INTRODUCTION
Hydroxyapatite is a biomaterial with superior properties such as low toxicity, osteoconductive, high bioactivity, and biocompatibility [1]. Hydroxyapatite also has the same chemical properties as human bone so it can be used in bone implants [2–4]. Hydroxyapatite (Ca10(PO4)6(OH)2) is bioceramic obtained from calcium, phosphate, and hydroxide precursors [2]. Calcium precursors can be obtained using natural sources, namely shellfish [5], limestone [6], eggshell [7], and common cockle (Cerastoderma edule) shells [2]. This study employed the green mussel shells as precursors of synthesized hydroxyapatite due to high calcium oxide (CaO) content, around 97.261%. Previous research reported that using shells as a biomaterial increases the strength and elasticity of the resulting product [8,9]. The limited antibacterial properties of hydroxyapatite mean that its potential in the implantation process cannot be utilized optimally [7]. Biofilm formation by Staphylococcus aureus and Escherichia coli bacteria on the implant surface can cause infection. It can cause clinical problems, the main problems in the bone implant process every year [10].
To overcome this problem, many researchers have synthesized hydroxyapatite materials composited or doped with other materials such as metals, polymers, or metal oxides to be applied more widely as biomedical materials. Some previous studies reported the synthesis of a biomedical material such as HAp/TiO2 as antiviral/antibacterial material [11], NiFe2O4@HAp-Ag as a prompt antibacterial material against pathogenic strains [12], cytocompatibility of biodegradable Zn-3Fe-HAP composites which be promising material for bone repair [13], Vanadium and Strontium co-doped HAp for tissue engineering applications [14], metals (Al, Fe, Ni, and Zn) doping in nano-crystalline hydroxyapatite for efficient biomedical applications [15] and strontium-substituted hydroxyapatite-CaO-CaCO3 nanofibers as drug delivery carriers [16].
In this study, the HAp-Sr doped was carried out to improve the antibacterial properties of HAp. The incorporation of strontium into bones occurs quickly (1–2 weeks). Strontium is also the main ingredient in the drug strontium ranelate, a drug currently being developed to treat cases of osteoporosis (bone fragility) [16]. In vitro, strontium is known to increase the number of osteoblasts (bone-forming cells) and can reduce the number of osteoclasts (bone-breaking cells) [17]. Strontium can also facilitate the process of bone resorption and stimulate bone formation [18]. Apart from that, strontium is also known to have good antibacterial properties because it can cause bacterial DNA to condense so that it loses its ability to replicate [19].
Synthesis of hydroxyapatite Sr-doped was carried out using the sol-gel method. The sol-gel method is carried out at low temperatures but produces products with high purity with a phase change from colloidal suspension (sol) to liquid (gel). Apart from that, using little equipment and cost-effectiveness are also advantages of the sol-gel method [20]. This approach also provides the capability to achieve molecular mixing of chemical precursors. Compared to conventional solid-state, wet precipitation, and hydrothermal processes, it has the capability to generate nanosized particles and nanocrystalline powders. This approach comprises multiple phases: formulation of the precursor solution, hydrolysis to initiate gelation, maturation, desiccation, and sintering. The precursor may be either an inorganic or organometallic compound [21].
Thus, this study aimed to examine the antibacterial characteristics of HAp doped with Sr. Concentration variations of Sr were also conducted during the doping process to determine the effective concentration required to inhibit bacterial growth. The employing of Sr-doped HAp can effectively reduce the reliance on pharmaceuticals in the field of medicine.
MATERIALS AND METHODS
Materials
Green mussel shells (Perna viridis), nitric Acid (HNO3), ammonium hydroxide (NH4OH), anhydrous strontium nitrate (Sr(NO3)2), diammonium hydrogen phosphate ((NH4)2HPO4), and nutrient agar (NA).
