INTRODUCTION
Enzyme biosynthesis is generally done using a recombinant DNA technology approach. Recombinant DNA technology itself is defined as the transfer of genetic materials of one organism into another to enable easier manipulation, research, and achieve products with desired characteristics en masse [1]. Escherichia coli serves as a frequently utilized host cell for the expression of recombinant proteins [2]. This is due to E. coli possessing advantages such as a rapid growth rate, affordable growth mediums, and a well-documented genetic profile [3,4]. Specifically, E. coli BL21(DE3) was selected as the host strain because it is widely employed in recombinant protein production due to its lower levels of proteases caused by deficiencies in the protease Lon and OmpT gene. Numerous types of enzymes, including the Thermus thermophilus (Tth) DNA Polymerase enzyme, have been effectively produced in E. coli hosts. Derived from the thermophilic bacterium T. thermophilus, Tth DNA polymerase exhibits dual functionality as both reverse transcriptase and DNA polymerase [5,6].
Protein production in E. coli is accomplished through two methods: intracellularly and extracellularly [1,7,8]. In intracellular expression, proteins are synthesized within the cytoplasm of the cell [9], whereas extracellular expression in E. coli entails protein translocation into the periplasm or culture medium through fusion with signal peptides such as OmpA, TorA, PhoA, and PelB [10,11,12]. According to Su et al. [13], extracellular expression generally yields lower levels of protein compared to intracellular expression. Consequently, intracellular protein expression is often employed as a method to enhance recombinant protein production. However, intracellular protein expression in E. coli is subject to certain limitations, such as the formation of inclusion bodies. Multiple factors influence the formation of these inclusion bodies, including misfolding of proteins, low levels of chaperones, which impede proper protein conformation under reduced cytoplasmic conditions, and susceptibility to degradation by proteases [14]. To address this challenge, various strategies have been devised, such as using MBP-tagged fusion proteins, refolding with the freeze-thawing method, adding a redox system (GSH/GSSG), and co-expression with chaperone [15–19].
Chaperones are proteins that aid in monitoring non-native conformations, stabilizing proteins, and facilitating the folding process, despite not being part of the final native structure of the protein [20]. The co-expression of chaperones with heterologous proteins in E. coli has been shown to elevate the soluble fraction and diminish the occurrence of inclusion body formation. As indicated by reference [21], concurrent co-expression of GroEL/ES and Trigger factor in E. coli facilitates protein folding and impedes the formation of inclusion bodies in recombinant proteins. GroE proteins are molecular chaperones derived from E. coli, with GroEL and GroES encoding proteins of sizes 57 kDa and 10–15 kDa, respectively [22]. The GroEL rings create a cavity serving as a folding chamber for polypeptide substrates [23]. GroES functions as a co-chaperone for GroEL. Another relevant chaperone is the Trigger Factor, which operates independently of ATP and has a molecular weight of 48 kDa [24]. In this study, GroEL/ES and Trigger Factor chaperones were constructed into the pG-Tf3 plasmid, which is resistant to the antibiotic chloramphenicol and can be induced with tetracycline and L-arabinose. The aim was to investigate whether co-expression using chaperones could facilitate proper protein folding and enhance the solubility of T. thermophilus DNA Polymerase enzyme.
Based on the explanation above, this research begins with E. coli BL21(DE3) [pD861-His-Tth DNA Pol/pG-Tf3] transformation. Thermus thermophilus DNA Polymerase enzyme is then expressed both with and without chaperone co-expression. Following that, the Tth DNA Polymerase enzyme is characterized using SDS-PAGE, and the total enzyme level is quantified using the Bradford Assay.
MATERIALS AND METHODS
Agar bacto (Oxoid), APS 10% (Sigma-Aldrich), acetic acid (Merck), hydrochloride acid (Merck), aquabidest, Bisacrylamide ready-to-use 30% (Sigma-Aldrich), β-mercaptoethanol (Merck), coomassie brilliant blue, EDTA (1’st Base), ethanol (Merck), E. coli BL21(DE3) (laboratory stock), glycerol (1’st Base), Glycine (Merck), calcium chloride (Merck), kanamycin sulfate (Sigma-Aldrich), chloramphenicol (SANBE Indonesia), L-rhamnose (Merck), L-arabinose (Merck), marker protein (Biorad), methanol (sigma-aldrich), plasmid [pD861- His-Tth DNA Pol] (ATUM, California), plasmid pG-Tf3, sodium hydroxide (Merck), sodium chloride (Merck), nuclease-free water (ThermoFisher), SDS (Merck), tetracycline (Merck), tris base (Merck), tryptone (1’st Base), and yeast extract (Oxoid).
