INTRODUCTION
Medicines, particularly essential ones, play a significant role in healthcare services, addressing healthcare needs based on factors such as disease prevalence, effectiveness, safety, and cost-effectiveness [1,2]. Essential medicines are a crucial component of the sustainable development goals (SDGs) aiming to ensure access to safe, effective, quality, and affordable essential medicines and vaccines for all [3]. They are also integral to achieving Universal health coverage, ensuring access to health services, including essential medicines, without financial hardship [4].
According to the 2000 World Health Report, approximately one-third of the world’s population lacks access to essential medicines [5]. This situation is more severe in less affluent nations in Africa and Asia, where up to 50% of the population lacks reliable access to necessary medications. Improving access to essential medicines and vaccines has the potential to save around 10 million lives annually, with 4 million specifically in Africa and Southeast Asia [6]. In 2019, about 5.2 million children under five, including 2.4 million newborns, died from preventable or treatable causes such as inadequate nutrition, lack of immunization, and insufficient treatment for common childhood illnesses [7].
Challenges in obtaining essential medicines are evident due to factors such as limited availability, excessively high medication prices, and restricted affordability [8,9]. National policies, medicine pricing, and procurement strategies are necessary to ensure affordable medicines [10]. Despite the obstacles posed by high medicine prices, access improvements can still be achieved, even in the face of weak infrastructure and poverty [11]. The difficulty in finding reliable information on medicine prices and availability hampers governments in constructing effective medicine pricing policies or evaluating their impact. It also makes it challenging to compare medicine expenditures with other countries at a similar stage of development. Those responsible for purchasing medicines struggle to negotiate better deals due to a lack of a solid negotiation basis. Even in countries with greater purchasing power, governments, insurance funds, and hospitals often face difficulties in selecting medicines due to a lack of information [12].
In 2003, the World Health Organization (WHO) and Health Action International (HAI) jointly published a manual detailing standardized approaches for measuring drug prices, aiding policymakers in identifying purchase prices and potential policy-related challenges. Consequently, healthcare institutions must ensure the accessibility of essential medicines, particularly for prevalent diseases with high incidence rates [5]. The WHO HAI method is a reliable approach for assessing the accessibility of pharmaceuticals. It involves utilizing core drug and supplementary drug references provided by established sources. Within the WHO/HAI methodology standards, there is a list of essential medications at both global and regional levels. This standardization of drugs, assessed globally and regionally, allows for valuable comparisons with other nations and international benchmarks. Typically, they survey global core medications, regional core medications, and supplementary drugs. The choice of supplementary drugs is made at the national level based on each country’s specific healthcare priorities or to gather data on particular therapeutic categories [13].
HAI surveys across 50 countries have revealed notably high drug prices, especially in the private sector, often exceeding 80 times the international reference price (IRP). Availability of essential medicines tends to be low, particularly in the public sector, sometimes resulting in their complete unavailability. The cost of healthcare is often unaffordable, with patients needing more than 15 days’ wages to cover the expenses of a 30-day treatment regimen [14]. This systematic review aimed to analyze the availability, pricing, and affordability of essential medicines across multiple Asian countries. The intention is to offer evidence-based findings to support policymaking endeavors focused on improving the accessibility and fairness in obtaining essential medicines.
METHODS
A systematic search was conducted across several databases, which included Scopus, PubMed, and Google Scholar. This search utilized a combination of Boolean operators (AND, OR) and specified fields (Title, abstract, and all fields) to ensure thorough coverage. The search terms employed were as follows: “Availability of medicine,” “Availability of drugs,” “Availability of key essential medicines,” “Drug availability,” “Essential medicines,” “Drug affordability,” and “WHO HAI.”
The research adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, ensuring a systematic and standardized approach. The inclusion criteria for this systematic review were as follows: the study had to focus on the availability and affordability of essential medicines in hospitals, clinics, or community pharmacies in various Asian countries and employ WHO-HAI methods in their assessment of drug availability and affordability. Conversely, the exclusion criteria included studies published before 2018 and studies not published in the English language. A total of seven articles were selected for comparative analysis to gather insights into the state of drug availability and affordability in Asia.