Methods
Calcium oxide preparation
The green mussel shells are cleansed of impurities, dried, and pulverized using a grinder. Subsequently, the material underwent calcination at a temperature of 900°C for 5 hours, producing powdered CaO. The CaO powder was subsequently analyzed using X-ray fluorescence (XRF) [20].
Synthesis of HAp by sol-gel method
CaO powder was weighed 4.2 g, dissolved in 75 ml of 2 M HNO3, and stirred at 250 rpm at 65°C for 15 minutes. Filtration is performed on the Ca(NO3)2 solution. By adding an NH4OH solution and getting a Ca(OH)2 sol, the Ca(NO3)2 filtrate was brought to pH 10. Then, dropwise 250 ml of 0.18 M diammonium phosphate ((NH4)2HPO4) was added and heated to 60°C for 5 hours while stirring. The hydroxyapatite sol formed was left for 24 hours until a gel formed. The gel obtained was filtered, dried at 110°C for 5 hours, then calcined at 600°C for 3 hours. The hydroxyapatite powder obtained was analyzed using X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR) characterization.
Synthesis of HAp-Sr doped by sol-gel method
A weighed solution of CaO powder is then dissolved in 75 ml of 2 M HNO3, and the mixture is agitated with a stirrer at 65°C for 15 minutes at 250 rpm. After preparing and filtering a Ca(NO3)2 solution, drop by drop of diammonium phosphate solution ((NH4)2HPO4) is added. A drop of 250 ml was added with NH4OH until a pH of 10 was obtained. A hydroxyapatite-strontium sol was formed by stirring Sr(NO3)2 at concentrations of 5%, 10%, and 20% in a temperature-controlled environment at 60°C for approximately 5 hours. After being aged for 24 hours, the HAp-Sr sol turned into a gel. Filtered, dried for 5 hours in an oven at 110°C, and then calcined for 3 hours at 600°C. The composition of precursor solution HAp-Sr doped composite synthesis is shown below in Table 1.
Antibacterial assay
Escherichia coli and S. aureus bacteria were regenerated for a full day in a sealed chamber. Then, antibacterial testing was carried out by adding rejuvenated bacteria to the NA medium. Add 0.5 g of HAp and HAp-Sr doped composite powder to the disc ring in a sterile petri dish containing media and bacteria. Then, the incubation was carried out for 24 hours after the control (amoxicillin) was used.
Characterizations
The determined element in the green mussel shell was analyzed using XRF (PANalytical type minimal 4). The functional group of the sample was analyzed using a Fourier Transform Infra-Red (Shimadzu IR Prestige-21) at a wavenumber of 4,000–500 cm−1. The structure properties of the HAp and HAp-Sr doped composite samples were analyzed using XRD (Brukker D8 Advance) at a 2θ value of 10°–90°.
RESULTS AND DISCUSSION
Preparation of CaO from green mussel shells (Perna viridis)
Hydroxyapatite and strontium hydroxyapatite were synthesized using green mussel shells (Perna viridis). Calcination of green mussel shells aims to release substances in the form of carbonates and hydroxides into substances in the form of oxides. In the calcination stage, the decomposition process (breakdown) of calcium carbonate compounds (CaCO3) into calcium oxide (CaO) will occur above a temperature of 800°C [22]. However, if the sample is calcined above a temperature of 900°C, the CaO yield obtained will decrease because the CaO compound begins to change into other compounds [23].