Plasmid [pG-TF3] transformation into E. coli BL21(DE3) [pD861-His-Tth DNA Pol] host using the heat shock method
The pG-Tf3 plasmid (1 µl) was delivered into a micro tube containing 100 µl competent cells. The creation of competent cells was carried out when the cell’s OD600 reached 0.45–0.5, indicating that E. coli was in the exponential growth phase. The mixture was homogenized and then cooled in an ice bath for 30 seconds. Subsequently, the mixture was subjected to heat treatment in a water bath at 42°C for 45 seconds, followed by transfer back to the ice bath for 2 minutes to cool. Following the heat shock process, 900 µl LB medium (1 % (w/v) tryptone, 0.5 % (w/v) yeast extract, and 1 % (w/v) NaCl) was added into the micro tube. The cells were resuspended and then incubated at 37°C for 3 hours with agitation at 200 rpm. Afterward, 100 µl of the transformed cells were spread onto solid LB media supplemented with kanamycin (75 µg/ml) and chloramphenicol (20 µg/ml) [26].
Tth DNA polymerase enzyme overexpression with and without combined co-expression of GroEl/ES and trigger factor chaperone
Stock glycerol E. coli BL21(DE3) [pD861-His-Tth DNA Pol/pG-Tf3] was cultured in 5 ml of LB medium supplemented with chloramphenicol (20 µg/ml) and kanamycin (75 µg/ml) at 37°C with 200 rpm for 16–18 hour. Subsequently, 1% (v/v) of the overnight culture was transferred to 50 ml of liquid LB medium supplemented with chloramphenicol (20 µg/ml) and kanamycin (75 µg/ml) at 37°C at 200 rpm to 0.4 OD600nm. For chaperone co-expression, L-arabinose (5 mg/ml), tetracycline (10 ng/ml), and L-rhamnose (4 mM) were added meanwhile without chaperone co-expression was only done with L-rhamnose inductor with 4 mM final concentration on the media. The expression culture obtained was harvested 4 hours post-induction, followed by centrifugation at 6,000 g for 20 minutes at 4°C. The resulting supernatant was collected as a soluble fraction. Subsequently, 8 ml of solubilization buffer (8 M urea, 20 mM Tris-HCl, and 5 mM β-mercaptoethanol, pH 8.5) was added for each gram of the pellet. The suspension was then incubated at 100 rpm for 1 hour at room temperature and centrifuged at 12,000 g for 20 minutes at 4°C. The supernatant obtained was collected as solubilized inclusion bodies and stored at −20°C for further analysis.
Determination total protein concentration
The total protein concentration was assessed utilizing the Bradford Assay. Bovine Serum Albumin (BSA) ranging from 0.2 to 2 mg/ml was used as the standard protein. Subsequently, 100 µl of each sample and standard was mixed with 1 ml of Bradford reagent, followed by incubation at room temperature for 10 minutes with intermittent inversion. The absorbance of each sample was measured at 595 nm, and the protein concentration was deduced by referencing the absorbance against the standard protein curve (Fig. 1).
RESULT AND DISCUSSION
[pG-TF3] plasmid transformation into host E. coli BL21(DE3) [pD861-His Tth DNA Pol] using heat shock method
In this research, the heat shock method was used due to its advantages, which include not requiring specialized equipment and being simple and cost-effective [27]. The results of the transformation are presented in Figure 2. Media control, positive control (media without antibiotics), and negative control (media with antibiotics) were used as controls to assess the quality of the competent cells to be utilized. The result indicates that the competent cells are in optimal condition and free from contamination. The E. coli BL21(DE3) [pD861-His-Tth DNA Polymerase/pG-Tf3] cells, following transformation, were examined on solid Luria-Bertani media supplemented with kanamycin and chloramphenicol as selection markers (Fig. 3D). Singular colonies, indicative of successful transformation, were observed, as the plasmids harbored the neomycin phosphotransferase II gene, conferring kanamycin resistance and the chloramphenicol acetyltransferase gene for chloramphenicol resistance [28,29]. This confirms the success of the transformation process.
