INTRODUCTION
Multi-species biofilms have a significant role in the pathogenesis of various infections [1] as bacteria grown in multi-species biofilms can interact synergistically, mutually, and antagonistic [2,3]. Infection-related multi-species biofilms caused an increase in mortality rate (70%) than mono-species biofilms (23%). Candida albicans (C. albicans) and Staphylococcus aureus (S. aureus) are the pathogens that most often cause increasing morbidity and mortality in hospitalized patients [2]. They are the most common microorganisms found in blood cultures, and both can act as co-infectors in various infections. About 27% of candidaemia cases are also found S. aureus in blood preparations [4]. Staphylococcus aureus can form microcolonies on the surface of biofilms covered with a matrix released by C. albicans in dual-species biofilm architecture [5].
The interaction between bacteria and fungi is clinically related to increasing the severity of the disease. Co-infections caused by dual-species biofilms are found in burns and cystic fibrosis. Dual-species biofilms are also found on the surface of medical devices such as dentures and prosthetic implants and are most often found on catheters [2].
Antibiotics and antifungals can be used as therapy for biofilm infections, i.e., cephalosporins, fluoroquinolones, amphotericin B, azole groups, and echinocandins. However, these antimicrobials had been reported to become resistant [6,7] and had severe side effects [8–10]. In other cases, C. albicans in polymicrobial biofilms can increase vancomycin resistance to S. aureus [5]. So far, no adequate and safe drugs for treating biofilm-associated infections have been available. Subsequently, discovering nontoxic, inexpensive, and more potent antibiofilm materials is very important.
Actinomycetes are Gram-positive, anaerobic, or facultative bacteria with a morphological appearance similar to fungi, widespread in water and land. Actinomycetes produce a variety of diverse secondary metabolites and functions to defend themselves from their environment. Several new compounds from Actinomycetes have been found as antifungal, i.e., Caerulomycin A, which has antifungal activity on Candida [11]; kasugamycin from Streptomyces kasugaensis, which acts as a protein biosynthetic inhibitor; 2,4-di-tert-butyl-phenol which has antifungal activity in Candida and other fungal pathogens [12]. In addition, Actinomycetes isolated from coral mucus Acropora digitifera had activity as an antibiofilm because it can reduce cell surface hydrophobicity, which is an essential factor in the formation of Streptococcus pyogenes biofilms [13]. The active compound of Actinomycetes in inhibiting biofilms is by inhibiting proliferation, adhesion, metabolic enzyme activity (protease and phospholipase), hypha formation, and biofilm development [14].
Actinomycetes InaCC A758 isolated from rhizosphere soil in Kepulauan Seribu, Jakarta, Indonesia, reported have wide-spectrum antimicrobial activities against C. albicans ATCC 10231, S. aureus ATCC 6538, Escherichia coli BTCC B 614, Bacillus subtilis, and Pseudomonas aeruginosa with minimum inhibitory concentration (MIC) ranged at 3.125–50 μg/ml [15]. The InaCC A758 has 99.85% similarity with Streptomyces badius strain NRRL B-2567 based on 16S rRNA gene identification [15]. In this study, we investigated the potency of Actinomycetes InaCC A758 extracts against dual-species biofilms C. albicans and S. aureus.
MATERIALS AND METHODS
Organisms and antibiofilm agents
This study used C. albicans ATCC 10231 and S. aureus ATCC 6538, acquired from the Regional Laboratory of Yogyakarta, Indonesia. The stock cultures were stored at −80°C, subcultured on sabouraud dextrose agar medium for C. albicans ATCC 10231 and Muller Hinton agar medium for S. aureus ATCC 6538.
We used ethyl acetate (EA) and chloroform extract from Actinomycetes InaCC A758 for antimicrobial and antibiofilm agents. InaCC A758 was obtained from the Indonesian Research Institute (LIPI) collection. Five milligrams of Actinomycetes extract was dissolved into 5 ml dimethyl sulfoxide (DMSO) 5% for the stock.
Extraction of InaCC A758
The extraction of InaCC A758 used the maceration method with EA and chloroform as solvents. Briefly, InaCC A758 were inoculated in 10 ml SYP (yeast extract 0.4%, peptone 0.2%, and starch 1%) medium at 30°C for 3 days in an incubator shaker (130 rpm). After incubation, 10 ml precultured of InaCC A758 was added into 90 ml SYP medium (1:9) in an Erlenmeyer baffle flask 500 ml and incubated at 30°C for 3 days in an incubator shaker (130 rpm) for cultivation.
The extraction process used a liquid–liquid multi-stage extraction method, which begins with maceration using a nonpolar solvent (chloroform). Two liters cultured of InaCC A758 were added to 2 l of solvent (1:1) and incubated for four hours in an orbital shaker (120 rpm). The solution was poured into a separatory funnel, and the organic phase was collected into the receiving flask and evaporated using a rotary evaporator at 40 mPa at 40°C [15]. The dry extract was put into a tube and stored at 4°C until used.
