INTRODUCTION
Ventilator-associated pneumonia (VAP), defined as pneumonia that occurs more than 48–72 hours after endotracheal intubation, is one of the most common infections found in the hospital, especially in the intensive care unit (ICU) setting
[1–4]. Methicillin-resistant Staphylococcus aureus (MRSA) has been identified as a common pathogen causing VAP [1,2,5]. Compared with the methicillin-susceptible strain, infection caused by MRSA afforded the worst outcome, i.e., higher mortality and cost of treatment [6–9]. Therefore, adequate management of MRSA infection should be ensured as early as possible after the infection has been recognized to prevent the occurrence of these worst outcomes.
Vancomycin has long been used as an antibiotic to treat MRSA infection. The American Thoracic Society guideline and the newest guideline from the Infectious Diseases Society of America (IDSA) recommended vancomycin as the core antibiotic for various types of infection caused by MRSA, including VAP [2,10]. The effectiveness of vancomycin treatment will be significantly determined by the achievement of pharmacokinetics-pharmacodynamic (PK-PD) indices, i.e., the area under the plasma drug concentration and time curve for 24 hours over minimum inhibitory concentration (AUC24/MIC) ≥400 mg.hour/l [10–12]. A greater proportion of patients who achieved these PK-PD indices received successful treatment in 30-day survival. Achieving this desired treatment target is challenging, especially in the era where the MIC value of MRSA strain has been shifted to a higher number even though it is still in the susceptible breakpoint value range ≤2 mg/l [13–15]. This phenomenon is also known as the “MIC Creep” phenomenon. Two recent meta-analysis revealed that high MIC value afforded high mortality events and treatment failure [16,17]. This finding emphasized the need to adjust the vancomycin dosage regimen to manage MRSA infection.
The need for dosage regimen adjustment is even more needed when vancomycin is used to treat MRSA infection among critically ill patients who are usually admitted to the ICU. Different physiological conditions among these patients may impact the different PK parameter profiles that, finally, may impact the achievement of AUC24/MIC [18,19]. Revilla et al. [20] found that a higher dose of vancomycin is needed to treat Staphylococcus aureus infection for critically ill patients with better renal function (Clcr >60 ml/minute). Meanwhile, for patients with worse renal function (Clcr <60 ml/minute), the standard vancomycin dose, defined as 1 g every 12 hours, might still be effectively used [20]. Accumulation of vancomycin would increase the total concentration of vancomycin in the body, which finally increases the value of AUC24.
One frightening factor of using high doses of vancomycin is the risk of nephrotoxicity. Lodise et al. [21] emphasized that a dose of vancomycin as high as 4 g/day might be possible for patients with a relatively tolerable risk of nephrotoxicity. Nephrotoxicity can cause a higher risk of in-hospital mortality, longer hospital stays, longer ICU stays, and increased health care costs [22,23]. Accumulation of vancomycin in the proximal tubular cells of renal leading to cell necrosis was postulated as the mechanism of vancomycin-induced nephrotoxicity [22–24]. Incidence of vancomycin-induced nephrotoxicity has been differently reported, ranging from 5% to 35%, and vancomycin trough level of ≥15 mg/l was documented as one of the risk factors of vancomycin-induced nephrotoxicity [24–29]. Conversely, this trough concentration was also recommended to ensure the achievement of desired PK-PD indices, particularly for deep-sited infections like VAP [10].
Because the MIC Creep MRSA phenomenon occurred in the last decades, we hypothesized that standard doses of vancomycin might not be effectively used to treat MRSA infection and a higher dose of vancomycin was needed. High doses of vancomycin, up to 4 g/day, could be given to the patients. Different vancomycin dosage regimens afford different efficacy and safety profiles. These different vancomycin dosage regimens might also influence the cost of the therapy that patients need to cover, including the risk of nephrotoxicity with higher doses that can cause additional cost burdens to the patients. At the time of this study being conducted, no literature could be used as the scientific foundation to address the clinical question about which vancomycin dosage regimen should be administered to treat MRSA infection in the era of “MIC creep”. Therefore, the aim was to identify the most cost-effective dosage regimen of vancomycin for VAP critically ill patients who were infected with “MIC Creep” MRSA.