Study quality assessment
By the assessment tools provided by the Joanna Briggs Institute, the researcher evaluated the methodological quality and risk of bias within the studies included in this systematic review. The assessment checklist encompasses eight criteria distributed across four key domains, which are: i) sampling techniques; ii) research subjects; iii) data collection; and iv) analytical methods [15]. Each criterion was assessed as “yes” (earning one point), “no” (zero points), “unclear” (zero points), or “not applicable” (one point). The methodological quality of each study was subsequently categorized as low (0–3 points), moderate (4–6 points), or high (7–8 points) [16].
Data extraction
The following data were collected from all the studies: i) basic information of literature including title, first author, publication year, the country where it took place, and the number of healthcare facilities involved. ii) Outcome measures including availability, median Median price ratio (MPR), and affordability of essential medicines based on the list of drugs from each paper.
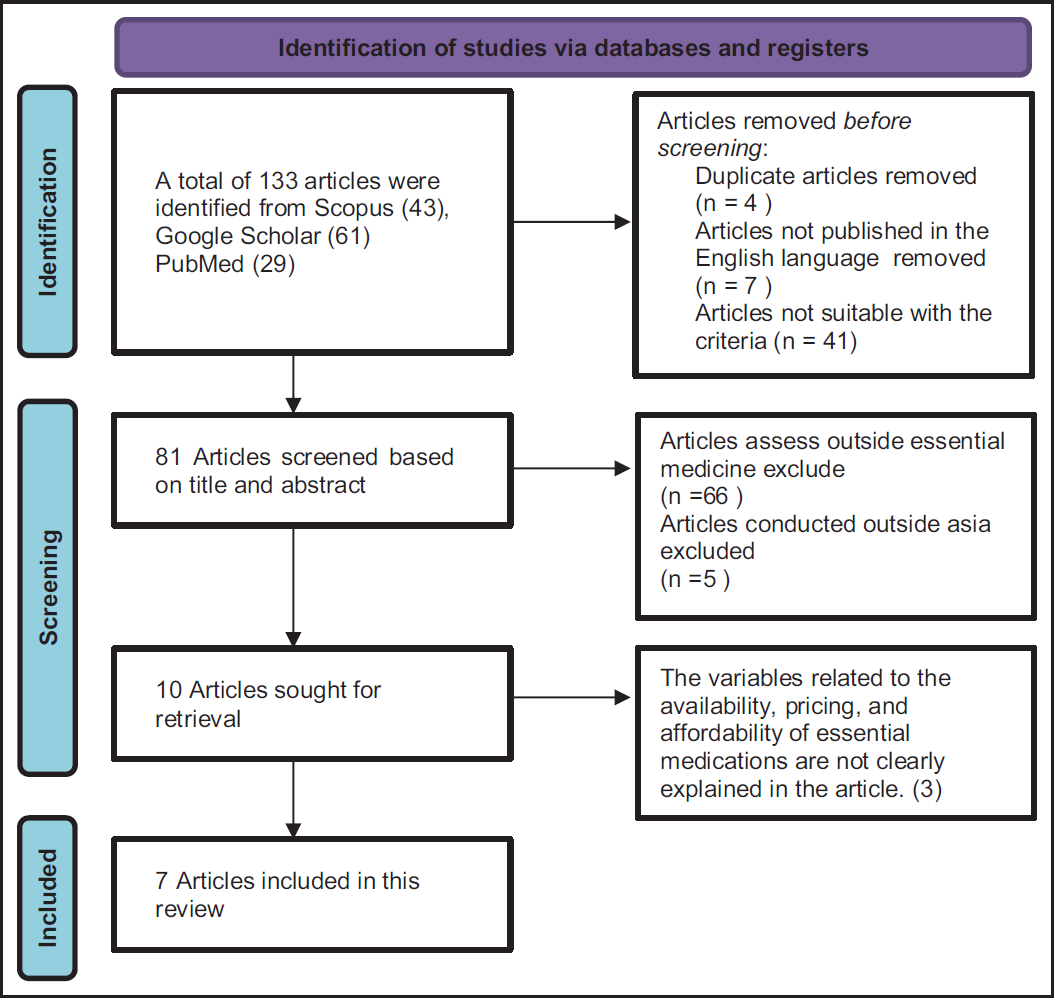 | Figure 1. PRISMA diagram of retrieved studies. [Click here to view] |
RESULTS
Search results
A PRISMA diagram illustrating the search process for this review is presented in Figure 1. As demonstrated in the PRISMA diagram, a total of 133 articles were identified in electronic databases, comprising 43 from Scopus, 61 from Google Scholar, and 29 from PubMed. Four duplicate articles, seven articles not published in the English language, and 41 articles not suitable with the criteria were excluded from this review, resulting in 81 articles. Screening based on the title and abstract, which specifically focused on essential medicines and studies conducted in Asia, led to the exclusion of 71 articles. Subsequently, 10 articles were assessed in full text, ultimately resulting in the eligibility of 7 articles for inclusion in the review.