Antimicrobial and antibiofilm testing of InaCC A758 extract
Inoculum preparation
Candida albicans ATCC 10231 was inoculated in 5 ml of yeast peptone dextrose (Sigma-Aldrich, USA) medium and incubated in a shaking incubator overnight at 35°C. Staphylococcus aureus ATCC 6538 was inoculated in brain heart infusion (BHI; Sigma-Aldrich, USA) medium and incubated in a shaking incubator at 37°C overnight. After overnight culture, the samples were centrifuged at 3,000 rpm for 5 minutes. The cells were washed twice with sterile phosphate buffered saline (PBS; Sigma-Aldrich, USA), and the pellets were resuspended with 5 ml of BHI medium (Sigma-Aldrich, USA). The final concentration of the cell suspension was adjusted at 1 × 108 CFU/ml according to McFarland standard 0.5 [16].
Antimicrobial activity assay of InaCC A758 extract
Antimicrobial activity assay used the micro broth dilution method based on the Clinical and Laboratory Standards Institute 2015 [17]. Candida albicans ATCC 10231 (∼106 CFU/ml) alone, S. aureus ATCC 6538 (∼105 CFU/ml) alone and dual-species C. albicans ATCC 10231 (∼106 CFU/ml), and S. aureus ATCC 6538 (∼105 CFU/ml) were added into microplate 96-wells in serial two-fold dilutions of InaCC A758 extracts (1.56–400 μg/ml). Negative controls used yeast or bacteria only, and positive controls used fluconazole alone, gentamicin alone, and fluconazole and gentamycin combined. The plates were incubated at 35°C for 24 hours, and the minimum inhibition concentration (MIC) values were determined by visual observation.
Biofilm inhibitory assay of InaCC A758 extracts against dual-species biofilms
Determination of the minimum biofilm inhibition concentration (MBIC) of dual-species biofilms C. albicans ATCC 10231 and S. aureus ATCC 6538 using the method from Kart et al. [18] with modification. One hundred microliters of suspension containing C. albicans 105 CFU/ml were added to each well’s microtiter plate and then incubated at 37°C for 90 minutes for the Candida biofilm attachment phase. After incubation, the plates were washed three times with 150 μl of sterile distilled water. One hundred microliters of BHI medium containing S. aureus 106 CFU/ml with various concentrations of test compounds (1.56–400 μg/ml) were put into wells and incubated at 37°C for 48 hours. Medium containing DMSO was used as solvent control, the microbial suspension was used as a negative control, fluconazole (0.39–100 μg/ml) and gentamycin (0.39–100 μg/ml) as a positive control, and BHI medium as a blank. After incubation, the medium was removed, washed with PBS three times, and dried at room temperature for 10 minutes. The biofilm formed was stained with 100 μl 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide solution and incubated at 37°C for 2 hours. The microplate was washed with sterile aquadest three times, and 125 μl of 96% ethanol was added to dissolve the biofilm formed. The optical density (OD) was obtained with an ELISA Reader at 570 nm [19]. The experiments were carried out three times replications. The percentage of biofilm-forming inhibition (% BFI) was calculated using the formula [20]:
ODc = optical density (570 nm) of the negative control (medium, yeast cells, and DMSO); ODt = optical density (570 nm) of the test well; ODbl = optical density of the blank.
Biofilm reduction assay of InaCC A758 extract against dual-species biofilms
The determination of minimum biofilm reduction concentration (MBRC) of dual-species C. albicans ATCC 10231, and S. aureus ATCC 6538 was the same as that of MBIC dual-species. Biofilms were grown together for 24 hours in the microplate, and then test compounds were added. The OD was obtained with an ELISA Reader at 570 nm [19]. The experiments were carried out three times replications. The percentage of biofilm-forming reduction (% BFR) was calculated using the formula [20]:
ODc = optical density (570 nm) of the negative control (medium, yeast cells, and DMSO); ODt = optical density (570 nm) of the test well; ODbl = optical density of the blank.
Scanning electron microscopy (SEM)
For SEM observations, dual-species C. albicans ATCC 10231 and S. aureus ATCC 6538 biofilm were grown on sterile polyvinyl chloride coverslips (with a thickness of 0.13–17 mm and a diameter of 22 mm) in 12-well microtiter plates (Corning® Costar®, Sigma-Aldrich, MO, USA) with EA and chloroform extract (1/4-1/2MIC), and untreated cells for 24 hours at 37°C. After that, the coverslips were washed twice with sterile PBS (0.1 M and pH 7.2). The samples were dehydrated with ethanol series (70% for 10 minutes, 95% for 10 minutes, and 100% for 20 minutes) and air-dried overnight in a desiccator [21,22]. The coverslip was coated twice with platinum vanadium using a sputter ion (Bal-Tec SCD 005), then bonded to carbon double-sided tape for examination by SEM (JEOL JED-2300, Japan).
Fractination, isolation, and identification of the compounds of Actinomycetes InaCC A758
The dried Actinomycetes extract was then fractinated using semi-preparative high-performance liquid chromatography (HPLC) (waters) with a C18 column. The mobile phase used methanol: water = 50:50 with a running time of 33 minutes. A total of 20 μl of Actinomycetes extract filtered with a syringe filter was injected into the HPLC machine. Each peak that appears is collected using a clean bottle and evaporated to be dried and tested for bioactivity. The active peak was then purified again similarly but with a different solvent composition, i.e., methanol: water = 80:20 with a running time of 20 minutes to obtain a single peak. Furthermore, the single peak is collected and dried for identification.
