDGMENTS
All research assistants from the Department of Pharmacology, FVM, UWKS were acknowledged for their assistance.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The Hibah Pasca Stroke Indonesia funded this study with grant number Hibah.01/18/PS/2022.
CONFLICTS OF INTEREST
This study has no conflict of interest to declare.
ETHICAL APPROVALS
The study protocol was approved by the Research Ethical Committee, Faculty of Veterinary Medicine, University of Wijaya Kusuma Surabaya, Indonesia with approval number 108-KKE/2023.
DATA AVAILABILITY
The data used to support the findings are available upon reasonable request to the corresponding author.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Donkor ES. Stroke in the 21st century: a snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat. 2018;2018:3238165. CrossRef
2. Murphy SJ, Werring DJ. Stroke: causes and clinical features. Medicine. 2020;48(9):561–6. CrossRef
3. Yan LL, Li C, Chen J, Miranda JJ, Luo R, Bettger J, et al. Prevention, management, and rehabilitation of stroke in low- and middle-income countries. eNeurologicalSci. 2016;2:21–30. CrossRef
4. Wilujeng S, Wirjaatmadja R, Prakoso YA. Effects of extraction, fermentation, and storage processes on the levels of choline derived from calabash fruit (Crescentia cujete L.). J Res Pharm. 2023;27(2):620–6. CrossRef
5. Hidayah JH, Prakoso YA, Widyarini S. Brain histopathological changes after treatment using calabash fruit (Crescentia cujete L.) in rat model with artificially induced ischemic stroke. Adv Anim Vet Sci. 2023;11(12):2003–9. CrossRef
6. Prakoso YA, Sigit M, Aliviameita A. Standardization of the simple methodology for experimentally induced ischemic stroke in rat models. World Vet J. 2023;13(4):510–9. CrossRef
7. Navarro-Sobrino M, Rosell A, Penalba A, Ribó M, Alvarez-Sabín J, Fernández-Cadenas I, et al. Role of endogenous granulocyte-macrophage colony stimulating factor following stroke and relationship to neurological outcome. Curr Neurovasc Res. 2009;6(4):246–51. CrossRef
8. Talwar T, Srivastava MV. Role of vascular endothelial growth factor and other growth factors in post-stroke recovery. Ann Indian Acad Neurol. 2014;17(1):1–6. CrossRef
9. Bertrand L, Dygert L, Toborek M. Induction of ischemic stroke and ischemia-reperfusion in mice using the middle artery occlusion technique and visualization of infarct area. J Vis Exp. 2017;120:54805. CrossRef
10. Sharma D, Spring KJ, Bhaskar SMM. Neutrophil-lymphocyte ratio in acute ischemic stroke: immunopathology, management, and prognosis. Acta Neurol Scand. 2021;144(5):486–99. CrossRef
11. Amalia L, Dalimonthe NZ. Clinical significance of platelet-to-white blood cell ratio (PWR) and National Institute of Health Stroke Scale (NIHSS) in acute ischemic stroke. Heliyon. 2020;6(10):e05033; CrossRef
12. Prakoso YA, Rini CS, Rahayu A, Sigit M, Widhowati D. Celery (Apium graveolens) as a potential antibacterial agent and its effect on cytokeratin-17 and other healing promoters in skin wounds infected with methicillin-resistant Staphylococcus aureus. Vet World. 2020;13(5):865–71. CrossRef
13. Xu RG, Ariëns RAS. Insights into the composition of stroke thrombi: heterogeneity and distinct clot areas impact treatment. Haematologica. 2020;105(2):257–9. CrossRef
14. Kuriakose D, Xiao Z. Pathophysiology and treatment of stroke: present status and future perspectives. Int J Mol Sci. 2020;21(20):7609. CrossRef
15. Spronk E, Sykes G, Falcione S, Munsterman D, Joy T, Kamtchum-Tatuene J, et al. Hemorrhagic transformation in ischemic stroke and the role of inflammation. Front Neurol. 2021;12:661955. CrossRef
16. Fischer U, Cooney MT, Bull LM, Silver LE, Chalmers J, Anderson CS, et al. Acute post-stroke blood pressure relative to premorbid levels in intracerebral haemorrhage versus major ischaemic stroke: a population-based study. Lancet Neurol. 2014;13(4):374–84. CrossRef
17. Haider L, Hametner S, Endmayr V, Mangesius S, Eppensteiner A, Frischer JM, et al. Post-mortem correlates of Virchow-Robin spaces detected on in vivo MRI. J Cereb Blood Flow Metab. 2022;42(7):1224–35. CrossRef
18. Zhao Y, Zhang X, Chen X, Wei Y. Neuronal injuries in cerebral infarction and ischemic stroke: from mechanisms to treatment (review). Int J Mol Med. 2022;49(2):15. CrossRef
19. Donatien P, Anand U, Yiangou Y, Sinisi M, Fox M, MacQuillan A, et al. Granulocyte-macrophage colony-stimulating factor receptor expression in clinical pain disorder tissues and role in neuronal sensitization. Pain Rep. 2018;3(5):e676. CrossRef
20. Li X, Lin S, Chen X, Huang W, Li Q, Zhang H, et al. The Prognostic value of serum cytokines in patients with acute ischemic stroke. Aging Dis. 2019;10(3):544–56. CrossRef
21. Huang X, Liu Y, Bai S, Peng L, Zhang B, Lu H. Granulocyte colony stimulating factor therapy for stroke: a pairwise meta-analysis of randomized controlled trial. PLoS One. 2017;12(4):e0175774. CrossRef
