INTRODUCTION
The genus Murraya has been split into Bergera and Murraya based on morphological and phytochemical differences [1]. Murraya koenigii (L.) Spreng. is now Bergera koenigii L., a small evergreen tropical tree of the family Rutaceae that is native to South, East, and Southeast Asia [2]. Fresh leaflets emit a unique sulphury and burnt aroma due to 1-phenylethanethiol, and are a compulsory spice for nearly all Indian curries and chutneys. Commonly known as the curry leaf tree, B. koenigii should be more appropriately called an Indian curry leaf tree.
The species is a shrub or small tree with a dark-brown stem and root bark. Leaves are pinnate, with individual leaflets having wavy margins and emitting a distinctive aroma (Fig. 1b). Inflorescences are axillary or terminal cymes, each bearing 60−90 flowers. Each flower is bisexual, white, funnel-shaped, and sweetly scented. Petals are five and whitish (Fig. 1a). Fruits are in close clusters, small berries, ovoid or sub-globose, turning purplish–black on ripening (Fig. 1c), and contain one or two green-colored seeds [1,3,4].
Alkaloids are a class of compounds containing at least one nitrogen atom. Carbazole alkaloids are characterized by a tricyclic aromatic basic skeleton with a central pyrrole ring fused between two benzene rings [5,6]. Carbazole alkaloids can broadly be divided into halogenated carbazole alkaloids, oxygenated carbazole alkaloids, carbazolequinone alkaloids, pyranocarbazole alkaloids, furocarbazole alkaloids, pyridocarbazole alkaloids, indolocarbazole alkaloids, dimeric carbazole alkaloids, tetrahydrocarbazole alkaloids, and other substituted carbazole alkaloids [6]. Carbazole alkaloids can also be divided into sub-classes based on the number of carbons. They include those with 13, 18, and 23 carbons; dimeric and trimeric carbazoles; and carbazoles with other moieties [5].
 | Figure 1. Flowers (a), leaves (b), and fruits (c) of Bergera koenigii. [Click here to view] |
In this article, the constituents of carbazole alkaloids in different plant parts of B. koenigii, and the anti-cancer properties of extracts, mahanine (MN), isomahanine (IMN), mahanimbine (MNB), and girinimbine (GNB) are reviewed. Other pharmacological properties of these carbazole alkaloids are briefly mentioned. The anti-cancer and other pharmacological properties of these four carbazole alkaloids have not been reviewed before. This short review is therefore justified. References for this article are mostly procured on databases such as Google, Google Scholar, Science Direct, PubMed, and J Stage. Search terms were based on the article title and keywords.
CHEMICAL CONSTITUENTS
A total of 101 carbazole alkaloids have been reported from different plant parts of B. koenigii (Table 1). They include carbazoles with 13 carbons and 2 methyl groups (e.g., murrayafoline A and murrayaquinone A); carbazoles with 18 carbons and 3 methyl groups (e.g., GNB and koenimbine); carbazoles with 23 carbons and 4 methyl groups (e.g., MN, IMN, and MNB); and dimeric carbazoles (e.g., bismahanine, bismurrayaquinone A, and bikoeniquinone A). From the leaf of B. koenigii, MNB (22) and koenimbine (16) are the two most dominant carbazole alkaloids, based on the number of reports. Most reports of these two compounds are from the leaf.
Carbazole alkaloids from B. koenigii selected for review are MN, IMN, MNB, and GNB (Fig. 2). They are classified as pyranocarbazoles because of their pyranocarbazole skeleton [7,8]. The pyranocarbazole is a tricyclic hetero-aromatic unit comprising two benzene rings A and C fused by a pyrrole ring B [9]. MN, IMN, and MNB are carbazoles with 23 carbons and four methyl groups [5]. MN (C23H25NO2 and 347.4 g/mol) was first isolated from the leaf of B. koenigii [10]. IMN (C23H25NO2 and 347.4 g/mol) was first isolated from the stem bark of M. euchrestifolia [11] and later from the leaf of B. koenigii [12]. MNB (C23H25NO, 331.4 g/mol) was first isolated from the leaf of B. koenigii [10]. GNB is another pyranocarbazole but with 18 carbons and three methyl groups. GNB (C18H17NO, 263.3 g/mol) was first isolated from the stem bark of B. koenigii [13].
From six climatic zones of India, 11 carbazole alkaloids were identified from the leaf of B. koenigii [14]. The contents of MN, GNB, MNB, and IMN were 0.01–7.34, 0.05–5.29, 0.01–1.67, and 0.01–0.11 mg/g, respectively. The contents of MN and MNB in the leaf of B. koenigii from Tamil Nadu were 9.6% and 4.3% w/w [15]. From the ethanol root extract of B. koenigii, the total phenolic content and total flavonoid content were reported to be 51.2 mg of gallic acid equivalent/g and 43.6 mg of catechin equivalent/g [16].
 | Table 1. Carbazole alkaloids from different plant parts of Bergera koenigii. [Click here to view] |
ANTI-CANCER PROPERTIES
Extracts
An earlier study reported that the aqueous methanol leaf extract of B. koenigii was cytotoxic against Caco2 colon, HeLa cervical, HepG2 liver, and LNCaP prostate cancer cells, with IC50 values of 8.07, 4.80, 17.5, and 16.4 μg/ml, respectively [17]. In terms of proteasome inhibition, a promising strategy for cancer therapy, their IC50 values were 12.5, 7.99, 43.4, and 12.4 μg/ml, respectively. With regard to the anti-cancer properties of B. koenigii extracts, breast cancer cells were the most commonly reported tumor cells. Glioma, colon, and cervical were the other cancer cells (Table 2). Five studies involved breast cancer cells [18–22] while the other cancer types were represented by single studies [23–25].