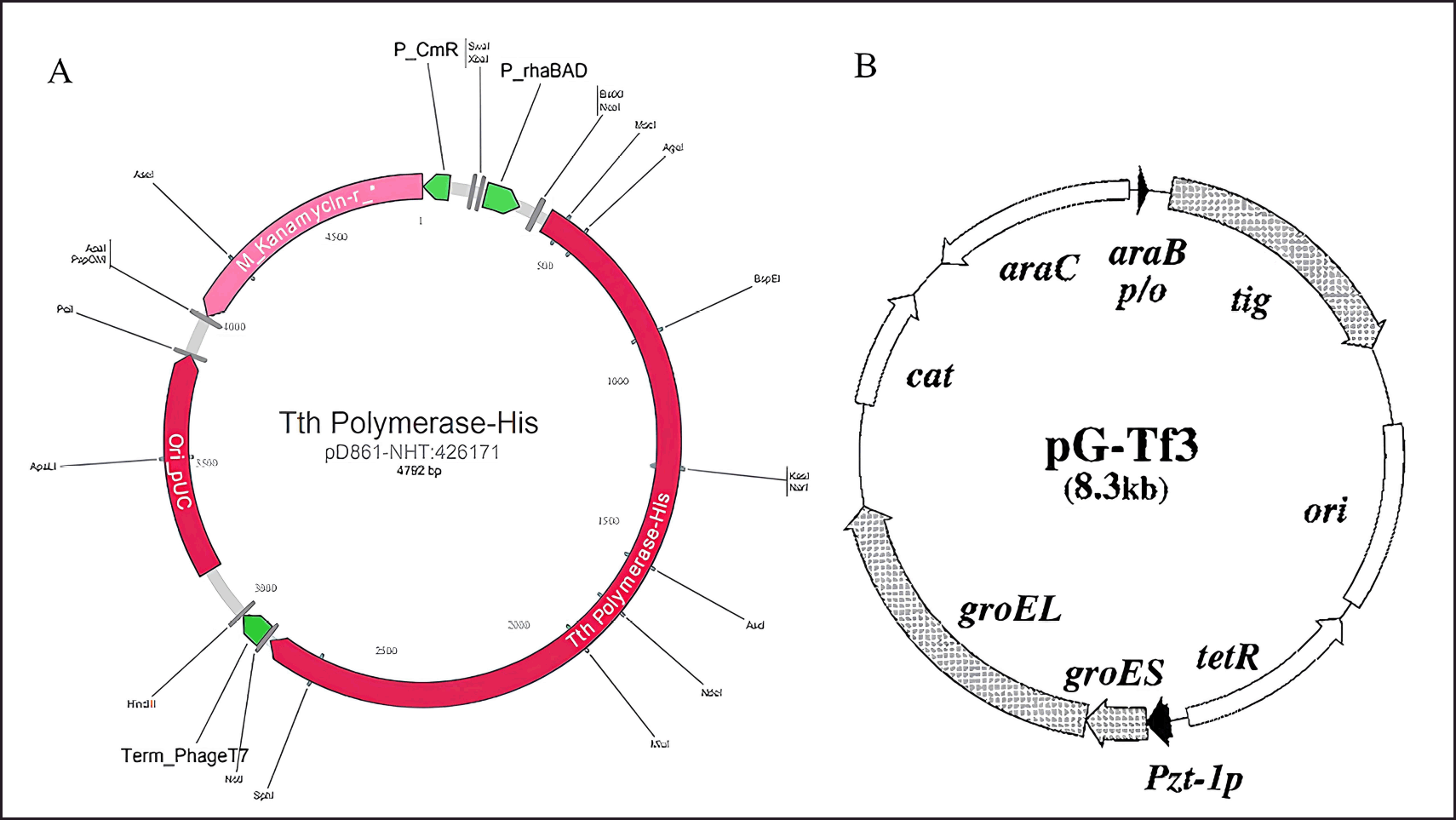 | Figure 1. A comprehensive illustration of (A) pD861-His-Tth DNA Pol plasmid construction, consisting of; Ori PUC (origin of replication); M_Kanamycin-r antibiotic, p_rhaBAD promotor (ATUM, 2021) and (B) pG-Tf3 expression plasmid construction consisting of; ori (origin of replication); cat (chloramphenicol acetyltransferase gene); araB p/o (araB promoter-operator); araC (araC repressor gene); Pzt-1p (Pzt-1 promoter); tetR (tetR repressor gene); tig (gene encoding trigger factor) [25] . [Click here to view] |
Thermus thermophilus DNA polymerase enzyme overexpression with GroEl/ES chaperone expression, trigger factor, and combination of both
An enzyme expression comparison was conducted to assess the impact of chaperone co-expression on the solubility of His-Tth DNA Polymerase. Three different chaperone conditions were tested: Trigger factor alone, GroEL/ES alone, and a combination of both. The results of protein characterization using SDS-PAGE are depicted in Figure 4A, where bands representing the target enzyme (His-Tth DNA Polymerase) at 96 kDa are observed. The area of this target band was quantified using densitometry analysis with ImageJ software (Fig. 4B).