MATERIAL AND METHODS
Cost-effectiveness model
This study conducted a simulation of the cost-effectiveness of several vancomycin dosage regimens for VAP critically ill patients infected with MRSA in this study using the decision tree model. It was a probabilistic study, meaning that the probability of the event, both clinical effectiveness and failure, in the decision tree model was not a fixed value. The model compared two different dosage regimens of vancomycin and a standard vancomycin dosing regimen, i.e., 1 g every 12 hours, was used as a standard comparator. The outcomes for each comparison were: 1) treatment successful of 30-day survival (indicated by the achievement of AUC24/MIC ≥ 400), and 2) treatment failure due to nephrotoxicity (indicated by the achievement of AUC24 >1,300). Since the most cost-effective dosage regimen of vancomycin was intended, a choice of changing to other antibiotics with MRSA coverage, such as linezolid, daptomycin, ceftaroline, ceftobiprole, and quinupristin-dalfopristin was not provided in our model. Figure 1 presents the decision model used in this study. The assumptions used in this study are listed below:
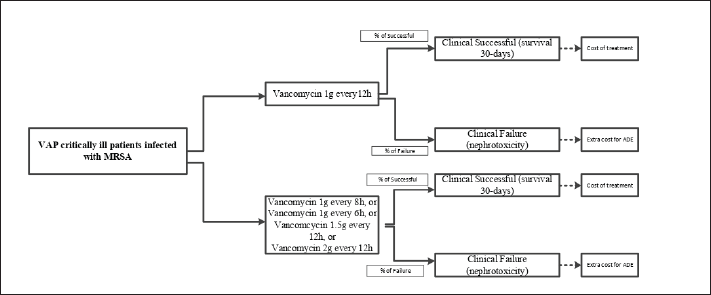 | Figure 1. Decision tree model for vancomycin treatment in ventilator-associated pneumonia infected with MRSA. [Click here to view] |
- Critically ill patients simulated in our model were those without severe renal impairment, defined as patients with creatinine clearance (CLCr) 60–120 mg/dl,
- MRSA strains were classified as vancomycin-susceptible MRSA, defined as strains with MIC ≤ 2 mg/l,
- Regardless of different dosage regimens, the duration was 11 days.
Perspective, time horizon, and discounting
The cost-effectiveness analysis was conducted from the perspective of the healthcare provider. VAP was generally classified as an acute disease; therefore, it might not be relevant to consider long-term complications. A 30-day survival rate was used in the analysis and no discounting method was applied to any cost.
Clinical data for simulation
Clinical data, both efficacy and safety data, were attained by Monte Carlo Simulation (MCS). A detailed explanation of each data required in the simulation is provided below:
a. Population PK parameters
Vancomycin was classified as a hydrophilic antibiotic with a main elimination process by glomerulus filtration. Volume distribution (Vd) and CLCr are essential in determining the body’s vancomycin concentrations. Vd and CLCr in this study were quantified using a valid and reliable PK model equation derived from a published population PK study among critically ill patients [20]. The final PK model equations from that study are listed below:
CL = θ1 X CLCr + Ageθ2 ...................................... (1)
CL stands for vancomycin clearance (ml/minute/kg) and CLCr stands for creatinine clearance (ml/minute/kg). The unit for age is years old.
Vd = θ3 × θ4 ............................................................ (2)
Vd stands for Vd (L). The values of θ1, θ2, θ3, and θ4 were 0.67, −0.24, 0.82, and 2.49, respectively.
b. MIC distribution
This study applied MIC distribution data from The European Committee on Antimicrobial Susceptibility Testing (EUCAST) [30]. There were three different values of MIC used in the analysis, i.e., 0.5, 1, and 2 mg/l, and the distribution of MRSA at each MIC value at the time of analysis being conducted can be found in Supplementary file Table 1.
PK-PD simulation of several doses of vancomycin
MCS with 5,000 replications was used to simulate the efficacy and safety of several vancomycin dosage regimens using Crystal Ball® 2000 (Decisioneering Inc., Denver, CO). Each simulated patient was created by a random assignment of several patients’ characteristics, including age, weight, and CLcr. The concentration of vancomycin each time during and post-infusion was calculated to calculate the AUC24. Since Revilla et al. [20] used a one-compartment model in their study and most vancomycin concentration was measured after the 5th dose, a one-compartment steady state condition intermittent infusion equation was used to calculate the concentration during the infusion time.
C = [k0/(keVd)](1−e−ket′)/(1− e−keτ)] ............(3) [31]
K0 stands for infusion rate (dose of vancomycin divided by duration of infusion; mg/hour); ke = elimination constant (hour-1); Vd = volume of distribution (L); t’ = time of infusion (hour); n = number of dose given; τ = dosing interval (hour).
While the equation to calculate concentration after stopping the infusion was [31]:
C = Cend . e-ke.t ............................................................(4)
Cend stands for concentration at the end of infusion; t = time after stopping infusion (hour).
The AUC during and post-infusion time was calculated using the linear trapezoidal (lin trap) rule [32]:
Lin trap AUC = {(C1+C2)/1} × (t2 – t1) ................(5)
C1 stands for concentration at time 1; C2 = concentration at time 2.
Five different vancomycin dosage regimens were simulated in this study, including 1 g every 12 hours, 1 g every 8 hours, 1 g every 6 hours, 1.5 g every 12 hours, and 2 g every 12 hours.
Analysis of the PK-PD model
Efficacy
The efficacy of a particular dosage regimen of vancomycin was presented as the probability of target attainment (PTA) and cumulative fraction response (CFR).
• The PTA is the probability of AUC24/MIC ≥400 mg.hour/l being achieved by giving a particular vancomycin dosage regimen at a specific MIC value [33]. Each vancomycin dosage regimen afforded some percentage of PTA for three different MIC values. Below is the equation used to calculate the PTA.