Carbazole alkaloids
MN is cytotoxic to leukemic, colon, lung, oral squamous, pancreatic, and breast cancer cells. The IC50 values of MN after 48 hours were 10.6 and 13.0 µM for MOLT-3 and K562 leukemic cells, respectively [26]. Against HCT116 (p53wt), HCT (p53null), and SW480 (p53mut) colon cancer cells, its IC50 values were 12.6, 13.9, and 16.6 µM [27]. The IC50 values of MN against A549, A549-TR, and H1299 lung cancer cells were 12.5, 12.5, and 10.0 µM, respectively [28]. IC50 values against A549 and H1299 lung cancer cells were 40.2 and 42.6 µM at 24 hours and 28.0 and 26.7 µM at 48 hours [28]. Against CLS-354 oral squamous carcinoma cells, the IC50 values of MN and IMN were 15.1 and 15.0 µM, respectively [67]. These values were slightly stronger than that of cisplatin, the anticancer drug, which has an IC50 value of 16.3 µM. MN enhanced cisplatin-induced apoptosis and reduced its effective concentration by 5–8 fold. IC50 values of MNB against CAPAN and SW119 pancreatic cancer cells were both 3.5 µM [68]. IC50 values against Hs172.T bladder and MCF-7 breast cancer cells were 32.5 µM [69] and 14 µM [70], respectively.
Anti-cancer effects of carbazole alkaloids of B. koenigii have been reported in nine types of cancer cells (Table 3). Four studies on MN involved prostate cancer cells [71–74], and two studies involved leukemia [26,75] and breast cancer cells [76,77]. Single studies on MN included colon [27], cervical [78], lung [79], pancreatic [80], and glioma [81] cancer cells. Cancer cells affected by MNB were lung [82], pancreatic [68], bladder [69], and breast [70] cancer cells. There was only one study on IMN involving oral squamous carcinoma cells [67]. Two studies on GNB involved colon cancer cells [83, 84] while one study each included anti-tumor [85], liver [86], lung [87], and breast [88] cancer cells.
The anti-cancer structure-activity relationship (SAR) of MN against five different cancer cell lines has been studied [89]. MN exhibited enhanced apoptosis compared to dehydroxymahanine, indicating a significant contribution of the C7−OH group. Methylation of the C7−OH group reduced its antiproliferative activity. The study provided evidence of the contribution of C7−OH and 9−NH groups of MN toward its cytotoxicity [89].
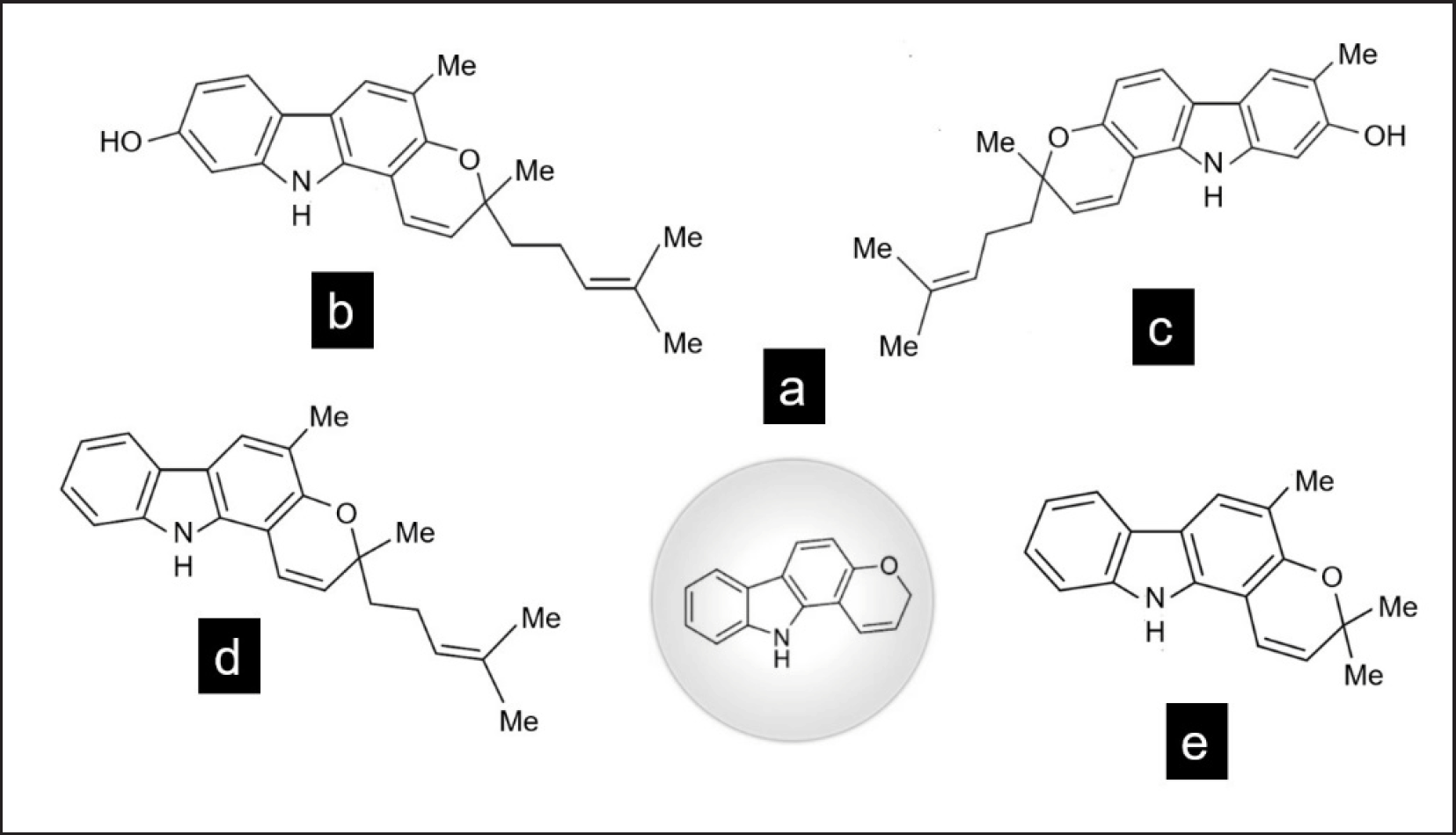 | Figure 2. Chemical structures of the pyranocarbazole skeleton (a), MN (b), IMN (c), MNB (d), and GNB (e). [Click here to view] |
 | Table 2. Anti-cancer properties of extracts from Bergera koenigii. [Click here to view] |
 | Table 3. Anti-cancer properties of MN, IMN, MNB, and GNB from Bergera koenigii. [Click here to view] |
OTHER PROPERTIES