The result of this analysis reveals that His-Tth DNA Polymerase enzyme expression using combined GroEL/ES and trigger factor chaperone co-expression is the best option to increase solubility. This finding aligned with the work of Hoffman et al. [30], who highlights the synergistic role of the Trigger Factor and GroEL/ES chaperones in the protein folding process. The Trigger factor serves as a chaperone facilitating the folding process of the nascent proteins within the cytoplasm by impeding premature aggregation and promoting proper folding. It achieves this by recognizing and binding to the peptide chain as it is being synthesized and stabilizing it in a partially folded intermediate state. This intermediate state enhances the efficiency and accuracy of protein folding, minimizing the likelihood of misfolding. Misfolded proteins are then recognized by Trigger Factor and directed to GroEL for further folding assistance. Inside the GroEL cavity, the folded protein is trapped, and GroES binds to the complex, inducing a conformational change that seals the folded protein inside. Release of the folded protein occurs upon ATP binding to GroEL, causing the detachment of the GroES cap and subsequent release of protein. Through this coordinated mechanism, GroEL and GroES play a pivotal role in facilitating the accurate folding of proteins and preventing the accumulation of misfolded proteins, which can be detrimental to cellular function [30].
His-Tth DNA polymerase enzyme expression with and without GroEl/ES, Trigger Factor chaperone combined co-expression and without combined co-expression
His-Tth DNA Pol expression in E. coli BL21(DE3) [pD861-His-Tth DNA Pol/pG-TF3] was done with and without chaperone co-expression as illustrated in Figure 5. Quantification using imageJ software revealed that in the absence of chaperone co-expression, the insoluble fraction still contained various proteins. This outcome can be attributed to an imbalance between the high-level gene expression required for target protein production and the insufficient folding capacity within the expression host. This imbalance leads to the development of inclusion bodies that accumulate in the insoluble fraction.
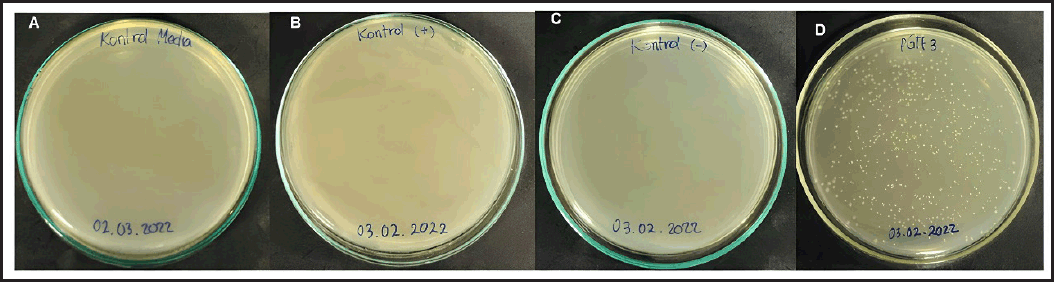 | Figure 2. Results of pG-Tf3 plasmid transformation on E. coli BL21(DE3) [pD861-His-Tth DNA Polymerase] host. A: media control; B: Positive control; C: Negative control; D: E. coli BL21(DE3) [pD861-His-Tth DNA Polymerase/pG-Tf3] transformed. [Click here to view] |
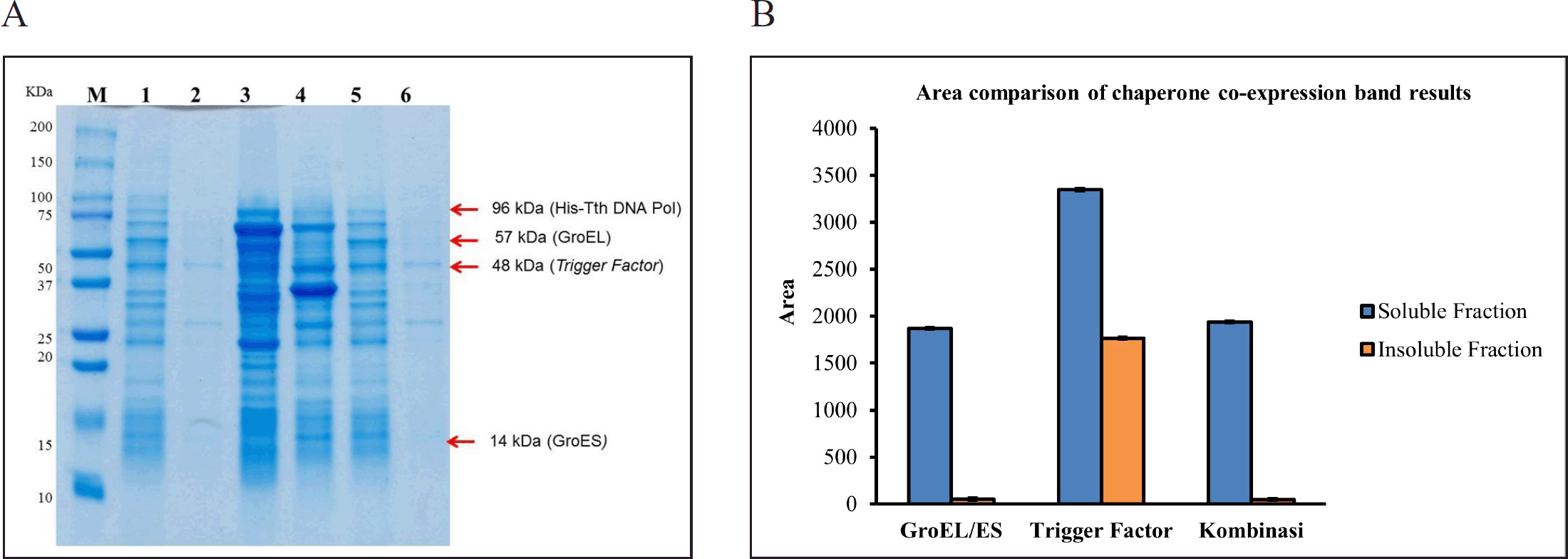 | Figure 3. A: Characterization results comparison of only GroEL/ES co-expression, Trigger Factor co-expression, combination of both using SDS-PAGE; B. Area Quantification using ImageJ “N = 3”; M: Protein marker; 1: GroEL/ES Chaperone Co-Expression Soluble Fraction Result; 2: GroEL/ES chaperone Co-Expression Insoluble Fraction Result; 3: Soluble Fraction Result from Trigger Factor chaperone