This study simulated 5 dosage regimens of vancomycin for 3 different values of MIC, and 15 different PTAs at the end of the study. Five dosage regimens simulated in this study included 1 g every 12 hours, 1 g every 8 hours, 1 g every 6 hours, 1.5 g every 12 hours, and 2 g every 12 hours. The main consideration in choosing the dosage regimens was related to the efficacy and safety profile attained by administering certain dosage regimens. The standard dosage regimen of vancomycin was 1 g every 12 hours, as also recommended in the IDSA guidelines [10]. Therefore, 1 g every 12 hours was used as the lowest dosage regimen in this study. Furthermore, the highest vancomycin dosage regimen found in the literature was 4 g/day, and this was also used as the highest dosage regimen in this study [29].
• CFR was defined as the expected population PTA for a specific vancomycin dosage regimen against the distribution of MRSA with different MIC values [33].
CFR of particular dosage regimen = (PTA for MIC 0.5 mg/l × percentage of MRSA with MIC 0.5 mg/l) + (PTA for MIC 1 mg/l × percentage of MRSA with MIC 1 mg/l) + (PTA for MIC 2mg/l × percentage of MRSA with MIC 2 mg/l)..........................(7).
Since this study simulated five dosage regimens of vancomycin, five different CFRs were presented at the end of this study.
Safety
Several studies have proven the association between vancomycin trough concentration of 15 mg/l and the risk of nephrotoxicity; however, it was debatable to use trough concentration as a suitable predictor of vancomycin-induced nephrotoxicity. Lodise et al. [21] found a higher percentage of nephrotoxicity in patients with AUC24 at steady state condition >1,300 mg.hour/l. Overall, 4.2% of patients with AUC24 >1,300 mg.hour/l developed nephrotoxicity. Therefore, AUC24 >1,300 mg.hour/l was used as the predictor of nephrotoxicity in this study.
Cost data for simulation
Only direct medical costs were counted in this study and expressed in Thai Bath. Direct cost is defined as the cost of the drug and any supporting material needed to administer the drug. There were several vancomycin products in Thailand. The chosen product in this study referred to the product used in a referral hospital in Thailand. The information about the material needed to administer the vancomycin was derived from a pharmacist who worked in a referral general hospital in Thailand and was supported with a reference [34]. The price of vancomycin and each supporting material was referred to the price list data recommended by the Health Intervention and Technology Assessment Program [35] Thailand and Drug Management System Information Centre (Supplementary file Table 2) [36]. When the analysis was conducted, the price for vancomycin was 131.3 Bath per vial of 500 mg vancomycin. Furthermore, the additional cost to manage nephrotoxicity adverse events was US$ 2,500, which was further adjusted in Thai Bath [36].
Each supporting material was used for a different number of days. The duration of using the infusion set, syringe, infusion pump, water for injection, and fluid for infusion was the same as the duration of the vancomycin prescription
[37–41]. The number of creatinine monitoring and therapeutic drug monitoring of vancomycin referred to the mean number of measurements used in the published study [38,40]. The total hospital stay was approximately 20 days and the ICU stay was 16 days [41]. The cost for the physician was the same for a total of 20 days [41]. In contrast, the cost of nurse services differed between the ICU and general wards. Therefore, ICU nurse services were multiplied by 16 days and general ward nurse services were multiplied by 4 days. Detailed duration of utilization for the drug, each supporting material, and hospital length of stay were presented in the Supplementary file Table 3.
Analysis of the cost-effectiveness model
The decision tree analysis was analyzed by using the path probability method. The clinical success for particular vancomycin dosage regimens was defined as the probability of 30-day survival.
- The probability of 30 days survival was calculated by multiplying the CFR for that particular vancomycin dosage regimen with the percentage of patients who survived when AUC24/MIC achieved ≥ 400 mg.hour/l. Moise-Broder et al. [12] found that 61% of patients survived when AUC24/MIC achieved ≥400 mg.hour/l.
- The expected cost for 30 days of survival was derived from the probability of 30 days of survival multiplied by the total direct cost.
- The probability of nephrotoxicity was calculated by multiplying the percentage of patients with AUC24 at steady state condition >1,300 with the incidence of nephrotoxicity. Lodise et al. [21] found that 4.2% of patients with AUC >1,300 got nephrotoxicity due to vancomycin.
- The expected cost for nephrotoxicity was derived from the probability of nephrotoxicity multiplied by the additional cost for nephrotoxicity [39].
- The expected cost for 30-day survival was added to the expected cost for nephrotoxicity management, and the result reflected the total expected cost for a particular dosage regimen of vancomycin. Finally, the total expected cost was used to calculate the incremental cost-effectiveness ratio (ICER).
Four ICERs were resulted at the end of this study and the lowest ICER was recommended as the most cost-effective dosage regimen of vancomycin.