Studies characteristics
Studies were conducted in several Asian countries, including China, Yemen, Pakistan, Indonesia, Vietnam, Jordan, and Bangladesh, between 2018 and 2020. All of these studies were carried out using a standardized survey instrument developed by the WHO and HAI. The research encompassed two major settings: the public sector and private sector, to assess the availability, pricing, and affordability of essential medicines as presented in Table 1. The results of the study quality assessment indicate that among the seven studies analyzed, three studies achieved a score of 6, two studies received a score of 7, and two studies scored 8. In summary, three studies (43%) were categorized as having moderate quality, while four studies (57%) were rated as high quality.
Drug availability in public and private sectors
The availability of each medication was represented as the percentage of the medicine’s presence in the facility on the day when data were collected. Several studies included in this review have provided insights into the availability of essential medicines across seven distinct regions presented in Table 2.
Research conducted in Zhejiang Province, China, revealed that the availability of Originator Brands (OBs) exceeded that of lowest-priced generics (LPGs) in the public sector, with OBs at 41.8% availability and LPGs at 35.1%. However, in the private sector, the situation reversed, with OBs at 36.7% availability and LPGs at 40.3% [17]. Contrastingly, in Jordan, the availability of essential medicines told a different story. In both the public and private sectors, the availability of OBs lagged behind that of LPGs. In the public sector, OBs were notably low at 9%, while LPGs had an average rate of 72%. In the private sector, both OBs and LPGs had higher availability, with rates of 57% and 67%, respectively [18].
Another study conducted a study in the Lahore Division, Pakistan, found that in the public sector, OBs were available at a mere 6.8%, while LPGs were more prevalent at 35.3%. In the private sector, OBs showed higher availability at an average of 55%, whereas LPGs were less available at 20.3% [19]. In Indonesia, there is a study on the availability of medicines that only assesses the availability of essential medicines, specifically LPGs, in both the public and private sectors. They found LPGs to be available at rates of 76.6% in the public sector and 60.58% in the private sector. However, no data were provided regarding the availability of OBs in either sector [20].