22. Dikmen HO, Hemmerich M, Lewen A, Hollnagel JO, Chausse B, Kann O. GM-CSF induces noninflammatory proliferation of microglia and disturbs electrical neuronal network rhythms in situ. J Neuroinflam. 2020;17(1):235. CrossRef
23. Prodjohardjono A, Vidyanti AN, Susianti NA, Sudarmanta, Sutarni S, Setyopranoto I. Higher level of acute serum VEGF and larger infarct volume are more frequently associated with post-stroke cognitive impairment. PLoS One. 2020;15(10):e0239370. CrossRef
24. Setyopranoto I, Sadewa AH, Wibowo S, Widyadharma IPE. Comparison of mean VEGF-A expression between acute ischemic stroke patients and non-ischemic stroke subjects. Open Access Maced J Med Sci. 2019;7(5):747–51. CrossRef
25. Hu ZB, Zhong QQ, Lu ZX, Zhu F. Association of platelet-to-white blood cell ratio and platelet-to-neutrophil ratio with the risk of fatal stroke occurrence in middle-aged to older Chinese. BMC Geriatr. 2022;22(1):430. CrossRef
26. Quan K, Wang A, Zhang X, Meng X, Chen P, Li H, et al. Neutrophil to lymphocyte ratio and adverse clinical outcomes in patients with ischemic stroke. Ann Transl Med. 2021;9(13):1047. CrossRef
27. Krittayaphong R, Chichareon P, Komoltri C, Kornbongkotmas S, Yindeengam A, Lip GYH. Low body weight increases the risk of ischemic stroke and major bleeding in atrial fibrillation: the COOL-AF registry. J Clin Med. 2020;9(9):2713. CrossRef
28. Derbyshire E, Obeid R. Choline, neurological development and brain function: a systematic review focusing on the first 1000 days. Nutrients. 2020;12(6):1731. CrossRef
29. Blusztajn JK, Slack BE, Mellott TJ. Neuroprotective actions of dietary choline. Nutrients. 2017;9(8):815. CrossRef
30. Tanabe J, Yamamoto DJ, Sutton B, Brown MS, Hoffman PL, Burnham EL, et al. Effects of alcohol and acetate on cerebral blood flow: a pilot study. Alcohol Clin Exp Res. 2019;43(10):2070–8. CrossRef
31. Kansakar U, Trimarco V, Mone P, Varzideh F, Lombardi A, Santulli G. Choline supplements: an update. Front Endocrinol. 2023;14:1148166. CrossRef
32. Liu L, Lu Y, Bi X, Xu M, Yu X, Xue R, et al. Choline ameliorates cardiovascular damage by improving vagal activity and inhibiting the inflammatory response in spontaneously hypertensive rats. Sci Rep. 2017;7:42553. CrossRef
33. Kusuda R, Carreira EU, Ulloa L, Cunha FQ, Kanashiro A, Cunha TM. Choline attenuates inflammatory hyperalgesia activating nitric oxide/cGMP/ATP-sensitive potassium channels pathway. Brain Res. 2020;1727:146567. CrossRef
34. Zhang LC, Jin X, Huang Z, Yan ZN, Li PB, Duan RF, et al. Protective effects of choline against hypoxia-induced injuries of vessels and endothelial cells. Exp Ther Med. 2017;13(5):2316–24. CrossRef
35. Theoret JK, Jadavji NM, Zhang M, Smith PD. Granulocyte macrophage colony-stimulating factor treatment results in recovery of motor function after white matter damage in mice. Eur J Neurosci. 2016;43(1):17–24. CrossRef
36. Olson KE, Namminga KL, Lu Y, Thurston MJ, Schwab AD, de Picciotto S, et al. Granulocyte-macrophage colony-stimulating factor mRNA and neuroprotective immunity in Parkinson’s disease. Biomaterials. 2021;272:120786. CrossRef
37. Dames C, Winek K, Beckers Y, Engel O, Meisel A, Meisel C. Immunomodulatory treatment with systemic GM-CSF augments pulmonary immune responses and improves neurological outcome after experimental stroke. J Neuroimmunol. 2018;321:144–9. CrossRef
38. Zhang N, Li Y, Feng Z. Inhibition effect of choline and parecoxib sodium on chronic constriction nerve injury-induced neuropathic pain in rats. BMC Anesthesiol. 2023;23(1):22. CrossRef
39. Winblad B. Piracetam: a review of pharmacological properties and clinical uses. CNS Drug Rev. 2005;11(2):169–82. CrossRef
40. Cohen PA, Zakharevich I, Gerona R. Presence of piracetam in cognitive enhancement dietary supplements. JAMA Intern Med. 2020;180(3):458–9. CrossRef
41. Olaizola I, Brodde MF, Kehrel BE, Evers S. The impact of levetiracetam and valproate on platelet functions-a double-blind, placebo-controlled crossover study. J Clin Med. 2023;12(3):933. CrossRef
42. Tuncer S, Ayhan S, Findikcioglu K, Ergun H, Tuncer I. Effect of systemic piracetam treatment on flap survival and vascular endothelial growth factor expression after ischemia-reperfusion injury. J Reconstr Microsurg. 2011;27(7):409–18. CrossRef
43. Fessel J. Cure of Alzheimer’s dementia requires addressing all of the affected brain cell types. J Clin Med. 2023;12(5):2049. CrossRef
44. Contreras-García IJ, Cárdenas-Rodríguez N, Romo-Mancillas A, Bandala C, Zamudio SR, Gómez-Manzo S, et al. Levetiracetam mechanisms of action: from molecules to systems. Pharmaceuticals. 2022;15(4):475. CrossRef
45. Mani V, Rabbani SI, Shariq A, Amirthalingam P, Arfeen M. Piracetam as a therapeutic agent for doxorubicin-induced cognitive deficits by enhancing cholinergic functions and reducing neuronal inflammation, apoptosis, and oxidative stress in rats. Pharmaceuticals. 2022;15(12):1563. CrossRef