Sensitivity analysis
The distribution of MIC plays an important role in achieving desired PK-PD indices. Since the MIC distribution in this study was derived from EUCAST, it might not always reflect the MIC distribution data in different settings. Therefore, sensitivity analysis was conducted by changing the proportion of strain with a particular MIC. Initially, the best scenario was set in which all MRSA strains were MIC 0.5 mg/l. Then, the proportion of MIC 0.5 mg/l was decreased by 10% and the proportion of MIC 1.0 mg/l was increased by 7.5%. The rest 2.5% incremental was for the strain with MIC 2 mg/l. After achieving proportions 0%, 75%, and 25% for the strain with MIC 0.5, 1.0, and 2.0 mg/l, respectively, the proportion of MIC 0.5 mg/l was held at 0% and the proportion of MIC 2 mg/l was increased by 5%. The proportion of MIC 1.0 mg/l was set to make the total proportion 100%.
RESULTS
MCS, using 5,000 replications, resulted in several PTAs and CFRs. The PTAs and CFRs of several vancomycin dosage regimens were presented in Tables 1 and 2, respectively. According to the data in Table 1, it was clearly shown that only vancomycin with a total daily dose of 4 g, either given as 1 g every 6 hours or 2 every 12 hours, could cover MRSA with MIC 2 mg/l. The results of the PTA calculation were multiplied by the proportion of MRSA with a particular MIC to get the CFRs. With the proportion of MIC of MRSA as presented in the Supplementary file Table 1, only vancomycin with a total dose of 4 g/day afforded acceptable CFR (≥ 90%). A total daily dose of vancomycin 4 g/day, either given as 1 g every 6 hours or 2 g every 12 hours, also afforded a higher risk of nephrotoxicity. Table 2 presents the probability of nephrotoxicity from several dosage regimens of vancomycin.
Giving vancomycin as 1 g every 6 hours regimen and standard dosage regimen afforded the highest and lowest total direct medical cost, i.e., 42,037.74686 and 34,676.36774 Thai Bath, respectively. The total direct cost for 1 g every 8 hours, 1.5 g every 12 hours, and 2 g every 12 hours were 38,357.0573; 38,027.0573; and 41,377.74686 Thai Bath, respectively. For any given dosage regimen, whenever nephrotoxicity occurs, the additional cost for nephrotoxicity management was 137,610.5795 Thai Bath. Table 3 presents the expected cost for 30 days survival, the expected cost for nephrotoxicity, and the total expected cost.
ICER analysis revealed that 1.5 g of vancomycin every 12 hours dosage regimen was the most cost-effective. Detailed information for ICER can be found in Table 4. Results of sensitivity analysis (Table 5) emphasized that vancomycin 1.5 g every 12 hours was the most cost-effective compared with other dosage regimens for different proportions of MIC of MRSA. In the best scenario analysis, i.e., all MRSA strains have MIC 0.5 mg/l, the ICER analysis revealed that vancomycin 1 g every 12 hours was dominant compared with any other dosage regimen. The difference in ICER value between the total daily dose of 3 g versus 4 g became lesser when the proportion of MRSA with higher MIC increased.
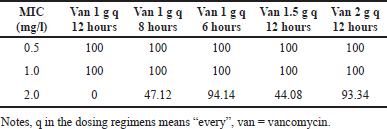 | Table 1. PTA for several dosage regimens of vancomycin. [Click here to view] |
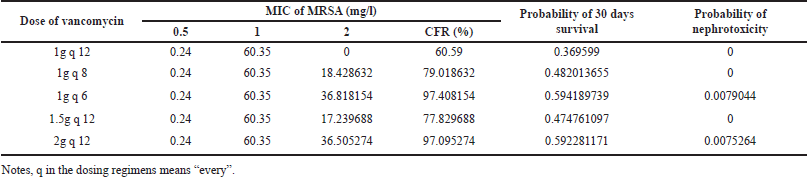 | Table 2. CFR, probability of 30 days survival, and probability of nephrotoxicity of several vancomycin dosage regimens. [Click here to view] |
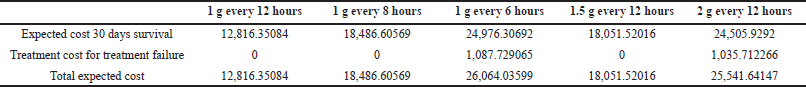 | Table 3. The expected cost for 30 days of survival, the expected cost for nephrotoxicity, and the total expected cost. [Click here to view] |
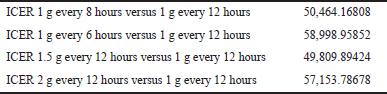 | Table 4. ICER of several vancomycin dosage regimens. [Click here to view] |
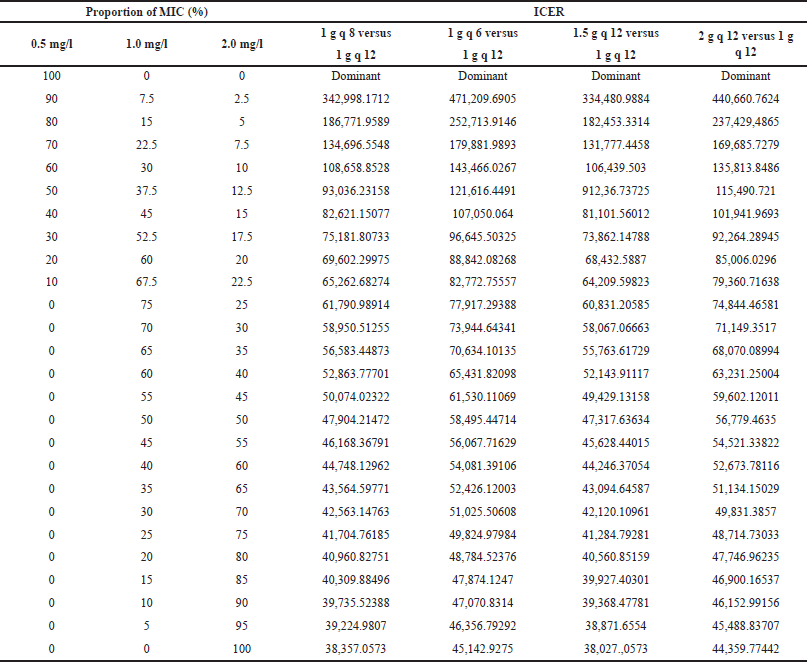 | Table 5. ICER for the sensitivity analysis. [Click here to view] |
DISCUSSION