Other pharmacological properties of MNB include anti-anxiety properties [90], anti-hyperglycemic and anti-lipidemic properties [91], neuroprotective [92], anti-obesity [93], acetylcholinesterase (AChE) inhibition [94], larvicidal [95], and reversal in age-related memory dysfunction [96]. Other pharmacological properties of GNB are anti-inflammatory [84], and anti-angiogenic activity [97], while MN stimulates glucose uptake [98] and promotes lipid-induced insulin resistance [99].
CONCLUSION
As concluding remarks, findings on the anti-cancer properties of extracts and carbazole alkaloids from B. koenigii that will generate interest for further research include: a) Extracts of B. koenigii inhibited proteasome and this led to cell cycle arrest, apoptosis, and reduced xenograft tumor. b) MN inhibited prostate cancer cells by down-regulating DNA methyltransferase (DNMT). c) Synthesis of silver nanoparticles using aqueous leaf extract of B. koenigii exerted potent cytotoxic activity. d) MN inhibited both drug-sensitive A549 and taxol-resistant A549-TR lung cancer cells with cytotoxicity of equal potency. e) Against CLS-354 oral squamous carcinoma cells, MN possessed cytotoxicity that was slightly stronger than cisplatin, and it enhanced cisplatin-induced apoptosis by 5–8 fold. f) GNB-induced apoptosis in HCT-15 colon cancer cells via the rapid decrease of mitochondrial transmembrane potential. g) MNB synergistically enhanced the efficiency of gefitinib by increasing its intracellular accumulation in A549 lung cancer cells. h) More in-depth studies on the anti-cancer SAR of MN and other carbazole alkaloids from B. koenigii are also needed.
AUTHOR CONTRIBUTIONS
The authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. The authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJEs) requirements/guidelines.
FINANCIAL SUPPORT
Assoc. Prof. Eric W. C. Chan, the Lead Author, acknowledges that the funds for the publication of this review article in the Journal of Applied Pharmaceutical Science (JAPS) as article processing charges (APC) are provided by UCSI University. The authors are grateful for the World’s Top 2% Scientist Research Grant, awarded by CERVIE (Grant Code: T2S-2024/004).
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Mou FJ, Hu X, Ha BT, Cuong NM. Taxonomic revision of Bergera J. Koenig ex L. (Rutaceae) based on the molecular phylogeny and morphology. Eur J Taxon. 2023;860:141−80. https://doi.org/10.5852/ejt.2023.860.2057
2. Steinhaus M. Characterization of the major odour-active compounds in the leaves of the curry tree Bergera koenigii L. by aroma extract dilution analysis. J Agric Food Chem. 2015;63(16):4060−7. CrossRef
3. Gahlawat DK, Jakhar S, Dahiya P. Murraya koenigii (L.) Spreng: an ethnobotanical, phytochemical and pharmacological review. J Pharmacogn Phytochem. 2014;3(3):109−19.