The formation reaction of hydroxyapatite is as follows:
CaO(s) + 2HNO3 (l) → Ca(NO3)2 (l) + H2O (l)
10Ca(NO3)2(l) + 6(NH4)2HPO4(l) + 8NH4OH(l) → Ca10(PO4)6(OH)2(s) + 20NH4NO3 + 6H2O
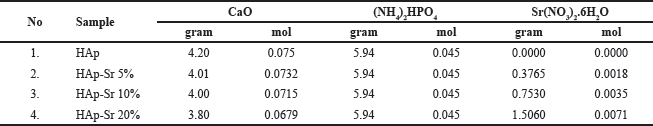 | Table 1. Composition of precursor solution HAp-Sr doped composite synthesis. [Click here to view] |
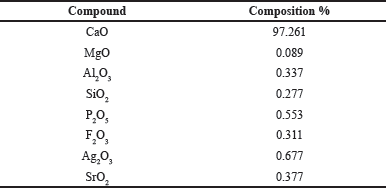 | Table 2. Composition of green mussel shells. [Click here to view] |
The reaction for the formation of strontium-hydroxyapatite is as follows:
(10-x)Ca(NO3)2 + x Sr(NO3)2 + 6(NH4)2HPO4 + 8NH4OH → Ca10-xSrx(PO4)6(OH)2 + 20NH4NO3 + 6H2O
Analysis of green mussel shell composition using XRF
Green mussel shells are utilized as a calcium source due to their high calcium oxide content of 97.261%. The green mussel shells can serve as raw materials for synthesizing hydroxyapatite due to their significant calcium content. The chemical composition of green mussel shells that underwent calcination at a temperature of 900°C for 5 hours was determined using XRF and is presented in Table 2.
Based on the results of XRF characterization, the percentage of CaO was found to be 97.261%, so it can be concluded that green mussel shells can be used as a calcium precursor in the synthesis process of HAp and HAp-Sr doped composites. Apart from CaO, other oxide compounds such as MgO, Al2O3, and SiO2 were also found. However, the content of these compounds is small enough that when reacted with HNO3, these compounds can dissolve and form a precipitate [23].
XRD analysis
XRD characterization was used to determine the structure of the synthesized samples. The samples analyzed on XRD consisted of HAp, HAp-Sr doped composite 5%, 10%, and 20%, as shown in Figure 1.
Based on the XRD graph in Figure 1, the spectra are conformed to ICDD standards (No. 96-101-1243). It can be concluded that Sr has succeeded in replacing or partially substituting Ca because no new peaks were found on the graph. Sr ions have a greater ion radius than Ca, which can be used as a substitute ion or ion to replace Ca2+. The Sr2+ ion is known to have an ionic radius of 1.18 Å. Meanwhile, the Ca2+ ion has an ionic radius of 1.00 Å32. The three prominent peaks of hydroxyapatite found at 2θ with values of 27.65°, 31.17°, and 34.50° have Miller indices of (002), (211), and (112), indicating that hydroxyapatite does not crystallize well [24]. In addition, XRD analysis was also used to determine the sample size through Scherer calculation.
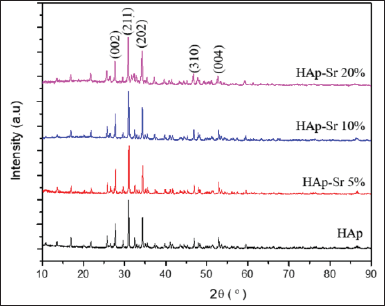 | Figure 1. XRD pattern HAp and HAp-Sr doped composite. [Click here to view] |
where D = crystal size (nm), k = constant (0.9), λ = X-ray wavelength, β = FWHM (Full Width at Half Maximum) at 2θ (π 180), and θ = Bragg angle. Based on Table 3, the crystallite size of HAp, HAp-Sr 5%, HAp-Sr 105%, and HAp-Sr 20% is 0.85 nm. It shows no change in the crystal size after doping with Sr. From the XRD data, it is known that the synthesized compounds are hydroxyapatite and hydroxyapatite-strontium composites.
Fourier transform infra-red
The synthesis results were analyzed using FT-IR to identify functional groups in absorption peaks and molecular interactions that play a role in the HAp and HAp-Sr doped composite synthesis process [25]. FT-IR analysis was carried out at wave numbers 3,500–500 cm−1. The FT-IR absorption of HAp and HAp-Sr doped composite is shown in Figure 2 as follows.