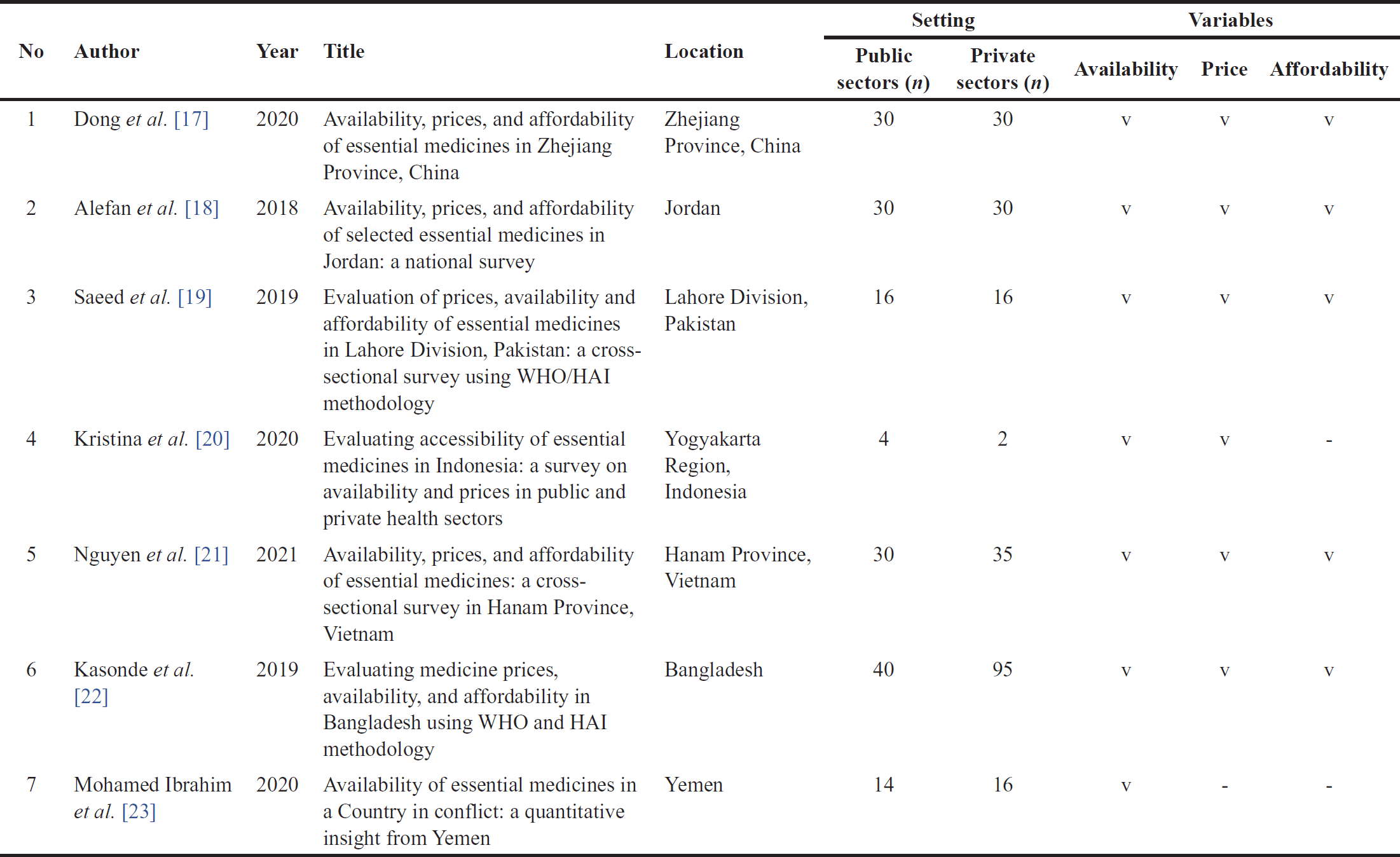 | Table 1. Characteristics of the studies. [Click here to view] |
Nguyen et al. [21] conducted a study in Hanam Province, Vietnam, revealing a pattern where OBs had lower availability than LPGs. In the public sector, OBs were only available at 0.7%, while LPGs had a 63.2% availability. In the private sector, OBs were slightly more available at 13.7%, whereas LPGs had 47.9% availability [21]. In Bangladesh, another study indicated that OBs were not found in the public sector. Only LPGs were available with a mean availability of 37%. In the private sector, which included retail pharmacies and private clinics, OBs were less available, with a 4% availability in retail pharmacies and 2% in clinics. Conversely, LPGs had higher availability, with a mean of 63% in retail pharmacies and 54% in clinics [22].
In Yemen, the last study included in this review focused on the availability of LPGs medications. The results showed that the availability of LPGs in public pharmacies was lower compared to private pharmacies, with mean availability rates of 53.3% in public hospitals and 18.9% in public health centers, while private hospitals and private pharmacies had higher availability rates of 73.3% and 79.7%, respectively [23].
Drug pricing in public and private sectors
In this review, pricing information for individual medications was presented as a ratio to an IRP, expressed as the MPR, to facilitate international comparisons. The MPR was calculated by dividing the median local price of the medication by the IRP. This ratio indicated the extent to which the MPR was higher or lower compared to the IRP. For instance, an MPR of 2 signifies that the local medication price is double the IRP.
Most of the studies indicate that the median MPR in the private sector was higher than in the public sector as presented in Table 2. Several studies from Indonesia, Vietnam, and Bangladesh did not assess the availability of OBs in the public sector and only focused on the LPGs [20–22]. In contrast, studies from Yemen did not assess drug pricing in either the public or private sector [23]. Another study from Pakistan states that in public sector health facilities, patient prices, i.e., the prices paid by patients to obtain medicines were not estimated due to the provision of free medicines [19].