4. Franyoto YD, Nurrochmad A, Nanang Fakhrudin N. Murraya koenigii L. Spreng: an updated review of chemical composition, pharmacological effects, and toxicity studies. J Appl Pharm Sci. 2024;14(4). CrossRef
5. Greger H. Phytocarbazoles: alkaloids with great structural diversity and pronounced biological activities. Phytochem Rev. 2017;16(6):1095−153. CrossRef
6. Ding YY, Zhou H, Zhang BQ, Zhang ZJ, Wang GH, Zhang SY, et al. Antimicrobial activity of natural and semi-synthetic carbazole alkaloids. Eur J Med Chem. 2023;259:115627. CrossRef
7. Kumar N, Singh KK, Luthra PM. A review on anticancer potential of some pyranocarbazole alkaloids and its derivatives Int J Adv Res. 2021;9:874−83. doi: http://dx.doi.org/10.21474/IJAR01 /13091
8. Song F, Liu D, Huo X, Qiu D. The anticancer activity of carbazole alkaloids. Arch Pharm. 2022;355(1):2100277. doi: http://dx.doi.org/10.1002/ardp.202100277
9. Viteritti E, Oliva E, Eugelio F, Fanti F, Palmieri S, Bafile E, et al. Analysis of carbazole alkaloids in Murraya koenigii by means of high-performance liquid chromatography coupled to tandem mass spectrometry with a predictive multi-experiment approach. J Chromatogr Open. 2022;2:100055. CrossRef
10. Narasimhan NS, Paradkar MV, Chitguppi VP, Kelkar SL. Alkaloids of Murraya koenigii: structures of mahanine, koenine, koenigine and koenidine. Indian J Chem. 1975;13:993−9.
11. Ito C, Nakagawa M, Wu TS, Furukawa H. New carbazole alkaloids from Murraya euchrestifolia. Chem Pharm Bull. 1991;39(10):2525−8. CrossRef
12. Tan SP, Ali AM, Nafiah MA, Awang K, Ahmad K. Isolation and cytotoxic investigation of new carbazole alkaloids from Murraya koenigii (Linn.) Spreng. Tetrahedron. 2015;71(23):3946−53. CrossRef
13. Chakraborty DP, Barman BK, Bose PK. On the structure of girinimbine, a pyrano-carbazole derivative, isolated from Murraya koenigii Spreng. Sci Cult. 1964;30:445.
14. Nandan S, Singh SK, Singh P, Bajpai V, Mishra AK, Joshi T, et al. Quantitative analysis of bioactive carbazole alkaloids in Murraya koenigii (L.) from six different climatic zones of India using UPLC/MS/MS and their principal component analysis. Chem Biodivers. 2021;18(12):e2100557. CrossRef
15. Pandit S, Kumar M, Ponnusankar S, Pal BC, Mukherjee PK. RP-HPLC-DAD for simultaneous estimation of mahanine and mahanimbine in Murraya koenigii. Biomed Chromatogr. 2011;25(9):959−62. CrossRef
16. Sindhu RK, Arora S. Evaluation of phenolic contents and antioxidant potential of Murraya koenigii (L) spreng roots. J Appl Pharm Sci. 2012;2(11):120−2. CrossRef
17. Noolu B, Ismail A. Anti-proliferative and proteasome inhibitory activity of Murraya koenigii leaf extract in human cancer cell lines. Discov Phytomed. 2015;2(1):1−9. doi: https:// doi.org/10.15562/phytomedicine.2015.18
18. Noolu B, Ajumeera R, Chauhan A, Nagalla B, Manchala R, Ismail A. Murraya koenigii leaf extract inhibits proteasome activity and induces cell death in breast cancer cells. BMC Complement Altern Med. 2013;13(1):1−7. CrossRef
19. Yeap SK, Abu N, Mohamad NE, Beh BK, Ho WY, Ebrahimi S, et al. Chemopreventive and immunomodulatory effects of Murraya koenigii aqueous extract on 4T1 breast cancer cell-challenged mice. BMC Complement Altern Med. 2015;15:306. CrossRef
20. Ismail A, Noolu B, Gogulothu R, Perugu S, Rajanna A, Babu SK. Cytotoxicity and proteasome inhibition by alkaloid extract from Murraya koenigii leaves in breast cancer cells—molecular docking studies. J Med Food. 2016;19(12):1155−65. CrossRef
21. Noolu B, Gogulothu R, Bhat M, SYH Qadri S, Sudhakar Reddy V, Bhanuprakash Reddy G, et al. In vivo inhibition of proteasome activity and tumour growth by Murraya koenigii leaf extract in breast cancer xenografts and by its active flavonoids in breast cancer cells. Anti-Cancer Agents Med Chem. 2016;16(12):1605−14.