co-expression; 4: Insoluble Fraction Result from Trigger Factor chaperone co-expression; 5: Soluble Fraction Result from combined chaperone co-expression 6: Insoluble Fraction Result from combined chaperone co-expression. [Click here to view] |
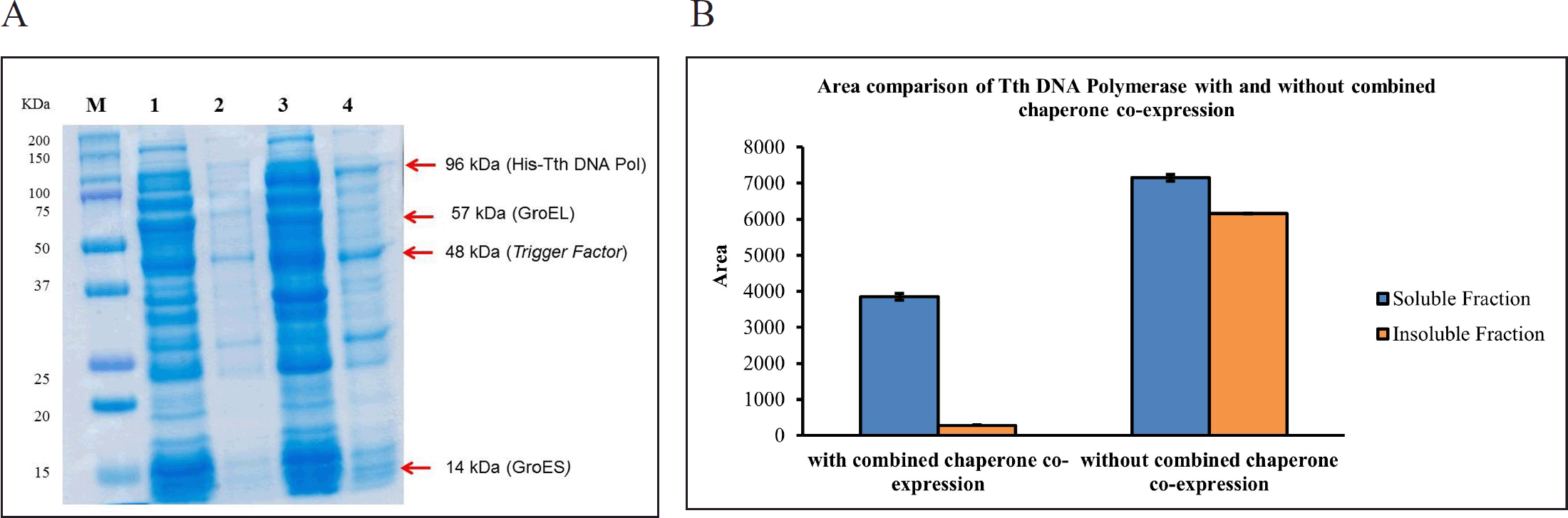 | Figure 4. A: Characterization comparison of His-Tth DNA Pol enzyme expression with and without combined Trigger Factor and GroEL/ES chaperone on SDS-PAGE; B. Area Quantification using ImageJ “N = 3”; M: Protein marker; 1: Soluble Fraction Result of combined chaperone co-expression; 2: Insoluble Fraction Result of chaperone co-expression; 3: Soluble Fraction Result without Trigger Factor chaperone co-expression; 4: Insoluble Fraction Result without chaperone co-expression. [Click here to view] |
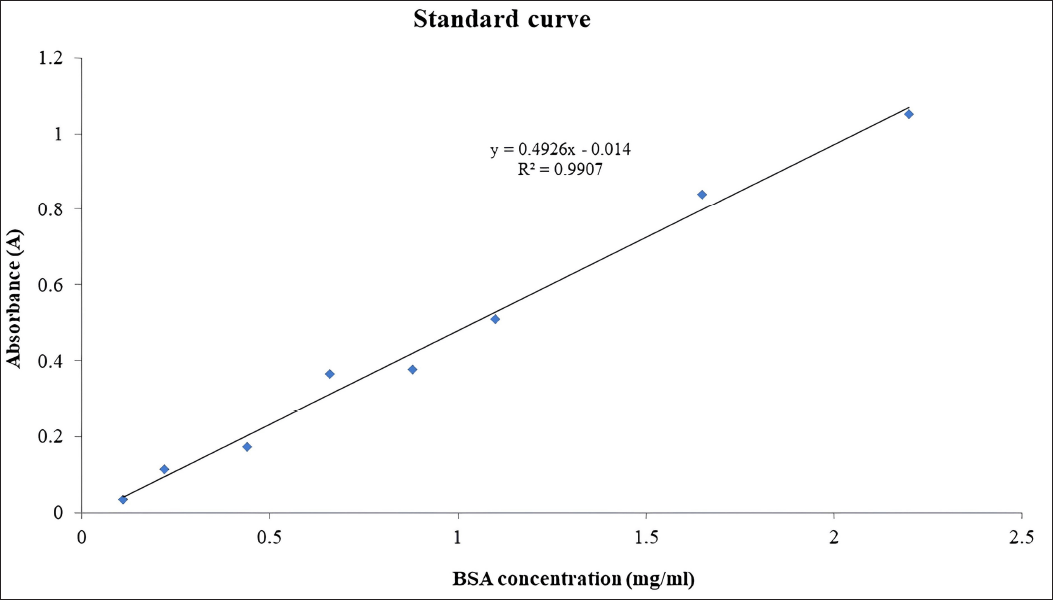 | Figure 5. Standard protein curve (BSA) with various concentration (0.2–2 mg/ml). [Click here to view] |
In comparison to His-Tth DNA polymerase expression without chaperone co-expression, the presence of chaperones resulted in a decreased width of bands within the insoluble fraction, while bands within the soluble fraction exhibited more prominence (Fig. 5A). This observation was further supported by area quantification using imageJ software (Fig. 5B), where the insoluble fraction with chaperone co-expression exhibited a significant reduction compared to that without co-expression, although some insoluble fractions still persisted.