The pure compound was determined [23] using GC-MS-QP2010S (Shimadzu, Japan) [23]. The column type used was Agilent HP-5MS UI (30 m length, 0.25 mm diameter, 0.25 m film thickness), and used helium carrier gas. The compound identification was analyzed based on m/z using chromeleonTM chromatography data system software.
Statistical analysis
We used probit analysis to determine 50% biofilm inhibition concentration (BIC50), 80% biofilm inhibition concentration (BIC80), 50% biofilm reduction concentration (BRC50), and 80% biofilm reduction concentration (BRC80).
RESULTS
Actinomycetes InaCC A758 extraction product
Two liters of fermented liquid from Actinomysetes InaCC A758 culture produced 290 mg of EA extracts (758 EA) and 187 mg of chloroform extracts (758 Cl).
Antimicrobial activity of InaCC A758
The minimum inhibitory (MIC) value of InaCC A758 ethyl acetate extract (758 EA) against S. aureus was eight times smaller than that of C. albicans (Table 1). This MIC indicated that A758 EA had better inhibitory activities against Gram-positive bacteria than fungi. In contrast, the MIC value of InaCC A758 chloroform extract (758 Cl) against C. albicans was twice as small as the MIC value of S. aureus. These results show that A758 Cl was more active in inhibiting fungi than Gram-positive bacteria. Meanwhile, the MIC values of both extracts, i.e., 758 EA and 758 Cl against dual-species C. albicans and S. aureus planktonic cells, were 8–64 times greater than MIC values in mono-species. Both EA and chloroform extracts have the same activities in inhibiting dual-species planktonic cells (MIC: 400 μg/ml) (Table 1).
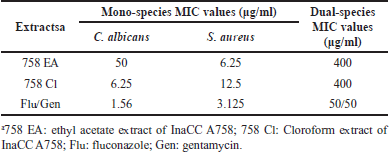 | Table 1. MIC values of InaCC A758 extracts against C. albicans and S. aureus planktonic cells (mono-species and dual-species). [Click here to view] |
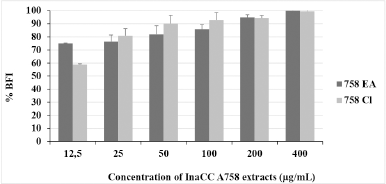 | Figure 1. BFI percentation of InaCC A758 extracts against dual-species biofilm: C. albicans ATCC 10231 and S. aureus ATCC 6538. Inhibitory activities were dose-dependent. Statistical analysis by probit analysis determined BIC50 and BIC80 value of EA extracts were 3.57 ± 1.384 and 30.5 ± 6.05 mg/ml, respectively. The BIC50 and BIC80 values of chloroform extracts ranged from 6.66 ± 1.59 and 29.25 ± 4.64 mg/ml, respectively. The experiments were carried out three times replication. 758 EA: ethyl acetate extract of InaCC A758; 758 Cl: chloroform extract of InaCC A758. [Click here to view] |
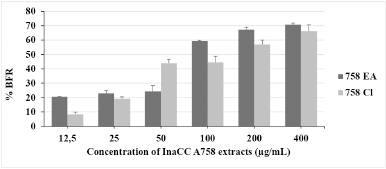 | Figure 2. BFR percentation of InaCC A758 extracts against dual-species biofilm: C. albicans ATCC 10231 and S. aureus ATCC 6538. Statistical analysis by probit analysis determined BRC50 and BRC80 of EA extracts were 100.62 ± 0.14 and 596.4 ± 24.4 mg/ml, respectively. The BIC50 and BIC80 value of chloroform extracts ranged from 131.85 ± 12.9 and 849.6 ± 29.03 mg/ml, respectively. The experiments were carried out three times replication. 758 EA: ethyl acetate extract of InaCC A758; 758 Cl: chloroform extract of InaCC A758. [Click here to view] |
Biofilm inhibition activity of InaCC A758 against dual-species biofilms
The biofilm inhibition percentage of InaCC A758 extracts is shown in Figure 1. The increase of dual-species biofilm inhibition is linear with adding the extract doses. These results indicated that the activities of Actinomycetes InaCC A758 extract were dose dependent. Both EA and chloroform extracts could inhibit 50% formation of dual-species biofilms at concentrations less than 12.5 μg/ml. In inhibiting 80% of dual-species biofilm formation, chloroform extract was better than EA extract (25 and 50 μg/ml, respectively). Both extracts could inhibit 100% dual-species biofilms at 400 μg/ml.
Biofilm reduction activity of InaCC A758 against dual-species biofilms
The biofilm reduction activity of InaCC A758 extracts is shown in Figure 2. EA extract reduced more than 50% of the biofilm formed at 100 μg/ml concentration, while chloroform extract required a two-fold concentration (200 μg/ml). At the highest concentrations tested (400 μg/ml), EA and chloroform extracts could reduce the formation of dual-species biofilms by more than 60%. However, EA extract has better activities than chloroform extract in reducing the dual-species biofilms.