22. Aisyah S, Handharyani E, Bermawie N, Setiyono A. Effects of ethanol extract of curry leaves (Murraya koenigii) on HER2 and caspase-3 expression in rat model mammary carcinoma. Vet World. 2021;14(8):1988. CrossRef
23. Sanaye M, Pagare N. Evaluation of antioxidant effect and anticancer activity against human glioblastoma (U373MG) cell lines of Murraya koenigii. Pharmacogn J. 2016;8(3):220−5. https://doi.org/10.5530/pj.2016.3.7
24. Roshni K, Younis M, Ilakkiyapavai D, Basavaraju P, Puthamohan VM. Anticancer activity of biosynthesized silver nanoparticles using Murraya koenigii leaf extract against HT-29 colon cancer cell line. J Cancer Sci Ther. 2018;10(4):72−5. CrossRef
25. Amna U, Wahyuningsih P, Saidi N, Nasution R. Evaluation of cytotoxic activity from Temurui (Murraya koenigii [Linn.] Spreng) leaf extracts against HeLa cell line using MTT assay. J Adv Pharm Technol Res. 2019;10(2):51−5. CrossRef
26. Bhattacharya K, Samanta SK, Tripathi R, Mallick A, Chandra S, Pal BC, et al. Apoptotic effects of mahanine on human leukemic cells are mediated through cross-talk between Apo-1/Fas signaling and the Bid protein and via mitochondrial pathways. Biochem Pharmacol. 2010;79(3):361−72. CrossRef
27. Das R, Bhattacharya K, Sarkar S, Samanta SK, Pal BC, Mandal C. Mahanine synergistically enhances cytotoxicity of 5-fluorouracil through ROS-mediated activation of PTEN and p53/p73 in colon carcinoma. Apoptosis. 2014;19:149−64. CrossRef
28. Kandimalla R, Aqil F, Moholkar DN, Samanta SK, Gupta RC. Mahanine, a carbazole alkaloid attenuates lung cancer progression. Cancer Res. 2022;82(12 Suppl):707. doi: https:// doi.org/10.1158/1538-7445.AM2022-707
29. Ma Q, Tian J, Yang J, Wang A, Ji T, Wang Y, et al. Bioactive carbazole alkaloids from Murraya koenigii (L.) Spreng. Fitoterapia. 2013;87:1−6. CrossRef
30. Sim KM, Teh HM. A new carbazole alkaloid from the leaves of Malayan Murraya koenigii. J Asian Nat Prod Res. 2011;13(10):972−5. CrossRef
31. Nalli Y, Khajuria V, Gupta S, Arora P, Riyaz-Ul-Hassan S, Ahmed Z, et al. Four new carbazole alkaloids from Murraya koenigii that display anti-inflammatory and anti-microbial activities. Org Biomol Chem. 2016;14(12):3322−32. CrossRef
32. Tan SP, Nafiah MA, Ahmad K. C23-carbazole alkaloids from Malayan Murraya koenigii (L.) Spreng. J Chem Pharm Res. 2014;6(4):1093−8.
33. Tachibana Y, Kikuzaki H, Lajis NH, Nakatani N. Comparison of antioxidative properties of carbazole alkaloids from Murraya koenigii leaves. J Agric Food Chem. 2003;51(22):6461–7. CrossRef
34. Balakrishnan R, Vijayraja D, Jo SH, Ganesan P, Su-Kim I, Choi DK. Medicinal profile, phytochemistry, and pharmacological activities of Murraya koenigii and its primary bioactive compounds. Antioxidants. 2020;9(2):101. https://doi.org/10.3390/antiox9020 101
35. Aniqa A, Kaur S, Sadwal S. A review of the anti-cancer potential of Murraya koenigii (curry tree) and its active constituents. Nutr Cancer. 2022;74(1):12−26. CrossRef
36. Chakraborty M, Nath AC, Khasnobis S, Chakrabarty M, Konda Y, Harigaya Y, et al. Carbazole alkaloids from Murraya koenigii. Phytochemistry. 1997;46(4):751−5. doi: https:// doi.org/10.1016/S0031-9422(97)00345-2
37. Chowdhury BK, Jha S, Bhattacharyya P, Mukherjee J. Two new carbazole alkaloids from Murraya koenigii. Indian J Chem. 2001;40B:490−4.
38. Tachibana Y, Kikuzaki H, Lajis NH, Nakatani N. Antioxidative activity of carbazoles from Murraya koenigii leaves. J Agric Food Chem. 2001;49(11):5589−94. CrossRef
39. Ito C, Itoigawa M, Nakao K, Murata T, Tsuboi M, Kaneda N, et al. Induction of apoptosis by carbazole alkaloids isolated from Murraya koenigii. Phytomedicine. 2006;13(5):359−65. CrossRef
40. Chatterjee D, Narzish F, Borade P, Singh IP. Simultaneous quantitation of nine carbazole alkaloids from Murraya koenigii (L.) Spreng by 1H qNMR spectroscopy. Nat Prod Res. 2023;1–9. CrossRef
41. Mehreen A, Kamal S, Musayeva S, Qaisar M, Urainab S, Ullah A. Novel carbazole alkaloid from Murraya koenigii (l.) Spreng. Int J Second Metab. 2023;10(3):354−60. CrossRef
42. Reisch J, Adebajo AC, Kumar V, Aladesanmi AJ. Two carbazole alkaloids from Murraya koenigii. Phytochemistry. 1994;36(4):1073−6. CrossRef
43. Chakraborty M. Identification of naturally occurring carbazole alkaloids isolated from Murraya koenigii and Glycosmis pentaphylla by the preparation of HPLC fingerprint. J Sci Res. 2022;14(1):289−300. CrossRef
44. Abu Bakar NH, Sukari MA, Rahmani M, Sharif AM, Khalid K, Yusuf UK. Chemical constituents from stem barks and roots of Murraya koenigii (Rutaceae). Malaysian J Anal Sci. 2007;11(1):173−6.