This study was the first to identify the most cost-effective treatment for managing VAP in critically ill populations, considering several vancomycin dosage regimens’ efficacy and safety profiles. Findings on the potential of achieving desirable outcomes by increasing the dose of vancomycin would provide important guidance for clinicians in settings where no other antibiotics with MRSA coverage have been listed in the national formulary, such as in Indonesia. It should be acknowledged that linezolid might be considered the first choice therapy recommended to manage MRSA in VAP as it has excellent tissue penetration into the lung based on existing published literature. Several previously published studies indicated the superiority of linezolid compared to vancomycin in managing VAP patients with MRSA [42–46]. However, it is worth noting that the efficacy was compared by incorporating a standard dose of vancomycin, i.e., 2 g/day [23, 45, 47–49]. Our study found that vancomycin could still be a promising agent for treating MRSA infection, a finding similar to the study by Niederman et al. [50]. Dose incremental according to MIC value is the key to maintaining the efficacy of vancomycin against MRSA and, therefore, MIC should be identified before deciding the appropriate treatment. Linezolid could be prescribed for more complex indications, including 1) patients who could not tolerate vancomycin, 2) patients who were infected with vancomycin-intermediate Staphylococcus aureus and vancomycin-resistant Staphylococcus aureus, and 3) patients with pre-existing renal disease.
We found vancomycin 1.5 g given every 12 hours to be the most cost-effective dosage regimen. This dosage regimen was higher than the most frequently prescribed dose of vancomycin, i.e., 1 g every 12 hours. This finding is similar to several published studies that have proposed a higher dose of vancomycin [42,51]. Chung et al. [42] conducted a study among critically ill patients with pneumonia in Korea. They emphasized the need for an incremental vancomycin dose, particularly in critically ill patients with CLCr ≥60 ml/minute [42]. Another study by Jeurissen et al. [51] suggested prescribing vancomycin 3 g/day for critically ill patients with normal renal function based on a retrospective study of the relationship between CLCr and vancomycin clearance. Our finding was in line with the recommendation from Jeurissen et al. [51] with the additional economic evaluation incorporating the percentage efficacy and safety of several vancomycin dosage regimens. Our recommendation also did not contradict the findings of Lodise et al. [29] who suggested that vancomycin should be given less than 4 g/day to minimize the risk of nephrotoxicity.
Vancomycin 1.5 g given every 12 hours might not only offer an advantage in the clinical outcome of the patients but also might prevent the development of further resistant mechanisms. Two in-vitro studies revealed a higher number of AUC24/MIC afforded prevention from developing further resistant strains and a higher rate of MRSA eradication [52,53]. The first in-vitro study by Zelenitsky et al. [53] found that the higher AUC24/MIC value afforded a lower percentage of strain with reduced susceptibility to vancomycin. There were 31% strains with reduced vancomycin susceptibility (characterized with MIC ≥3 mg/l) when fAUC24/MIC ≤120 mg.hour/l was attained, while only 5% when the fAUC24/MIC was at ≥240 mg.hour/l (p = 0.003). Since vancomycin, usually defined as has 50% of protein binding, the value of fAUC24/MIC 120 and 240 mg.hour/l is the same with total AUC24/MIC 240 and 480 mg.hour/l, respectively. Moreover, no strain with reduced vancomycin susceptibility was found with the fAUC24/MIC between 480 and 960 mg.hour/l equals the total AUC24/MIC 960–1,920 mg.hour/l. The second in-vitro study also reported a similar finding. The fAUC24/MIC as high as 225 mg.hour/l afforded strain with lower MIC and less mean cell wall thickening than lower fAUC24/MIC [52]. A thicker cell wall is one characteristic of resistant strain [52]. These studies pointed out an important concept, i.e., the lower AUC24/MIC may afford a greater chance to develop further resistant strains of MRSA. The development of further resistant strains will impact the unfavorable treatment outcome and the economic burden on society.