The probit analysis for determining the value of dual-species BIC50 and BIC80 and BRC50 and BRC80 was shown in Table 2. The potency of EA extract to reduce dual-species biofilm was better than chloroform extract.
Biofilm structures observation by SEM
The structures of dual-species biofilm without treatment (control) were pseudohifa of C. albicans with smooth surfaces. The structure of S. aureus cells was round-shaped, clustered, and attached to the surface of pseudohifa. The surface of S. aureus cells was also smooth (Fig. 3a).
The structure of dual-species biofilm cells exposed by 758 EA at a concentration of 1/4 MIC (100 μg/ml) showed a reduced number of C. albicans and S. aureus cells. Yeast cells with oval shapes were stuck together and had varying sizes and smooth surfaces. Staphylococcus aureus cells appeared round-shaped, clustered, and partially attached to the surface of the yeast cells. Some bacterial cells seen have structural damage (Fig. 3b). Cells exposed to 758 EA at a concentration of 1/2 MIC (200 μg/ml) appeared to undergo morphological changes where yeast cells became wrinkled and damaged (Fig. 3c). Cells were exposed to 758 Cl at a concentration of 1/4 MIC (100 μg/ml), and the yeast cells were attached by S. aureus cells. The surface of the yeast cells began to wrinkle and become uneven. Bacterial cells appeared round-shaped, and the membrane cells seemed to be in intake, but the cells’ numbers were reduced (Fig. 3d). Yeast cells with irregular oval-shaped surfaces were wrinkled, uneven, and damaged when exposed to 758 Cl at a concentration of 1/2 MIC (200 mg/ml) (Fig. 3e).
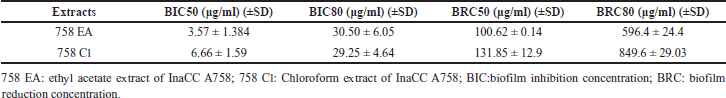 | Table 2. BIC and BRC of InaCC A758 extracts against dual-species C. albicans and S. aureus biofilm based on probit analysis. [Click here to view] |
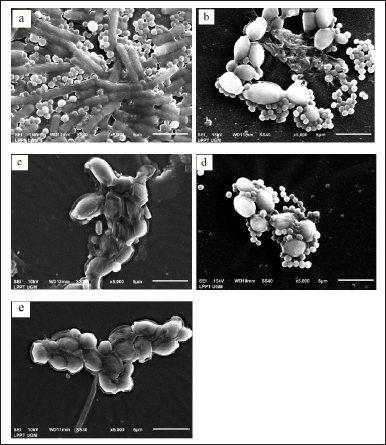 | Figure 3. SEM observation of dual-species biofilm: C. albicans ATCC 10231 and S. aureus ATCC 6538. (a) Cells without pre-treatment (control) seemed pseudo hyphae of C. albicans with smooth surfaces and overlapping. Staphylococcus aureus cells were round in shape and clique. Bacterial cells are attached to the surface of pseudo hypha. The membrane surface of S. aureus cells was smooth; (b) cells were exposed to EA extract of InaCC A758 at a concentration of 1/4 MIC (100 mg/ml), which showed a reduced amount of yeast and bacterial cells. Bacterial cells are attached to the surface of the yeast cells partially. There was structural damage of bacterial cells; (c) cells were exposed to EA extract of InaCC A758 at concentration 1/2 MIC (200 mg/ml) appeared to undergo morphological changes where yeast cells become wrinkled and damaged; (d) cells were exposed to chloroform extract at concentration 1/4 MIC (100 mg/ml) seemed the surface of the yeast cells was wrinkled and uneven; (e) cells were exposed to chloroform extract at concentration 1/2 MIC (200 mg/ml) appeared only yeast cells with irregular oval-shaped, surface wrinkled, uneven, and damaged. [Click here to view] |
Fractination, isolation, and identification of active compounds of InaCC A758
Fractionation of InaCC A758 EA extract resulted in eight fractions (F1 EA–F8 EA). In comparison, chloroform extract obtained seven fractions (F1 Cl–F7 Cl) with the retention time (Rt) of each peak and its area percentage shown in Table 3. The chromatograms of the fractions results using semipreparative HPLC can be seen in Figure 4. The Identification of the active compound InaCC A758 using gas chromatography-mass spectrometry (GC-MS) is shown in Figure 5 and Table 4. Figure 5 shows that the EA fraction has seven peaks with possible compounds that can be seen in Table 4.