45. Ng RC, Kassim NK, Yeap YS, Ee GC, Yazan SL, Musa KH. Isolation of carbazole alkaloids and coumarins from Aegle marmelos and Murraya koenigii and their antioxidant properties. Sains Malays. 2018;47(8):1749−56. doi: http://dx.doi.org/10.17576/ jsm-2018-4708-14
46. Chakraborty M, Saha S, Mukhapadhyay S. Murrayakoeninol—a new carbazole alkaloid from Murraya koenigii (Linn) Spreng. Nat Prod Commun. 2009;4(3):355−8. CrossRef
47. Reisch J, Goj O, Wickramasinghe A, Herath HB, Henkel G. Carbazole alkaloids from seeds of Murraya koenigii. Phytochemistry. 1992;31(8):2877−9. CrossRef
48. Joshi BS, Kamat VN, Gawad DH. On the structures of girinimbine, mahanimbine, isomahanimbine, koenimbidine and murrayacine. Tetrahedron. 1970;26(5):1475−82. CrossRef
49. Bhattacharyya P, Maiti AK, Basu K, Chowdhury BK. Carbazole alkaloids from Murraya koenigii. Phytochemistry. 1994;35(4):1085−6. CrossRef
50. Patel OP, Mishra A, Maurya R, Saini D, Pandey J, Taneja I, et al. Naturally occurring carbazole alkaloids from Murraya koenigii as potential antidiabetic agents. J Nat Prod. 2016;79(5):1276−84. CrossRef
51. Arun A, Patel OP, Saini D, Yadav PP, Konwar R. Anti-colon cancer activity of Murraya koenigii leaves is due to constituent murrayazoline and O-methylmurrayamine A induced mTOR/AKT down-regulation and mitochondrial apoptosis. Biomed Pharmacother. 2017;93:510−21. CrossRef
52. Wang YS, He HP, Hong X, Zhao Q, Hao XJ. A new binary carbazole alkaloid from Murraya koenigii. Chin Chem Lett. 2002;13(9):849−50.
53. Abeysinghe DT, Alwis DD, Kumara KA, Chandrika UG. Nutritive importance and therapeutics uses of three different varieties (Murraya koenigii, Micromelum minutum, and Clausena indica) of curry leaves: an updated review. Evid-Based Complement Altern Med. 2021;2021:23. CrossRef
54. Fiebig M, Pezzuto JM, Soejarto DD, Kinghorn AD. Koenoline, a further cytotoxic carbazole alkaloid from Murraya koenigii. Phytochemistry. 1985;24(12):3041−3. doi: https:// doi.org/10.1016/0031-9422(85)80052-2
55. Mandal S, Nayak A, Banerjee SK, Banerji J, Banerji A. A new carbazole alkaloid from Murraya koenigii Spreng (Rutaceae). Nat Prod Commun. 2008;3(10):1679−82. doi: https:// doi.org/10.1177/1934578X08003010
56. Nagappan T, Ramasamy P, Wahid ME, Segaran TC, Vairappan CS. Biological activity of carbazole alkaloids and essential oil of Murraya koenigii against antibiotic resistant microbes and cancer cell lines. Molecules. 2011;16(11):9651−64. CrossRef
57. Ramsewak RS, Nair MG, Strasburg GM, DeWitt DL, Nitiss JL. Biologically active carbazole alkaloids from Murraya koenigii. J Agric Food Chem. 1999;47(2):444−7. doi: https: //doi.org/10.1021/jf9805808
58. Ito C, Thoyama Y, Omura M, Kajiura I, Furukawa H. Alkaloidal constituents of Murraya koenigii. Isolation and structural elucidation of novel binary carbazolequinones and carbazole alkaloids. Chem Pharm Bull. 1993;41(12):2096−100. CrossRef
59. Mukherjee M, Mukherjee S, Shaw AK, Ganguly SN. Mukonicine, a carbazole alkaloid from leaves of Murraya koenigii. Phytochemistry. 1983;22(10):2328−9. CrossRef
60. Chakraborty M. Mumunine—a new carbazole alkaloid from Murraya koenigii (Linn.) Spreng. J Sci Res. 2020;12(4):665−72. CrossRef
61. Sukari MA, Ahmad K, Haron MJ, Muse R. Carbazole alkaloids from roots of Murraya koenigii (Rutaceae). Malaysian J Anal Sci. 2001;7(1):263−5.