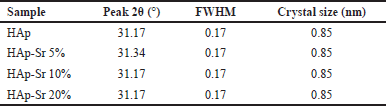 | Table 3. The crystallite size of HAp and HAp-Sr doped sample. [Click here to view] |
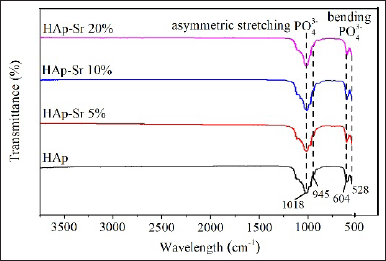 | Figure 2. Spectrum HAp and HAp-Sr doped composite. [Click here to view] |
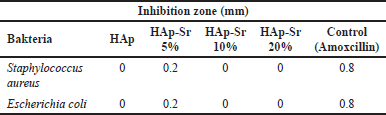 | Table 4. Inhibition zone of HAp and HAp-Sr doped composite. [Click here to view] |
There is a typical peak in the FT-IR spectrum of hydroxyapatite, namely the PO43− group peak. At wave numbers 604 cm−1 and 528 cm−1, it is the vibrational bending of PO43−. The peak at wave number 1,018 cm−1 is vibrational stretching (asymmetry) of the PO43− group. Meanwhile, wave number 945 cm−1 is vibrational stretching (symmetry) of the PO43− group. Identifying the PO43− group and the OH− group, functional groups found in hydroxyapatite, suggests that the sample contains Hap [26].
Antibacterial assay
Antibacterial tests were carried out on samples of HAp, HAp-Sr 5%, HAp-Sr 10%, and HAp-Sr 20% using amoxicillin as a control as shown in Figure 3. In this antibacterial test, two types of bacteria are used: the Gram-positive bacteria S. aureus and the Gram-negative bacteria E. coli. Inoculum was prepared from fresh culture after incubation for 24 hours at 37°C. Antibacterial activity was observed using the disc ring method, measuring the minimum inhibition zone in millimeters. Inhibition Zone of HAp and HAp-Sr doped composite is shown in Table 4.
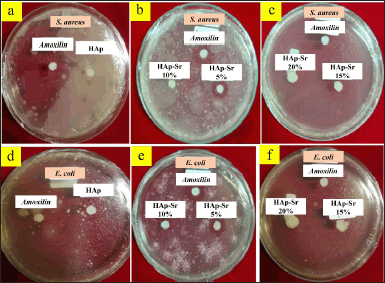 | Figure 3. Antibacterial activity of HAp-Sr doped composite against S. aureus bacteria (a–c) and E. coli (d–f). [Click here to view] |
The test results found that the antibacterial activity of HAp-Sr 5% had an inhibition zone compared to HAp, HAp-Sr 10%, and HAp-Sr 20%. The more Sr is added, the less antibacterial activity it becomes. Therefore, the Sr added to the sample must be in a certain amount. Previous research revealed that strontium-hydroxyapatite will have good bioactivity if the level of Sr added in hydroxyapatite doping is 3%–7% [27]. When the added Sr exceeds the specified level, it can be toxic in the body. These data show that HAp-Sr 5% has an inhibition zone value and can act as an antibacterial. It proves that doping strontium ions on hydroxyapatite can increase the bioactivity of hydroxyapatite [28]. Nagyné-Kovács et al. [29] reported that In vitro cytotoxicity tests of HAp-doped revealed evident antibacterial activity compared to pure HAp against E. coli bacteria [29].
CONCLUSION
The HAp-Sr doped composites have been successfully synthesized using the sol-gel method. The XRD and FTIR analysis results show that HAp-Sr doped has been appropriately synthesized. HAp-Sr doped with several Sr concentration variations (5%, 10%, 20%) showed different antibacterial activities. Only HAp-Sr 5% has antibacterial activity, while HAp-Sr 10% and HAp-Sr 20% have no antibacterial activity. The overall characterization and tests implied that HAp-Sr 5% has antibacterial activity, so it can be applied to biomedical materials such as bone grafts.