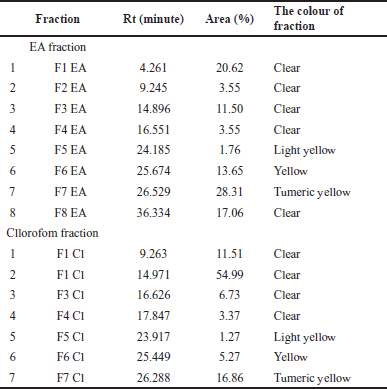 | Table 3. Retention time (Rt) and percentage of the area of the InaCC A758 fraction. [Click here to view] |
DISCUSSION
This study showed that the MIC values of Actinomycetes InaCC A758 extract against dual-species C. albicans and S. aureus were higher than those of monospecies (Table 1). Interactions between microbes in multi-species communities could increase virulences and drug tolerance [24] and require higher doses for treatment. The co-infection of C. albicans and S. aureus could increase mortality because there was “lethal synergy” when they were together [24]. Todd et al. [25] reported an increase in mortality range at 80%–100% in mice with intraabdominal infection models inoculated with dual-species C.albicans and S. aureus, while in monospecies infections, there was no mortality in the mouse model. The ability of C. albicans to form hyphae is an essential factor in increasing mortality because the hyphal can invade and penetrate human tissue [26]. It was difficult to eradicate the dual-species biofilm because the polysaccharides contained in the biofilm matrix of C. albicans could influence the tolerance of S. aureus to antimicrobials when they were grown together [27]. Extracellular biofilm matrix could protect microbes against antimicrobial agents. The protection effect of the extracellular matrix against antimicrobial agents in dual-species biofilm communities occurred through two mechanisms: the first is that the extracellular matrix binds drug components; thus, the drugs could not penetrate biofilms. The second is the presence of the matrix material itself, which induces drug tolerance due to the expression of related genes [24]. Interestingly, the Actinomycetes InaCC A758 extracts in this study could inhibit the dual-species biofilm up to 80% at concentrations 30.50 ± 6.05 μg/ml (Table 2). This result is an important finding regarding the potential of Actinomycetes extract as an anti-biofilm agent.
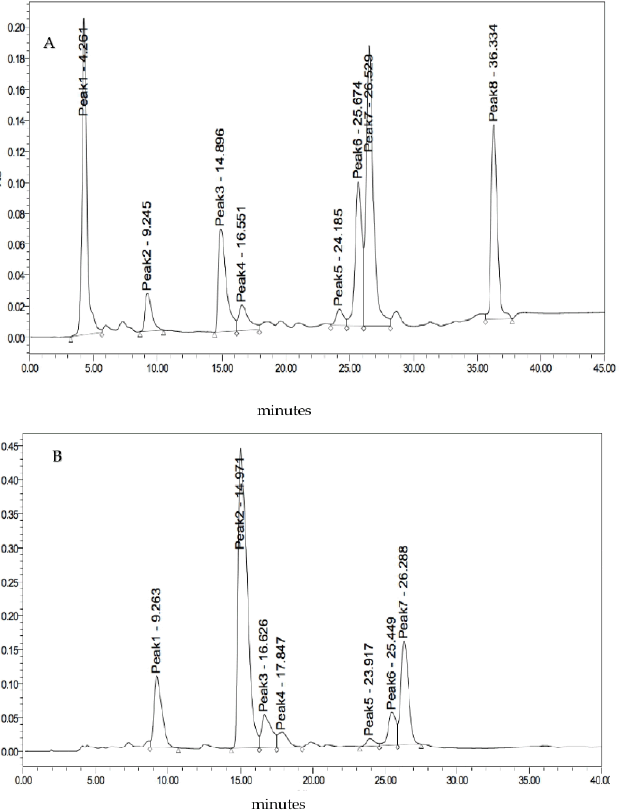 | Figure 4. Chromatograms of the InaCC A758 isolated fractions. (A) EA extracts. (B) Chloroform extracts. [Click here to view] |
This study found the unique structure of dual-species C. albicans and S. aureus biofilm (Fig. 3). This result is related to Peters et al. [2], which reported the physical interactions within the dual-species biofilm communities. The dual-species of S. aureus and C. albicans biofilms formed a unique architecture where S. aureus attached to the C. albicans hypha. Staphylococcus aureus would form microcolonies attached to the surface of the biofilm with C. albicans as the basis [5]. The S. aureus-hypha binding would influence the formation of yeasts into hyphae in the biofilm communities [24]. The specific interactions between S. aureus and C. albicans could affect the growth of biofilms [27]. In the dual-species community, S. aureus is attached to the hyphal of C. albicans specifically [2], and this hypha acts as an S. aureus courier to invade human tissue [28]. Staphylococcus aureus was bound in the matrix secreted by C. albicans, i.e., β-1,3-glucan [27], affecting its resistance to vancomycin [5]. Protein adhesin plays an essential role in bacterial-fungal interaction in biofilm communities. The Als3 protein was a specific receptor secreted by C. albicans hypha, which could bind S. aureus [28]. Kean et al. [29] reported another component in the biofilm matrix, i.e., extracellular DNA, which supported the attachment of S. aureus to fungal cells and affected the stability of the biofilm matrix.