62. Wang YS, He HP, Shen YM, Hong X, Hao XJ. Two new carbazole alkaloids from Murraya koenigii. J Nat Prod. 2003;66(3):416−8. CrossRef
63. Chakraborty DP, Barman BK, Bose PK. On the constitution of murrayanine, a carbazole derivative isolated from Murraya koenigii Spreng. Tetrahedron. 1965;21(2):681−5. doi: https: //doi.org/10.1016/S0040-4020(01)82240-7
64. Uvarani C, Sankaran M, Jaivel N, Chandraprakash K, Ata A, Mohan PS. Bioactive dimeric carbazole alkaloids from Murraya koenigii. J Nat Prod. 2013;76(6):993−1000. doi: https:// doi.org/10.1021/np300464t
65. Rahman MM, Gray AI. A benzoisofuranone derivative and carbazole alkaloids from Murraya koenigii and their antimicrobial activity. Phytochemistry. 2005;66(13):1601−6. CrossRef
66. Sampath SN, Jayasinghe S, Attanayake AP, Karunaratne V, Yaddehige ML, Watkins DL. A new dimeric carbazole alkaloid from Murraya koenigii (L.) leaves with α-amylase and α-glucosidase inhibitory activities. Phytochem Lett. 2022;52:87−91. CrossRef
67. Utaipan T, Athipornchai A, Suksamrarn A, Chunsrivirot S, Chunglok W. Isomahanine induces endoplasmic reticulum stress and simultaneously triggers p38 MAPK-mediated apoptosis and autophagy in multidrug-resistant human oral squamous cell carcinoma cells. Oncol Res. 2017;37(2):1243−52. CrossRef
68. Pei C, He Q, Liang S, Gong X. Mahanimbine exerts anticancer effects on human pancreatic cancer cells by triggering cell cycle arrest, apoptosis, and modulation of AKT/mammalian target of rapamycin (mTOR) and signal transducer and activator of transcription 3 (STAT3) signalling pathways. Med Sci Monit. 2018;24:6975. doi: https://doi. org/10.12659/MSM.911013
69. Xie H, Zhang T, Yang N, Li Z, Liu Y. Anticancer effects of mahanimbine alkaloid on the human bladder cancer cells are due to the induction of G0/G1 cell cycle arrest, apoptosis and autophagy. J BUON. 2020;25:1166−71.
70. Hobani YH. Cytotoxicity of mahanimbine from curry leaves in human breast cancer cells (MCF-7) via mitochondrial apoptosis and anti-angiogenesis. Molecules. 2022;27(3):971. CrossRef
71. Jagadeesh S, Sinha S, Pal BC, Bhattacharya S, Banerjee PP. Mahanine reverses an epigenetically silenced tumor suppressor gene RASSF1A in human prostate cancer cells. Biochem Biophys Res Commun. 2007;362(1):212−7. CrossRef
72. Agarwal S, Amin KS, Jagadeesh S, Baishay G, Rao PG, Barua NC, et al. Mahanine restores RASSF1A expression by down-regulating DNMT1 and DNMT3B in prostate cancer cells. Mol Cancer. 2013;12:99. CrossRef
73. Amin KS, Jagadeesh S, Baishya G, Rao PG, Barua NC, Bhattacharya S, et al. A naturally derived small molecule disrupts ligand-dependent and ligand-independent androgen receptor signaling in human prostate cancer cells mahanine disrupts AR signaling in prostate cancer cells. Mol Cancer Ther. 2014;13(2):341−52. CrossRef
74. Sinha S, Pal BC, Jagadeesh S, Banerjee PP, Bandyopadhaya A, Bhattacharya S. Mahanine inhibits growth and induces apoptosis in prostate cancer cells through the deactivation of Akt and activation of caspases. Prostate. 2006;66(12):1257−65. CrossRef
75. Roy MK, Thalang VN, Trakoontivakorn G, Nakahara K. Mechanism of mahanine-induced apoptosis in human leukemia cells (HL-60). Biochem Pharmacol. 2004;67(1):41−51. doi: https: //doi.org/10.1016/j.bcp.2003.07.021
76. Samanta SK, Choudhury P, Kandimalla R, Aqil F, Moholkar DN, Gupta RC, et al. Mahanine mediated therapeutic inhibition of estrogen receptor-α and CDK4/6 expression, decipher the chemoprevention-signaling cascade in preclinical model of breast cancer. J Ethnopharmacol. 2024;319:117235. CrossRef
77. Das M, Kandimalla R, Gogoi B, Dutta KN, Choudhury P, Devi R, et al. Mahanine, a dietary phytochemical, represses mammary tumor burden in rat and inhibits subtype regardless breast cancer progression through suppressing self-renewal of breast cancer stem cells. Pharmacol Res. 2019;146:104330. CrossRef
78. Das R, Bhattacharya K, Samanta SK, Pal BC, Mandal C. Improved chemosensitivity in cervical cancer to cisplatin: synergistic activity of mahanine through STAT3 inhibition. Cancer Lett. 2014;351(1):81−90. CrossRef
79. Chatterjee P, Seal S, Mukherjee S, Kundu R, Bhuyan M, Barua NC, et al. A carbazole alkaloid deactivates mTOR through the suppression of rictor and that induces apoptosis in lung cancer cells. Mol Cell Biochem. 2015;405:149−58. CrossRef
80. Bhattacharya SS, Mandal C, Albiez RS, Samanta SK, Mandal C. Mahanine drives pancreatic adenocarcinoma cells into endoplasmic reticular stress-mediated apoptosis through modulating sialylation process and Ca2+-signaling. Sci Rep. 2018;8(1):3911. CrossRef
81. Chen M, Yin X, Lu C, Chen X, Ba H, Cai J, et al. Mahanine induces apoptosis, cell cycle arrest, inhibition of cell migration, invasion and PI3K/AKT/mTOR signalling pathway in glioma cells and inhibits tumor growth in vivo. Chem-Biol Interact. 2019;299:1−7. doi: https:// doi.org/10.1016/j.cbi.2018.11.009
82. Mondal P, Natesh J, Salam AA, Meeran SM. Mahanimbine isolated from Murraya koenigii inhibits P-glycoprotein involved in lung cancer chemoresistance. Bioorg Chem. 2022;129:106170. CrossRef
83. Wang SL, Cai B, Cui CB, Yan SY, Wu CF. Study on induction of apoptosis by girinimbine in HCT-15 cell in vitro. Chin J Pharm Anal. 2008;28(2):176−81.