Results from the sensitivity analysis emphasized better coverage of MRSA activity in a higher total daily dosage regimen when the proportion of higher MIC of MRSA increased. The ICER differences between the total daily dose of 3 g versus 4 g became narrow following the increase in the proportion of higher MIC of MRSA. For any proportion of MIC value, the total daily dose of 3 g was more cost-effective than the total daily dose of 4 g. This result was afforded because of the different risks of nephrotoxicity between these regimens. Even though a total daily dose of 4 g afforded higher efficacy than 3 g/day, this regimen also had a higher risk of nephrotoxicity. This study found no nephrotoxicity risk from a total daily dose of 3 g. The higher cost of a total daily dose of 4 g resulted from the additional cost of managing nephrotoxicity adverse events that would not be found in the 3 g/day dosage regimen. Therefore, this study never found a dosage regimen of 4 g/day more cost-effective than 3 g/day.
In our study, the ICER was calculated according to direct medical cost only, including the cost of products, supporting materials, physician and nurse fees, and additional costs to manage the adverse event of nephrotoxicity. This study did not consider productivity loss as the cost component in the ICER analysis, as commonly found in studies with chronic diseases (such as diabetes and Chronic Obstructive Pulmonary Disorder (COPD) [54,55]. It should be noted that, in general, VAP might not always result in severe morbidity as compared with other degenerative diseases such as diabetes and COPD. Survival was referred to as one of the fundamental goals when treating patients with VAP. Future studies might consider the economic burden of mortality rate and productivity loss, particularly if a high mortality rate occurs among people in their productive age.
The findings in our study should be interpreted with caution because of some limitations. First, our recommendation might not apply to this population since we used an assumption for patients without advanced renal impairment. Vancomycin is usually prescribed every alternate day for patients with advanced renal impairment. Second, the cost-effectiveness of a dosage regimen of 1.5 g every 12 hours in our study might be overestimated because of the same duration of treatment regardless of the total daily dose. Since different dosage regimens afforded different efficacy profiles, it would be possible that a shorter duration of treatment is needed by giving a higher vancomycin dosage regimen. Third, the nephrotoxicity from vancomycin with a total daily dose of 3 g might be underestimated because we just incorporated one cut-off point, i.e., AUC >1,300 mg.hour/l. Unfortunately, at the time of the analysis, we could not find other studies that revealed the association between AUC and risk nephrotoxicity. We also did not relate the duration of vancomycin treatment with the risk of nephrotoxicity. In this study, the discount rate was not considered in the analysis of the Montecarlo simulation for direct medical costs; therefore, the finding of our simulation might not always be relevant in all situations in the future. Finally, we did not consider other important factors that might influence the total expected cost, such as the severity of VAP, underlying condition, and comorbid condition. Finally, clinical studies are required to justify our recommendation.
CONCLUSION
This probabilistic simulated cost-effectiveness study suggested that vancomycin 1.5 g given every 12 hours is the most cost-effective dosage to treat VAP patients infected with “MIC Creep” MRSA. However, this cost-effective dosage regimen could not be generalized for all conditions and settings, as it might be influenced by renal function and comorbidities status. In the hospitals where most MRSA strains have MIC 0.5 mg/l, the standard dose of vancomycin might still be effective in treating MRSA infections. Patients without deep-site infection, such as urinary tract infection caused by MRSA, might not necessarily prescribed a higher dose of vancomycin.
AUTHOR CONTRIBUTIONS
Concept and design: ES, SVH, BP. Data acquisition: ES, SVH. Data analysis/interpretation: ES. Drafting manuscript: ES. Critical revision of manuscript: SVH, BP. Statistical analysis: ES, BP. All authors have read and agreed to the published version of the manuscript.
FINANCIAL SUPPORT
There is no source of funding for this study.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the journal’s website: Link here [https://japsonline.com/admin/php/uploadss/4358_pdf.pdf].