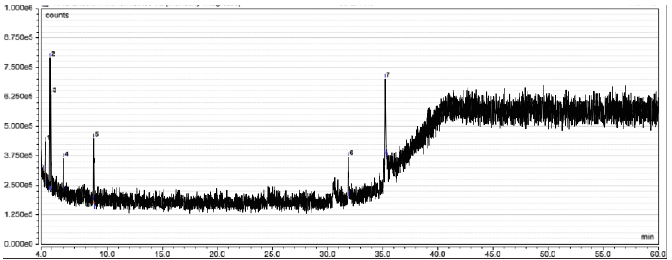 | Figure 5. The GC-MS spectra of EA InaCC A758 fraction. [Click here to view] |
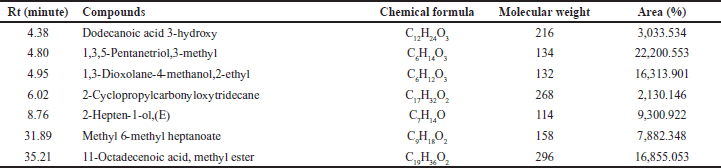 | Table 4. Molecular weight and compounds contained of the EA InaCC A758 fraction. [Click here to view] |
The structures of dual-species biofilm cells exposed by Actinomycetes InaCC A758 extracts displayed morphological damage to the cells (Fig. 3). Alkylphenol compounds found in Actinomycetes could inhibit the germination of spores [30]. Polyketide compounds such as Forazaline A can damage the integrity of the cell membrane by deregulating phospholipids. Phenolic compounds worked as antifungals by influencing the morphological changes of fungal cells. This study also showed that untreated cells appeared in the pseudohyphae while treated cells appeared only in yeast cells (Fig. 2). This result was possible because Actinomycetes extracts act as antimicrobial and antibiofilm in dual-species by damaging the membrane cells and inhibiting the yeast-hyphal transition. Phenolic compounds found in Actinomycetes effectively inhibited and damaged C. albicans biofilms because they inhibited hyphal development. Hyphae were very involved in the initial adhesion stage of biofilms [31]. Glycolipid compounds produced from actinobacterium Brevibacterium casei MS419 isolated from the Dendrilla nigra sponge inhibited the initial attachment phase of E. coli and Vibrio alginolyticus biofilm cells [32].
The GC-MS results showed that the EA of InaCC A758 fraction contained seven compounds, i.e., 3-hydroxy dodecanoic acid; 1,3,5-pentanetriol,3-methyl; 1,3-dioxolane-4-methanol,2-ethyl; 2-cyclopropylcarbonyloxytridecane; 2-hepten-1-ol,(E); methyl 6-methyl heptanoate; and 11-octadecenoic acid, a methyl ester. Dodecanoic acid 3-hydroxy is a medium-chain saturated fatty acid compound. Fatty acids are compounds that are found in many living organisms. Fatty acids are essential in forming cell structures and play an important role in energy for living things.
Several studies have reported that fatty acid compounds are potential antimicrobials [33] and antibiofilms [34]. Several fatty acids are reported to be able to damage and inhibit biofilm formation from various pathogens such as S. aureus [35], P. aeruginosa [36], and C. albicans [37]. Lee et al. [38] reported the activity of dodecanoic acid, which could inhibit C. albicans biofilms up to more than 75% with MIC values ranging from 100 to 200 μg/ml. This compound inhibited the growth of hyphae and cell aggregation and reduced the production of farnesol and sterols. Farnesol plays an essential role in quorum sensing activity, which affects the formation of biofilms, and sterols play an important role in the structure of cell membranes.
The 1,3,5-pentane-triol,3-methyl compound is an alkane compound class of aliphatic compounds. The 1,3,5-pentanetriol,3-methyl compounds are contained in several extracts from the stems and stalks of the Buchanania cocinchinensis plant and are reported to have antioxidant and antimicrobial activity [39]. The 1,3-dioxolane is a compound that is widely used in the synthesis of compounds that have biological activity. In synthesizing compounds of natural origin, these compounds are often used as protective groups for ketones and aldehydes. Several compounds containing ring 1,3 dioxolane were reported to have antifungal, antibacterial, antiviral, and antineoplastic activity [40] succeeded in synthesizing compounds derived from 1,3-dioxolane and reported that these compounds have activity against Gram-positive bacteria (S. aureus ATCC 29213, Staphylococcus epidermidis ATCC 12228, and Enterococcus faecalis) and Gram-negative bacteria (P. aeruginosa ATCC 27853, E. coli ATCC 25922, Klebsiella pneumoniae ATCC 4352, and Proteus mirabilis ATCC 14153). The compound also has antifungal activity against C. albicans 10231. The compound 11-octadecenoic acid, methyl ester, has anti-inflammatory, anticancer, and hypocholesterolemic activity.
However, research on secondary metabolites of Actinomycetes as dual-species antibiofilm is very rare. In fact, in the human body, biofilms are usually formed in multi-species biofilms. This result can lead to further studies to develop the Actinomycetes InaCC A758 as an antibiofilm, especially in dual-species.
CONCLUSION
Actinomycetes InaCC A758 have activities in inhibiting and reducing dual-species biofilms. The activities of EA extract and chloroform extract as antibiofilm were almost similar. The InaCC A758 extracts affect the morphological shape of bacteria and fungal cells. However, this research was still fundamental, so further study of the fractionation and isolation of bioactive compounds is still needed.
ACKNOWLEDGMENTS
The experiment was performed in the Research Centre for Biotechnology, Indonesian Institute of Sciences (LIPI), and the Microbiology Department, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada. We want to thank the Department for all the facilities.