84. Iman V, Mohan S, Abdelwahab SI, Karimian H, Nordin N, Fadaeinasab M, et al. Anticancer and anti-inflammatory activities of girinimbine isolated from Murraya koenigii. Drug Des Devel Ther. 2016;11:103−21. CrossRef
85. Kok YY, Mooi LY, Ahmad K, Sukari MA, Mat N, Rahmani M, et al. Anti-tumour promoting activity and antioxidant properties of girinimbine isolated from the stem bark of Murraya koenigii S. Molecules. 2012;17(4):4651−60. CrossRef
86. Syam S, Abdul AB, Sukari MA, Mohan S, Abdelwahab SI, Wah TS. The growth suppressing effects of girinimbine on HepG2 involve induction of apoptosis and cell cycle arrest. Molecules. 2011;16(8):7155−70. CrossRef
87. Mohan S, Abdelwahab SI, Cheah SC, Sukari MA, Syam S, Shamsuddin N, et al. Apoptosis effect of girinimbine isolated from Murraya koenigii on lung cancer cells in vitro. Evid-Based Complement Altern Med. 2013;2013:12. CrossRef
88. Yang L, Yu X. Naturally occurring girinimbine alkaloid inhibits the proliferation, migration, and invasion of human breast cancer cells via induction of apoptosis and inhibition of MEK/Erk and STAT3 signalling pathways. Acta Biochim Pol. 2021;68(4):647−52. CrossRef
89. Samanta SK, Dutta D, Roy S, Bhattacharya K, Sarkar S, Dasgupta AK, et al. Mahanine, a DNA minor groove binding agent exerts cellular cytotoxicity with involvement of C7−OH and −NH functional groups. J Med Chem. 2013;56(14):5709−21. CrossRef
90. Dahiya J, Singh J, Kumar A, Sharma A. Isolation, characterization and quantification of an anxiolytic constituent—mahanimbine from Murraya koenigii Linn. Spreng leaves. J Ethnopharmacol. 2016;193:706−11. CrossRef
91. Dineshkumar B, Mitra A, Mahadevappa M. Antidiabetic and hypolipidemic effects of mahanimbine (carbazole alkaloid) from Murraya koenigii (Rutaceae) leaves. Int J Phytomed. 2010;2:22−30. CrossRef
92. Azahan NS, Mani V, Ramasamy K, Lim SM, James RM, Alsharidah M, et al. Mahanimbine-induced neuroprotection via cholinergic system and attenuated amyloidogenesis as well as neuroinflammation in lipopolysaccharides-induced mice. Pharmacogn Mag. 2020;16(68):57−63. CrossRef
93. Birari R, Javia V, Bhutani KK. Antiobesity and lipid lowering effects of Murraya koenigii (L.) Spreng leaves extracts and mahanimbine on high fat diet induced obese rats. Fitoterapia. 2010;81(8):1129−33. CrossRef
94. Kumar NS, Mukherjee PK, Bhadra S, Saha BP, Pal BC. Acetylcholinesterase inhibitory potential of a carbazole alkaloid, mahanimbine, from Murraya koenigii. Phytother Res. 2010;24(4):629−31. CrossRef
95. Sukari MA, Noor HM, Bakar NA, Ismail IS, Rahmani M, Abdul AB. Larvicidal carbazole alkaloids from Murraya koenigii against dengue fever mosquito Aedes aegypti Linnaeus. Asian J Chem. 2013;25(14):7719−21.
96. Mani V, Mohd Azahan NS, Ramasamy K, Lim SM, Abdul Majeed AB. Mahanimbine improved aging-related memory deficits in mice through enhanced cholinergic transmission and suppressed oxidative stress, amyloid levels, and neuroinflammation. Brain Sci. 2021;12(1):12. CrossRef
97. Iman V, Karimian H, Mohan S, Hobani YH, Noordin MI, Mustafa MR, et al. In vitro and in vivo anti-angiogenic activity of girinimbine isolated from Murraya koenigii. Drug Des Devel Ther. 2015;9:1281−92. CrossRef
98. Nooron N, Athipornchai A, Suksamrarn A, Chiabchalard A. Mahanine enhances the glucose-lowering mechanisms in skeletal muscle and adipocyte cells. Biochem Biophy Res Commun. 2017;494(1-2):101−6. CrossRef
99. Biswas A, Bhattacharya S, Dasgupta S, Kundu R, Roy SS, Pal BC, et al. Insulin resistance due to lipid-induced signaling defects could be prevented by mahanine. Mol Cell Biochem. 2010;336:97−107. CrossRef