ACKNOWLEDGMENT
The author would like to send their gratitude to LPPM (Lembaga Penelitian dan Pengabdian kepada Masyarakat) of Universitas Andalas for supporting the researcher in publishing this work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Adamu DB, Zereffa EA, Segne TA, Razali MH, Lemu BR. Synthesis and characterization of bismuth-doped hydroxyapatite nanorods for fluoride removal. Environ Adv. 2023;12:100360. CrossRef
2. Nareswari TL, Juniatik M, Aminatun A, Sari M, Utami RA, Sari YW, et al. A facile technique for overcoming seeding barriers of hydrophobic polycaprolactone/hydroxyapatite-based nanofibers for bone tissue engineering. J Appl Pharm Sci. 2023;13:49–60. CrossRef
3. Martínez-Gracida NO, Esparza-González SC, Castillo-Martínez NA, Serrano-Medina A, Olivas-Armendariz I, Campos-Múzquiz LG, et al. Synergism in novel silver-copper/hydroxyapatite composites for increased antibacterial activity and biocompatibility. Ceram Int. 2020;46:20215–25. CrossRef
4. Salam N, Gibson IR. Lithium ion doped carbonated hydroxyapatite compositions: synthesis, physicochemical characterisation and effect on osteogenic response in vitro. Biomater Adv. 2022;140:213068. CrossRef
5. Wulandari W, Wellia DV, Jamarun N. The effect of pH on the synthesis and characterization hydroxyapatite from bamboo shell (Sollen spp. ) with emulsion method. J Appl Chem. 2021;10:872–9.
6. Jamarun N, Miftahurrahmi, Septiani U. Synthesis of hyroxyapatite from halaban limestone by sol-gel method. RJPBCS. 2016;7:2956–61.
7. Sayed M, El-Maghraby HF, Bondioli F, Naga SM. 3D carboxymethyl cellulose/hydroxyapatite (CMC/HA) scaffold composites based on recycled eggshell. J Appl Pharm Sci. 2018;8:23–30. CrossRef
8. Ate? HG, Kaygili O, Bulut N, Osmanl?o?lu F, Keser S, Tatar B, et al. Investigation of the structural, thermal, magnetic and cell viability properties of Ce/Sr co-doped hydroxyapatites. J Mol Struct. 2023;1283:135318. CrossRef
9. Sangwaranatee NW, Teanchai K, Kongsriprapan S, Siriprom W. Characterization and analyzation of chitosan powder from Perna viridis shell. Mater Today Proc. 2018;5:13922–5. CrossRef
10. Iqbal N, Kadir MR, Mahmood NH, Salim N, Froemming GR, Balaji HR, et al. Characterization, antibacterial and in vitro compatibility of zinc–silver doped hydroxyapatite nanoparticles prepared through microwave synthesis. Ceram Int. 2014;40:4507–13.