AUTHOR CONTRIBUTIONS
Concept and design: Mustofa Mustofa and Puspita Lisdiyanti.; data acquisition: Titik Nuryastuti and Setiawati.Setiawati; data analysis, Setiawati Setiawati, Eti Nurwening Sholikhah, and Jumina Jumina; drafting manuscript: Setiawati Setiawati, Titik Nuryastuti, Eti Nurwening Sholikhah.; critical revision of manuscript: Mustofa Mustofa, Jumina Jumina, and Puspita Lisdiyanti.; Statistical analysis: Setiawati Setiawati, Eti Nurwening Sholikhah; funding acquisition: Eti Nurwening Sholikhah. Project administration, Setiawati Setiawati; supervision: Mustofa Mustofa; Titik Nuryastuti; final approval: Eti Nurwening Sholikhah.
FINANCIAL SUPPORT
This research was funded by a final project reconciliation grant from the Faculty of Medicine, Public Health and Nursing of Universitas Gadjah Mada.
CONFLICTS OF INTEREST
The authors declare no conflict of interest. The funders had no role in the study’s design, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1 Pammi M, Zhong D, Johnson Y, Revell P, Versalovic J. Polymicrobial bloodstream infections in the neonatal intensive care unit are associated with increased mortality: a case-control study. BMC Infect Dis. 2014;14:1–8. CrossRef
2. Peters BM, Jabra-Rizk MA, Scheper MA, Leid JG, Costerton JW, Shirtliff ME. Microbial interactions and differential protein expression in Staphylococcus aureus-Candida albicans dual-species biofilms. FEMS Immunol Med Microbiol. 2010;59:493–503. CrossRef
3. Yang L, Liu Y, Wu H, Høiby N, Molin S, Song Z. Current understanding of multi-species biofilms. Int J Oral Sci. 2011;3:74–81. CrossRef
4. Klotz SA, Chasin BS, Powell B, Gaur NK, Lipke PN. Polymicrobial bloodstream infections involving Candida species: analysis of patients and review of the literature. Diagn Microbiol Infect Dis. 2007;59:401–6. CrossRef
5. Harriott MM, Noverr MC. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrob Agents Chemother. 2009;53:3914–22. CrossRef
6. Dumitru R, Hornby JM, Kenneth W, Nickerson KW. Defined anaerobic growth medium for studying Candida albicans basic biology and resistance to eight antifungal drugs. Antimicrob Agents Chemother. 2004;48:2350–4. CrossRef
7. Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum M, et al. Biofilm formation by the fungal pathogen Candida albicans: development, architecture and drug resistance. Natl Rev Microbiol. 2001;183:5385–94. CrossRef
8. Khanna N. Antimicrobial agents: antifungal & antiviral drugs. Delhi, India: University College of Medical Sciences Shahdara; 2007.
9. Larabi M, Legrand P, Appel M, Gil S, Lepoivre M, Devissaguet JP, et al. Reduction of NO synthase expression and tumor necrosis factor alpha production in macrophages by amphotericin B lipid carriers. Antimicrob Agents Chemother. 2001;45:553–62. CrossRef
10. Dodds Ashley ES, Lewis R, Lewis JS, Martin C, Andes D. Pharmacology of systemic antifungal agents. Clin Infect Dis. 2006;43:S28–39. CrossRef
11. Ambavane V, Tokdar P, Parab R, Sreekumar ES, Mahajan G, Mishra PD, et al. Caerulomycin a—an antifungal compound isolated from marine actinomycetes. Adv Microbiol. 2014;04:567–78. CrossRef
12. Belghit S, Driche EH, Bijani C, Zitouni A, Sabaou N, Badji B, et al. Activity of 2,4-di-tert-butylphenol produced by a strain of Streptomyces mutabilis isolated from a Saharan soil against Candida albicans and other pathogenic fungi. J Mycol Med. 2016;26:160–9. CrossRef
13. Nithyanand P, Thenmozhi R, Rathna J, Karutha Pandian S. Inhibition of Streptococcus pyogenes biofilm formation by coral-associated actinomycetes. Curr Microbiol. 2010;60:454–60. CrossRef
14. Guo Y, Wei C, Liu C, Li D, Sun J, Huang H, et al. Inhibitory effects of oral Actinomyces on the proliferation, virulence and biofilm formation of Candida albicans. Arch Oral Biol. 2015;60:1368–74. CrossRef
15. Setiawati S, Nuryastuti T, Sholikhah ETIN. The potency of actinomycetes extracts isolated from Pramuka Island, Jakarta, Indonesia as antimicrobial agents. Biodiversitas. 2021;22:1104–11. CrossRef
16. Setiawati S, Nuryastuti T, Ngatidjan N, Mustofa M, Jumina J, Fitriastuti D. In vitro antifungal activity of (1)-N-2-methoxybenzyl-1,10-phenanthrolinium bromide against Candida albicans and its effects on membrane integrity. Mycobiology. 2017;45:25–30. CrossRef
17. Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Wayne, PA: CLSI; 2015. CrossRef
18. Kart D, Tavernier S, Acker H Van, Nelis HJ, Coenye T. Activity of disinfectants against multispecies biofilms formed by Staphylococcus aureus, Candida albicans and Pseudomonas aeruginosa. Biofouling. 2014;30:377–83. CrossRef
19. Taff HT, Mitchell KF, Edward JA, Andes DR. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013;8:1325–37. CrossRef
20. Wang D, Wang C, Gui P, Liu H, Khalaf SMH, Elsayed EA, et al. Identification, bioactivity, and productivity of actinomycins from the marine-derived Streptomyces heliomycini. Front Microbiol. 2017;8:1–12. CrossRef
21. Torex A, Sasidharan S. Anti-Candida albicans biofilm activity by Cassia spectabilis standardized methanol extract: an ultrastructural study. Eur Rev Med Pharmacol Sci. 2011;15:875–82.