Determination of total protein concentration using Bradford Assay
The total protein concentration without chaperone co-expression was measured as 3.7019 mg/ml, while with combined GroEL/ES and Trigger Factor chaperone co-expression, it increased to 3.9258 mg/ml. The total protein concentration showed an increase of 0.2239 mg/ml. These results indicate that co-expression using chaperones leads to an enhanced production of protein.
CONCLUSION
Based on the findings of the conducted research, it can be inferred that as per the SDS-PAGE analysis and densitometric analysis using imageJ, the co-expression of Trigger Factor and GroEL/ES chaperones alongside Tth DNA Polymerase enzyme results in a reduction in inclusion body formation and a decrease in the proportion of insoluble fractions compared to expression without co-expression. The total protein concentration resulting from co-expression is measured at 3.9258 mg/ml.
ACKNOWLEDGEMENT
This study received support from grant No.116/IV/KS/11/2022 provided by the National Innovation Research Agency, Ministry of Technology Research and Higher Education, as well as the Academic Leadership Grant No.1549/UN6.3.1/PT.00/2023, held by Padjadjaran University.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Maksum IP, Utama E, Sriwidodo, and Subroto T. Extracellular secretion of recombinant human epidermal growth factor by using trimethylamine N-Oxide reductase a (TORA) signal peptide in Escherichia coli BL21(DE3). J Pharm Sci Res. 2017;9(6):1007–16.
2. Maksum IP, Lestari A, Fauzia RP, Rachman SD, Soedjanaatmadja UMS. Escherichia coli BL21(DE3) expression system using TorA signal peptide for recombinant human albumin (rHA) secretion. Int J Res Pharm Sci. 2019;10(4):19–24.
3. Chae YK, Kim SH, Markley JL. Relationship between recombinant protein expression and host metabolome as determined by two-dimensional NMR spectroscopy. PLoS One. 2017;12(5):1–12.
4. Sriwidodo S, Subroto T, Maksum IP, Wathoni N, Rostinawati T, Ulya H. Optimization of secreted recombinant human epidermal growth factor production using pectate lyase B from Escherichia coli BL21(DE3) by central composite design and its production in high cell density culture. J Pharm Bioallied Sci. 2019;11(Suppl 4):562–6.
5. Myers TW, Gelfand DH. Reverse transcription and DNA amplification by a Thermus thermophilus DNA Polymerase. Biochemistry. 1991;30(31):7661–6.
6. Auer T, Landre PA. Properties of the 5′→3′ Exonuclease/Ribonuclease H activity of thermus thermophilus DNA polymerase. Biochemistry. 1995;34(15):4994–5002.
7. Sriwidodo, Maksum IP, Riswanto N, Rostinawati T, and Subroto T. Extracellular secretion recombinant of human epidermal growth factor (hEGF) using pectate lyase B (PelB) signal peptide in Escherichia coli BL21 (DE3). Int J Res Pharm Sci. 2017;8(1):1–8.
8. Silaban S, Gaffar S, Simorangkir M, Maksum IP, Subroto T. Effect of IPTG concentration on recombinant human prethrombin-2 expression in Escherichia coli BL21(DE3). IOP Conf Ser Earth Environ Sci. 2019;217(1):012039.
9. Maksum IP, Latifah FPU, Nabiel A, Lestari NF, Sriwidodo S, Azizah MI, et al. An overview of culture conditions for recombinant protein expression in Escherichia coli. J Appl Biotechnol Rep. 2023;10(1):864–75.
10. Indriyani A, Anggraeni NI, Sriwidodo, Maksum IP. Optimization extracellular secretion of recombinant human epidermal growth factor (hEGF) in Escherichia coli BL21(DE3) pD881-OmpA-hEGF by using response surface method (RSM). Int J Res Pharm Sci. 2019;10(3):1824–31.
11. Indriyani A, Gaffar S, Latifah FP, Maksum IP. Co-expression of recombinant human epidermal growth factor (rhEGF) in Escherichia coli BL21 (DE3) with Bacillus cereus phospholipase C. In AIP Conference Proceedings, Melville, NY: AIP Publishing LLC; 2020;2243(1):020009.