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Alp E, Voss A. Ventilator associated pneumonia and infection control. Ann Clin Microbiol Antimicrob. 2006;5:1–11. CrossRef
2. American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388. CrossRef
3. Doyle JS, Buising KL, Thursky KA, Worth LJ, Richards MJ, editors. Epidemiology of infections acquired in intensive care units. Semin Respir Crit Care Med. 2011;32(2):115–38. CrossRef
4. Tao L, Hu B, Rosenthal VD, Gao X, He L. Device-associated infection rates in 398 intensive care units in Shanghai, China: International nosocomial infection control consortium (INICC) findings. Int J Infect Dis. 2011;15(11):e774–80. CrossRef
5. Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51(Supplement_1):S81–7. CrossRef
6. Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166–74. CrossRef
7. Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36(1):53–9. CrossRef
8. Filice GA, Nyman JA, Lexau C, Lees CH, Bockstedt LA, Como-Sabetti K, et al. Excess costs and utilization associated with methicillin resistance for patients with Staphylococcus aureus infection. Infect Control Hosp Epidemiol. 2010;31(4):365–73. CrossRef
9. Wolkewitz M, Frank U, Philips G, Schumacher M, Davey P, Group BS, et al. Mortality associated with in-hospital bacteraemia caused by Staphylococcus aureus: a multistate analysis with follow-up beyond hospital discharge. J Antimicrob Chemother. 2011;66(2):381–6. CrossRef
10. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–55. CrossRef
11. Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52(8):975–81. CrossRef
12. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43:925–42. CrossRef
13. Pitz AM, Yu F, Hermsen ED, Rupp ME, Fey PD, Olsen KM. Vancomycin susceptibility trends and prevalence of heterogeneous vancomycin-intermediate Staphylococcus aureus in clinical methicillin-resistant s. aureus isolates. J Clin Microbiol. 2011;49(1):269–74. CrossRef
14. Robert J, Bismuth R, Jarlier V. Decreased susceptibility to glycopeptides in methicillin-resistant Staphylococcus aureus: a 20 year study in a large french teaching hospital, 1983–2002. J Antimicrob Chemother. 2006;57(3):506–10. CrossRef
15. Steinkraus G, White R, Friedrich L. Vancomycin mic creep in non-vancomycin-intermediate Staphylococcus aureus (visa), vancomycin-susceptible clinical methicillin-resistant s. aureus (mrsa) blood isolates from 2001–05. J Antimicrob Chemother. 2007;60(4):788–94. CrossRef
16. Jacob JT, DiazGranados CA. High vancomycin minimum inhibitory concentration and clinical outcomes in adults with methicillin-resistant Staphylococcus aureus infections: a meta-analysis. Int J Infect Dis. 2013;17(2):e93–100. CrossRef
17. Van Hal S, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis. 2012;54(6):755–71. CrossRef
18. Pea F, Viale P, Furlanut M. Antimicrobial therapy in critically ill patients: a review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin Pharmacokinet. 2005;44:1009–34. CrossRef
19. Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37(3):840–51. CrossRef
20. Revilla N, Martín-Suárez A, Pérez MP, González FM, Fernández de Gatta MDM. Vancomycin dosing assessment in intensive care unit patients based on a population pharmacokinetic/pharmacodynamic simulation. Br J Clin Pharmacol. 2010;70(2):201–12. CrossRef
21. Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis. 2009;49(4):507–14. CrossRef
22. Beringer PM, Wong-Beringer A, Rho JP. Economic aspects of antibacterial adverse effects. Pharmacoeconomics. 1998;13:35–49. CrossRef
23. Mullins CD, Kuznik A, Shaya FT, Obeidat NA, Levine AR, Liu LZ, et al. Cost-effectiveness analysis of linezolid compared with vancomycin for the treatment of nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Ther. 2006;28(8):1184–98. CrossRef
24. Rybak MJ, Albrecht L, Berman J, Warbasse L, Svensson C. Vancomycin pharmacokinetics in burn patients and intravenous drug abusers. Antimicrob Agents Chemother. 1990;34(5):792–5. CrossRef
25. Cano EL, Haque NZ, Welch VL, Cely CM, Peyrani P, Scerpella EG, et al. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: retrospective analysis of the impact-hap database. Clin Ther. 2012;34(1):149–57. CrossRef
26. Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68:1243–55. CrossRef
27. Gupta A, Biyani M, Khaira A. Vancomycin nephrotoxicity: Myths and facts. Neth J Med. 2011;69(9):379–83.
28. Jeffres MN, Isakow W, Doherty JA, Micek ST, Kollef MH. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther. 2007;29(6):1107–15. CrossRef
29. Lodise TP, Lomaestro B, Graves J, Drusano G. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008;52(4):1330–6. CrossRef
30. European Committee on Antimicrobial Susceptibility Testing. Antimicrobial wild type distributions of microorganisms. Växjö, Sweden: European Committee on Antimicrobial Susceptibility Testing. Available from: http://mic.eucast.org/ Eucast2/SearchController/search.jsp?action= perform Search& BeginIndex= 0&Micdif=mic&NumberIndex=50&Antib=38&Specium=-1
31. Bauer LA. Applied clinical pharmacokinetics. 2nd ed. New York, NY: The McGraw-Hill Companies, Inc; 2008.
32. DeRyke CA, Alexander DP. Optimizing vancomycin dosing through pharmacodynamic assessment targeting area under the concentration-time curve/minimum inhibitory concentration. Hosp Pharm. 2009;44(9):751–65. CrossRef
33. Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. Standardization of pharmacokinetic/pharmacodynamic (pk/pd) terminology for anti-infective drugs: an update. J Antimicrob Chemother. 2005;55(5):601–7. CrossRef
34. Trissel L. Handbook on injectable drugs. 15th ed. Bethesda, MD: American Society of Health-System Pharmacists, Inc; 2009.
35. Health Intervention and Technology Assessment Program. Standard cost lists for health technology assessment. Nonthaburi, Thailand: Thailand; 2009.