Studies conducted in Zhejiang province, China, reveal that the median MPR in the public sector for OBs was 13.41, and for LPGs was 5.21. In the private sector, the MPR for OBs was 14.75, and for LPGs was 4.94. This indicates that the price for OBs is higher than the lowest-priced generics [17]. Studies from Jordan show similar results, with both OBs and LPGs priced higher in both the public and private sectors. The median MPR for OBs and LPGs in the public sector was 5.8 and 1.16, respectively. In the private sector, the median MPR for OBs and LPGs was 9.7 and 7.5, respectively [18].
As explained above, a study conducted in Pakistan only assessed the median MPR in the private sector for both OBs and LPGs, with the median MPR for OBs at 2.45 and for LPGs at 1.36, respectively [19]. Another study from Indonesia, only evaluated the median MPR of LPGs in both the public and private sectors, resulting in 0.98 and 2.46, respectively [20]. The next study from Vietnam evaluated the median MPR for both the public and private sectors, but in the public sector, only the median MPR of LPGs was assessed with a result of 0.95. In the private sector, the median MPR appeared to be higher for both OBs and LPGs when compared to the public sector, with the median MPR for OBs at 6.24 and for LPGs at 1.65 [21]
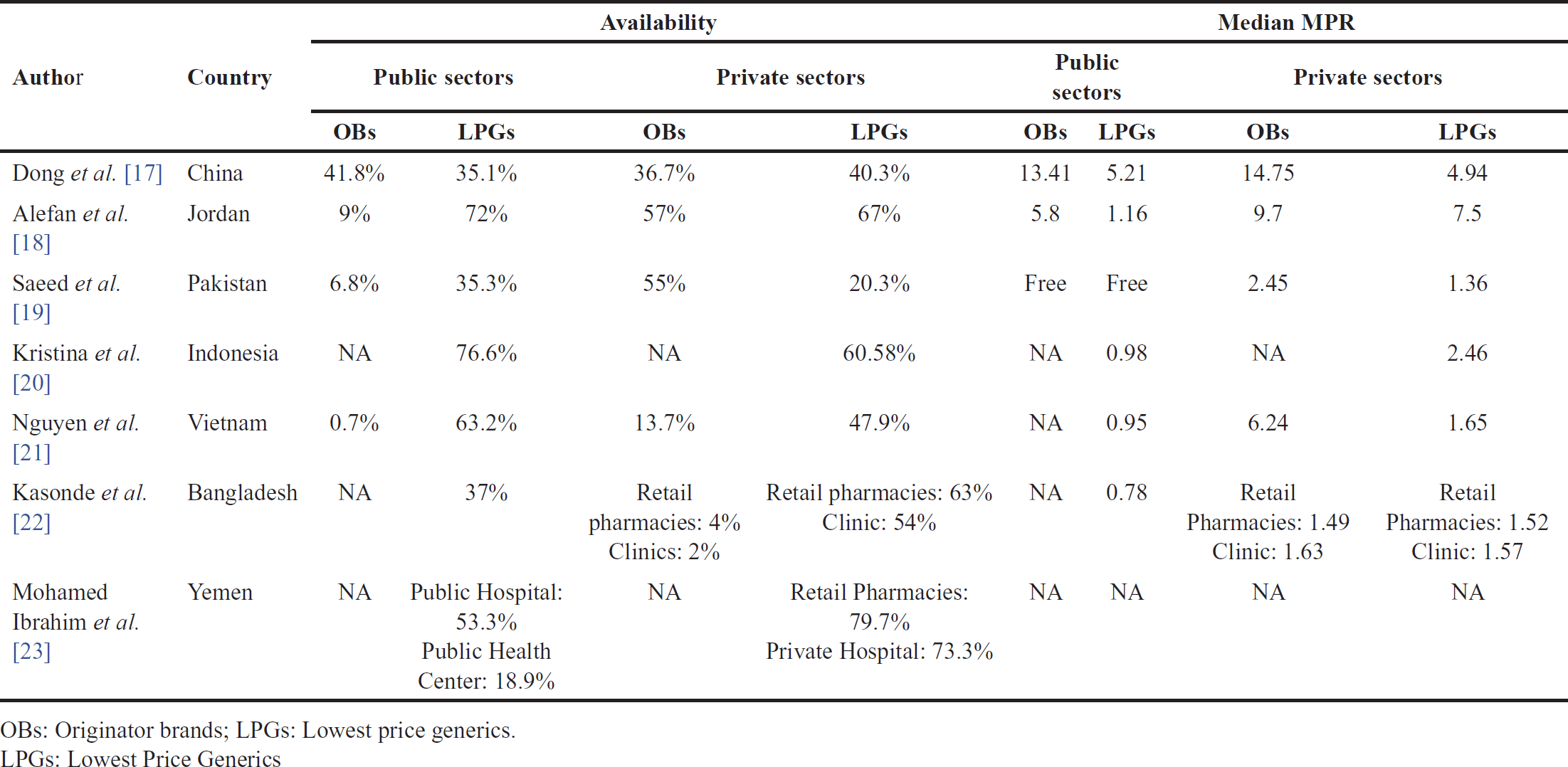 | Table 2. The availability and prices of essential medicines. [Click here to view] |
The study from Bangladesh also evaluated the median MPR for both the public and private sectors, but in the public sector, only LPGs were assessed with a median MPR of 0.78. In the private sector, the researchers not only evaluated the median MPR in retail pharmacies but also in private clinics. For OBs, the results for retail pharmacies and clinics were 1.49 and 1.63, respectively, while for LPGs, the results for retail pharmacies and clinics were 1.52 and 1.57, respectively. This shows that the median MPR in the clinics for both OBs and LPGs was higher than in retail pharmacies [22].
Drug affordability in private sectors
The subsequent variable examined in this review was drug affordability. Affordability was determined by comparing the total cost of acquiring standard treatments for common conditions to the daily wage of the lowest-paid unskilled government worker. In this review, drug affordability in the private sector was exclusively assessed as presented in Table 3. This decision was primarily made because the majority of papers included in this review did not evaluate affordability in the public sector, especially for branded medications. In addition, two studies from Indonesia and Yemen did not assess drug affordability [20,23].