11. Otsuka Y, Minh NQ, Ishiguro K, Miyashita Y. Antiviral/antibacterial and mechanical properties of HAp/TiO2 porous composite. Mater Today Proc. 2023. CrossRef
12. Kalita J, Das A, Bharali L, Chakraborty D, Dhar SS, Pandey P. Prompt antibacterial activity of silver nanoparticle decorated on HAp embedded NiFe2O4 nanocomposite (NiFe2O4@HAp-Ag) against pathogenic strains and investigation of its photocatalytic activity towards degradation of antibiotics. Mater Today Sustain. 2023;24:100552. CrossRef
13. Shan R, Yao R, Wang H, Liu L, Zhao Y, Yao X, et al. Microstructure, mechanical properties, in vitro degradation behavior and cytocompatibility of biodegradable Zn-3Fe-HAP composites prepared by vacuum heating-press sintering. J Alloys Compd. 2023;942:168832. CrossRef
14. Megha M, Joy A, Unnikrishnan G, Haris M, Thomas J, Deepti A, et al. Structural and biological properties of novel Vanadium and Strontium co-doped HAp for tissue engineering applications. Ceram Int. 2023;49:30156–69. CrossRef
15. Habib ML, Disha SA, Hossain MS, Uddin MN, Ahmed S. Enhancement of antimicrobial properties by metals doping in nano-crystalline hydroxyapatite for efficient biomedical applications. Heliyon. 2024;10:e23845. CrossRef
16. Tsai SW, Yu WX, Hwang PA, Huang SS, Lin HM, Hsu YW, et al. Fabrication and characterization of strontium-substituted hydroxyapatite-CaO-CaCO3 nanofibers with a mesoporous structure as drug delivery carriers. Pharmaceutics. 2018;10:179. CrossRef
17. Ivankovic T, Turk H, Hrenovic J, Schauperl Z, Ivankovic M, Ressler A. Antibacterial activity of silver doped hydroxyapatite toward multidrug-resistant clinical isolates of Acinetobacter baumannii. J Hazard Mater. 2023;458:131867. CrossRef
18. Bigi A, Boanini E, Capuccini C, Gazzano M. Strontium-substituted hydroxyapatite nanocrystals. Inorganica Chim Acta. 2007;360:1009–16. CrossRef
19. Brauer DS, Karpukhina N, Kedia G, Bhat A, Law RV, Radecka I, et al. Bactericidal strontium-releasing injectable bone cements based on bioactive glasses. J R Soc Interface. 2013;10:20120647. CrossRef
20. Jamarun N, Trycahyani NA, Arief S, Septiani U, Sisca V. Synthesis of hydroxyapatite-polyethylene glycol with in-situ method using calcium oxide from blood shells (Anadara granosa). Indones J Chem. 2023;23:618. CrossRef
21. Sidiqa AN, Djustiana N, Sunendar B, Febrida R. Surface modification of multilayer coatings Ti-Al-Cr and hydroxyapatite on calcium phosphate cement with Sol-Gel method. J Dent Indones. 2013;19:43–6. CrossRef
22. Wei Z, Xu C, Li B. Application of waste eggshell as low-cost solid catalyst for biodiesel production. Bioresour Technol. 2009;100:2883–5. CrossRef
23. Edwin N, Wilson P. Investigations on sonofragmentation of hydroxyapatite crystals as a function of strontium incorporation. Ultrason Sonochem. 2019;50:188–99. CrossRef
24. Liao J, Li Y, Zou Q, Duan X, Yang Z, Xie Y, et al. Preparation, characterization and properties of nano-hydroxyapatite/polypropylene carbonate biocomposite. Mater Sci Eng C. 2016;63:285–91. CrossRef
25. Yuan Q, Zhang Z, Yang Y, Jian Y, Li R, Dai X, et al. Synthesis, characterization and biological performance study of Sr-doped hydroxyapatite/chitosan composite coatings. Mater Chem Phys. 2021;270:124752. CrossRef
26. Prakash VC, Venda I, Thamizharasi V, Sathya E. A new attempt on synthesis of spherical nano hydroxyapatite powders prepared by dimethyl sulfoxide—poly vinyl alcohol assisted microemulsion method. Mater Chem Phys. 2021;259:124097. CrossRef
27. Capuccini C, Torricelli P, Boanini E, Gazzano M, Giardino R, Bigi A. Interaction of Sr-doped hydroxy apatite nanocrystals with osteoclast and osteoblast-like cells. J Biomed Mater Res Part A. 2009;89:594–600. CrossRef
28. Ehret C, Aid-Launais R, Sagardoy T, Siadous R, Bareille R, Rey S, et al. Strontium-doped hydroxyapatite polysaccharide materials effect on ectopic bone formation. PLoS One. 2017;12:1–21.
29. Nagyné-Kovács T, Mészáros B, Molnár M, Tolner M, Lukács IE, Szilágyi IM, et al. Hydrothermal synthesis of Sr-doped hydroxyapatite and its antibacterial activity. Period Polytech Chem Eng. 2019;64:54–60. CrossRef