36. Ministry of Public Health. Drugs and medical supplies information center (DMSIC). Ministry of Public Health, Thailand; 2013. Available from: http://dmsic.moph.go.th/price/price1_1.php?method=drug
37. Chan JD, Pham TN, Wong J, Hessel M, Cuschieri J, Neff M, et al. Clinical outcomes of linezolid vs vancomycin in methicillin-resistant Staphylococcus aureus ventilator-associated pneumonia: retrospective analysis. J Intensive Care Med. 2011;26(6):385–91. CrossRef
38. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–70. CrossRef
39. Kollef MH, Rello J, Cammarata SK, Croos-Dabrera RV, Wunderink RG. Clinical cure and survival in gram-positive ventilator-associated pneumonia: retrospective analysis of two double-blind studies comparing linezolid with vancomycin. Intensive Care Med. 2004;30:388–94. CrossRef
40. Rojas L, Bunsow E, Munoz P, Cercenado E, Rodriguez-Creixems M, Bouza E. Vancomycin mics do not predict the outcome of methicillin-resistant Staphylococcus aureus bloodstream infections in correctly treated patients. J Antimicrob Chemother. 2012;67(7):1760–8. CrossRef
41. Wunderink RG, Mendelson MH, Somero MS, Fabian TC, May AK, Bhattacharyya H, et al. Early microbiological response to linezolid vs vancomycin in ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus. Chest. 2008;134(6):1200–7. CrossRef
42. Chung J, Oh J, Cho E, Jang H, Hong S, Lim C, et al. Optimal dose of vancomycin for treating methicillin-resistant Staphylococcus aureus pneumonia in critically ill patients. Anaesth Intensive Care. 2011;39(6):1030–7. CrossRef
43. De Cock E, Krueger W, Sorensen S, Baker T, Hardewig J, Duttagupta S, et al. Cost-effectiveness of linezolid vs vancomycin in suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia in germany. Infection. 2009;37:123–32. CrossRef
44. Grau S, Alvarez-Lerma F, Del Castillo A, Neipp R, Rubio-Terres C. Cost-effectiveness analysis of the treatment of ventilator-associated pneumonia with linezolid or vancomycin in spain. J Chemother. 2005;17(2):203–11. CrossRef
45. Machado AR, Arns CDC, Follador W, Guerra A. Cost-effectiveness of linezolid versus vancomycin in mechanical ventilation-associated nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus. Braz J Infect Dis. 2005;9:191–200. CrossRef
46. Shorr AF, Susla GM, Kollef MH. Linezolid for treatment of ventilator-associated pneumonia: a cost-effective alternative to vancomycin. Crit Care Med. 2004;32(1):137–43. CrossRef
47. Patel DA, Shorr AF, Chastre J, Niederman M, Simor A, Stephens JM, et al. Modeling the economic impact of linezolid versus vancomycin in confirmed nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus. Crit Care. 2014;18:1–9. CrossRef
48. Wan Y, Li Q, Chen Y, Haider S, Liu S, Gao X. Economic evaluation among chinese patients with nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus and treated with linezolid or vancomycin: a secondary, post-hoc analysis based on a phase 4 clinical trial study. J Med Econ. 2016;19(1):53–62. CrossRef
49. Buendía JA, Patiño DG, Zuluaga Salazar AF. Cost-effectiveness of linezolid to ventilator-associated pneumonia in colombia. BMC Infect Dis. 2024;24(1):98. CrossRef
50. Niederman MS, Chastre J, Solem CT, Wan Y, Gao X, Myers DE, et al. Health economic evaluation of patients treated for nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus: secondary analysis of a multicenter randomized clinical trial of vancomycin and linezolid. Clin Ther. 2014;36(9):1233–43. e1. CrossRef
51. Jeurissen A, Sluyts I, Rutsaert R. A higher dose of vancomycin in continuous infusion is needed in critically ill patients. Int J Antimicrob Agents. 2011;37(1):75–7. CrossRef
52. Rose WE, Knier RM, Hutson PR. Pharmacodynamic effect of clinical vancomycin exposures on cell wall thickness in heterogeneous vancomycin-intermediate Staphylococcus aureus. J Antimicrob Chemother. 2010;65(10):2149–54. CrossRef
53. Zelenitsky S, Alkurdi N, Weber Z, Ariano R, Zhanel G. Preferential emergence of reduced vancomycin susceptibility in health care-associated methicillin-resistant Staphylococcus aureus isolates during continuous-infusion vancomycin therapy in an in vitro dynamic model. Antimicrob Agents Chemother. 2011;55(7):3627–30. CrossRef
54. Hsieh HM, Gu SM, Shin SJ, Kao HY, Lin YC, Chiu HC. Cost-effectiveness of a diabetes pay-for-performance program in diabetes patients with multiple chronic conditions. PLoS One. 2015;10(7):e0133163. CrossRef
55. Bohingamu Mudiyanselage S, Stevens J, Watts JJ, Toscano J, Kotowicz MA, Steinfort CL, et al. Personalised telehealth intervention for chronic disease management: a pilot randomised controlled trial. J Telemed Telecare. 2019;25(6):343–52. CrossRef