The focus of drug affordability in this review centered on the eight most frequently examined diseases from each study, including asthma, diabetes, hypertension, hypercholesterolemia, pediatric respiratory infection, adult respiratory infection, arthritis, and ulcers, each treated with standard medication. Affordability for each standard treatment was evaluated for both branded drugs OBs and generic drugs (LPGs). Generally, treatments that cost only one day’s income or less are deemed affordable, but if they exceed 1 day’s income, it is categorized as unaffordable.
Based on the reviewed data on drug affordability across various countries, a general observation was that branded drugs OBs were less affordable than the lowest-priced medicines. The affordability values for OBs for bisoprolol were 1.5–2.7, simvastatin 1.3–4.3, ciprofloxacin 1.5–4.4, diclofenac 1.5–2.3, and omeprazole 1.9–5.2. Almost all branded drugs had affordability values above 1, except for drugs to treat asthma (salbutamol inhaler) and pediatric respiratory infection (cotrimoxazole). Furthermore, some generic drugs were still not affordable, such as bisoprolol (2.18) and omeprazole (4). This indicates that patients would need to spend more than a day’s wage to acquire these drugs [18,22].
DISCUSSION
Overall, the availability of essential medicines in both the public and private sectors, as indicated by the reviewed studies, was below the WHO global action plan targets of achieving 80% availability of essential medicines by 2025 [24,25]. Medicines must be adequately accessible to patients in both public and private sectors to ensure appropriate treatment and to achieve the overarching goal of improving their quality of life [1]. The average availability of branded drugs OBs in both the public and private sectors is categorized as very low (<30%), while in the public sector, the average availability of generic drugs (LPGs) is classified as low (30%–49%), and in the private sector, it is relatively high (50%–80%) [26]. This result was because most people tend to purchase drugs at lower prices compared to innovator brands. In addition, in the public sector, the availability of generic drugs is higher compared to branded drugs due to the limited health budget provided by the government to public hospitals. As a result, public hospitals need to allocate funds efficiently by purchasing generic drugs [13].