22. Tsang PWK, Bandara HMHN, Fong WP. Purpurin suppresses Candida albicans biofilm formation and hyphal development. PLoS One. 2012;7:1–8. CrossRef
23. Wu P-S, Kuo Y-T, Chen S, Li Y, Lou B-S. Gas chromatography-mass spectrometry analysis of photosensitive characteristics in citrus and herb essential oils. J Chromatogr Sep Tech. 2014;6:1–9. CrossRef
24. Carolus H, Van Dyck K, Van Dijck P. Candida albicans and Staphylococcus species: a threatening twosome. Front Microbiol. 2019;10:2162. CrossRef
25. Todd OA, Fidel PL, Harro JM, Hilliard JJ, Tkaczyk C, Sellman BR, et al. Candida albicans augments Staphylococcus aureus virulence by engaging the staphylococcal agr quorum sensing system. MBio. 2019;10:1–16. CrossRef
26. Sudbery P, Gow N, Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004;12:317–24. CrossRef
27. Kong EF, Kucharíková S, Van Dijck P, Peters BM, Shirtliff ME, Jabra-Rizka MA. Clinical implications of oral candidiasis: host tissue damage and disseminated bacterial disease. Infect Immun. 2015;83:604–13. CrossRef
28. Peters BM, Ovchinnikova ES, Krom BP, Schlecht LM, Zhou H, Hoyer LL, et al. Staphylococcus aureus adherence to Candida albicans hyphae is mediated by the hyphal adhesin Als3p. Microbiology (United Kingdom). 2012;158:2975–86. CrossRef
29. Kean R, Rajendran R, Haggarty J, Townsend EM, Short B, Burgess KE, et al. Candida albicans mycofilms support Staphylococcus aureus colonization and enhances miconazole resistance in dual-species interactions. Front Microbiol. 2017;8:1–11. CrossRef
30. Dharni S, Gupta S, Maurya A, Samad A, Srivastava SK, Sharma A, et al. Purification, characterization and in vitro activity of 2, 4-di-tert-butylphenol from Pseudomonas monteilii PsF84: conformational and molecular docking studies. J Agric Food Chem. 2014;62(26):6138–46.
31. Padmavathi AR, Abinaya B, Pandian SK. Phenol, 2,4-bis(1,1-dimethylethyl) of marine bacterial origin inhibits quorum sensing mediated biofilm formation in the uropathogen Serratia marcescens. Biofouling. 2014;30:1111–22. CrossRef
32. Kiran GS, Sabarathnam B, Selvin J. Biofilm disruption potential of a glycolipid biosurfactant from marine Brevibacterium casei. FEMS Immunol Med Microbiol. 2010;59:432–8. CrossRef
33. Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. App Micobiol Biotechnol. 2010;85:1629–42. CrossRef
34. Kumar P, Lee J, Beyenal H, Lee J. Fatty acids as antibiofilm and antivirulence agents. Trends Microbiol. 2020;28:1–16. CrossRef
35. Kim Y, Lee J, Raorane CJ, Oh ST, Park JG, Lee J, et al. Herring oil and omega fatty acids inhibit Staphylococcus aureus biofilm formation and virulence. Front Microbiol. 2018;9:1–10. CrossRef
36. Wenderska IB, Chong M, Mcnulty J, Wright GD. Palmitoyl-DL-carnitine is a multitarget inhibitor of Pseudomonas aeruginosa biofilm development. Chembiochem. 2011;12:2759–66. CrossRef
37. Prasath KG, Sethupathy S, Pandian SK. Proteomic analysis uncovers the modulation of ergosterol, sphingolipid and oxidative stress pathway by myristic acid impeding biofilm and virulence in Candida albicans. J Proteomics. 2019;208:103503. CrossRef
38. Lee J, Kim Y, Kiran S, Lee J. Antibiofilm and antifungal activities of medium-chain fatty acids against Candida albicans via mimicking of the quorum-sensing molecule farnesol. Microb Biotechnol. 2020;14(4):1353–66. CrossRef
39. Dixit AK, Kumar V, Maloni N, Sarkar M, Shrivastava B, Nair PG, et al. Comparative assessment of phytochemicals, antioxidant, and antimicrobial potential of stem bark and small branches of Buchanania cochinchinensis (Lour.). J Drug Res Ayuverdic Sci. 2019;4(2):72–8.doi: https://doi.org/10.5005/jp-journals-10059-0069
40. Kucuk H, Yusufoglu A, Mataraci E, Dosler S. Synthesis and biological activity of new 1,3-dioxolanes as potential antibacterial and antifungal compounds. Moleculs. 2011;16(8):6806–15. CrossRef.