12. Latifah FPU, Indriyani A, Pratiwi RD, Sriwidodo, Maksum IP. Effects of growth medium on extracellular secretion of human epidermal growth factor in Escherichia coli by co-expression with Bacillus cereus phospholipase C. IOP Conf Ser: Mater Sci Eng. 2020;833:012021
13. Su Z, Huang Y, Zhou Q, Wu Z, Wu X, Zheng Q, et al. High-level expression and purification of human epidermal growth factor with SUMO fusion in Escherichia coli. Protein Peptide Lett. 2006;13(8):785–92.
14. Wang Y, van ON, Ali AM, Adawy A, Anindya AL, Dömling ASS, et al. A systematic protein refolding screen method using the DGR approach reveals that time and secondary TSA are essential variables. Sci Rep. 2017;7(1):9355.
15. Subroto T, Maksum IP, Yusuf M, Kusuma SA, Opratami W, Maharani M. Purity of maltose-binding protein—recombinant streptavidin expressed in Escherichia coli BL21 (pD861-MBP: 327892). J Adv Pharm Technol Res. 2022;13:117–22.
16. Maksum IP, Utami DF, Nurhakim EA, Yosua Y, Yusuf M, Fadhlillah M, et al. Overexpression of soluble recombinant Thermus thermophilus (Tth) DNA polymerase in Escherichia coli BL21(DE3) using an MBP fusion tag as a solubility enhancer. J Appl Pharm Sci. 2022;12(09):017–24.
17. Maksum IP, Yosua Y, Nabiel A, Pratiwi RD, Sriwidodo S, Soedjanaatmadja UM. Refolding of bioactive human epidermal growth factor from E. coli BL21 (DE3) inclusion bodies & evaluations on its in vitro & in vivo bioactivity. Heliyon. 2022;8(4):e09306.18.Nabiel A, Yosua Y, Sriwidodo S, Maksum IP. Overview of refolding methods on misfolded recombinant proteins from Escherichia coli inclusion bodies. J App Biol Biotech. 2023;11(3):47–52.
19. Maksum IP, Wildan DA, Hasan K, Subroto T. The effect of single co-expression of The DnaK-DnaJ-GrpE and GroEL/ES Chaperones and their combination on expression Intein-pretrombin-2 in Escherichia coli ER2566. J Kim Val. 2020;6(1):47–54.
20. Ellis, J. Proteins as molecular chaperones. Nature. 1987;328:378–9.
21. Nishihara K, Kanemori M, Yanagi H, Yura T. Overexpression of trigger factor prevents aggregation of recombinant proteins in Escherichia coli. Appl Environ Microbiol. 2000;66(3):884–9.
22. Buchner J, Grallert H. Review: A structural view of the GroE chaperonee cycle. J Struct Biol, 2001;135(2):95–103.
23. Xu Z, Horwich AL, Sigler PB. The crystal structure of the asymmetric GroEL/ES-(ADP)7 chaperonein complex. Nature. 1997;388:741–50.
24. Valent QA, Kendall DA, High S, Kusters R, Oudega B, Luirink J. Early events in preprotein recognition in E. coli: Interaction of SRP & trigger factor with nascent polypeptides. EMBO J. 1995;14:5494–505.
25. Nishihara K, Kanemori M, Kitagawa M, Yanagi H, Yura T. Chaperone co-expression plasmids: differential & synergistic roles of DnaK-DnaJ-GrpE & GroEL-GroES in assisting folding of an allergen of Japanese Cedar Pollen, Cryj2, in Escherichia coli. Appl Environ Microbiol. 1998;64:1694–9.
26. Bollag DM, Royzicki MD, Edelstein SJ. Protein methods. 2nd ed. New york, NY: A John Willey and Sons Inc; 1996.
27. Liu X, Liu L, Wang Y, Wang X, Ma Y, Li Y. The study on the factors affecting transformation efficiency of E. coli competent cells. Pak J Pharm Sci. 2014;3:679–84.
28. Yenofsky RL, Fine M, Pellow JW. A mutant neomycin phosphotransferase II gene reduces the resistance of transformants to antibiotic selection pressure. Proc Natl Acad Sci. 1990;87(9):3435–9.
29. Shaw WV, Packman LC, Burleigh BD, Dell A, Morris HR, Hartley BS. Primary structure of a chloramphenicol acetyltransferase specified by R plasmids. Nature. 1979;282(5741):870–2.
30. Hoffmann A, Bukau B, Kramer G. Structure and function of the molecular chaperone Trigger Factor. Biochim Biophys Acta. 2010;1803(6):650–61.