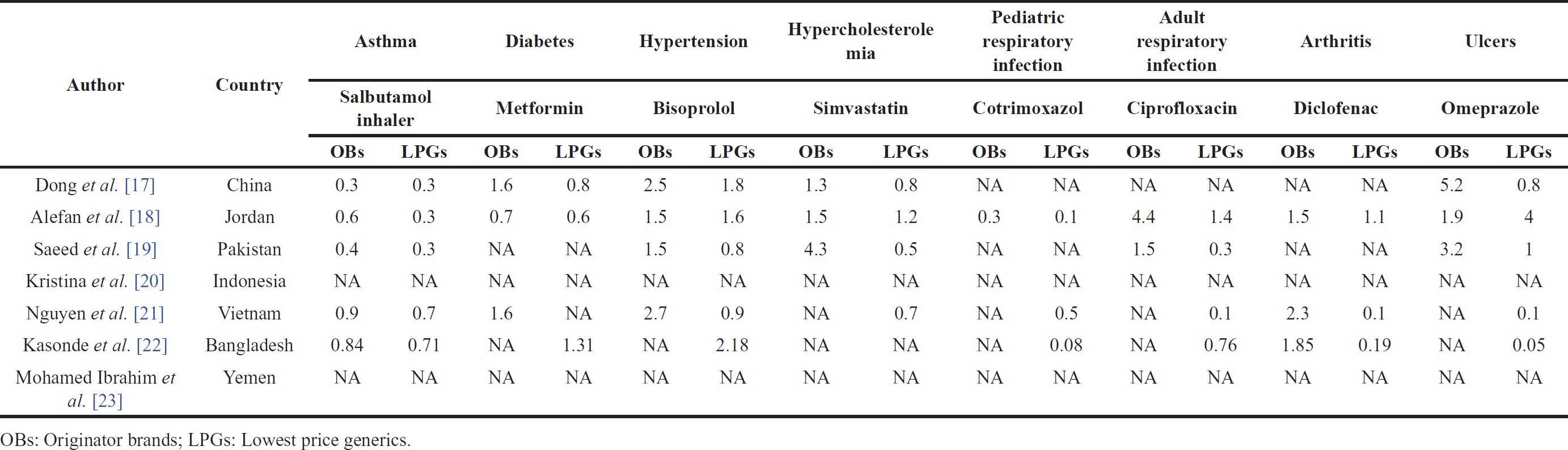 | Table 3. The affordability of core medicines for common diseases in the private sector. [Click here to view] |
This systematic review also indicates that the overall availability of essential medicines in the private sector surpasses that in the public sector. This aligns with findings from a study conducted across six low and middle-income countries (Bangladesh, Brazil, Malawi, Nepal, Pakistan, and Sri Lanka), revealing significantly lower overall medicine availability in public sectors across all countries when compared to the private sectors [27]. This happens because retail pharmacies have flexible ways of getting medicines. The groups that buy medicines have more options, and pharmacy operators can choose distribution companies that suit their needs. The different ways medicines are distributed can better and more flexibly meet the needs of these medicine-buying groups in retail pharmacies [28].
In terms of pricing assessed using the MPR, it is generally observed that the prices of medicines in the private sector tend to be higher than those in the public sector. Some papers also do not evaluate the prices of branded drugs OBs in the public sector. This might be because healthcare facilities in the public sector in some countries rarely provide branded medications. According to a study conducted in Pakistan, in public sector health facilities, patient prices—meaning the prices paid by patients to obtain medicines—were not estimated, as medicines were provided free of charge by the government [19].
From the public sector, the median MPR values vary across countries, ranging from 0.78× IRP for generic drugs (LPGs) to 13.41× IRP for branded medications. Meanwhile, in the private sector, the median MPR values range from 1.49× IRP for branded drugs in retail pharmacies to 14.75× IRP for branded drugs. All median MPR values from the private sector are above the IRP values, indicating that the set prices in each country are still higher than the international standard prices. The lack of price regulations may contribute to these disparities in the private sector [20,29]. These inconsistencies underscore notable variations in the markup of prices among different medicines. Therefore, emphasizing the role of IRPs as a benchmark is essential to ensuring lower procurement prices in the public sector [18]. The higher the purchase price of a drug, the greater the impact of various price components on the overall cost of the drug. Patient prices are influenced by factors such as patent rights for drugs within the country, the extent of generic drug production, the presence of national policies safeguarding local companies, and the imposition of various taxes and duties on essential medicines [30].
In terms of affordability, our review focused exclusively on the private sector, assessing eight drugs associated with the most frequently discussed diseases in each paper. These diseases include asthma, diabetes, hypertension, hypercholesterolemia, pediatric respiratory infection, adult respiratory infection, arthritis, and ulcers. A treatment is considered affordable if its cost is equal to or less than one day’s income. This criterion applies to a 7-day supply of medicine for acute conditions or a 1-month supply of medicine for chronic diseases [18].
It can be observed that branded drugs are less affordable compared to generic drugs. Most of the medications still require more than 1 day’s income (1.1–5.2) to purchase the needed drugs. Therefore, this result raises concerns that patients with low income may not be able to afford these medicines in the private sector. For instance, a patient may need to allocate up to 5.2 days’ income for the treatment of conditions such as ulcers with the originator drug omeprazole, or a patient may need to spend up to 4.3 days’ income to afford the treatment of conditions like hypercholesterolemia with the originator drug simvastatin in the private sector. The issue of affordability within the private sector holds significant importance, especially in light of the limited accessibility of medications in the public sector. Affordability also can be substantially compromised by the presence of multiple simultaneous illnesses, such as diabetes and hypertension, as well as by the occurrence of illnesses affecting multiple family members [22].
Crucial considerations for policymakers in crafting medicine pricing policies include periodic reviews of national essential medicines list (NEML)-based procurement. It is essential to implement innovative financing mechanisms for essential medicine sales in private sector pharmacies, along with an efficient procurement system supported by sustainable financing to enhance availability in public sectors. Establishing a reasonable pricing mechanism, such as the reference pricing policy applied in many countries, is key to addressing challenges in the pharmaceutical sector. Collaboration between drug regulators and the health department is crucial. Measures such as lowering procurement prices, exempting essential medicines from tariffs, promoting local manufacturing, and improving affordability need to be implemented. Combining these steps with consumer awareness campaigns and advocating regressive markups on costly medicines can significantly reduce drug prices [31,19].
This systematic review has several limitations. First, it exclusively evaluates drug availability, pricing, and affordability variables without delving into the factors that influence these variables in detail. Moreover, this study incorporates only seven studies conducted in different Asian countries. It is important to acknowledge that there is a chance that certain studies might not have been included in this review due to our search terms possibly missing some of the diverse terminology used in the WHO-HAI studies.
CONCLUSION
The availability of essential medicines in several Asian countries is still relatively low and falls far below the standards set by the WHO. In addition, the prices of these medicines tend to be high, surpassing the income of the lowest-paid unskilled government workers. Relevant authorities must continue to evaluate and exercise control over these two aspects to ensure that essential medicines are accessible to all segments of the population. This way, the overall health levels in each country can be progressively improved.
ACKNOWLEDGMENT
The authors express their gratitude to the PMDSU under the Ministry of Research, Technology and Higher Education Indonesia (Kemenristekdikti) for the financial support.
LIST OF ABBREVIATIONS
HAI, Health Action International; IRP, Internasional reference pricing; LPGs, Lowest price generics; MPR, Median price ratio; Obs, Originator Brands; WHO, World Health Organization.
AUTHOR CONTRIBUTIONS
SAK and S conceptualized the study, and SAK critically revised the manuscript. MQA drafted the article, and reviewed, and edited the manuscript. All authors have read and approved the final version of the manuscript.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Hogerzeil H. Essential medicines and human rights: what can they learn from each other? Bull World Health Organ [Internet]. 2006 May 1;84(5):371–5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2627335/pdf/16710546.pdf/
2. Perehudoff S, Laing R, Hogerzeil H. Access to essential medicines in national constitutions. Bull World Health Organ [Internet]. 2010 Nov 1;88(11):800–800. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2971519/pdf/BLT.10.078733.pdf/
3. United Nations. 2019. About the sustainable development goals. New York, NY: United Nation. Available from: https://www.un.org/sustainabledevelop ment/sustainable-development-goals/
4. World Health Organization. 2019. What is health financing for Universal health coverage? Geneva, Switzerland: WHO.
5. Yang H, Dib HH, Zhu M, Qi G, Zhang X. Prices, availability and affordability of essential medicines in rural areas of Hubei Province, China. Health Policy Plan [Internet]. 2010 May 1;25(3):219–29. Available from: https://academic.oup.com/heapol/article-lookup/doi/10.1093/heapol/czp056
6. DFDI. Increasing access to essential medicines in the developing world: UK Government policy and plans [Internet]. London, UK: DFDI; 2004. 8 p. Available from: https://www.rhsupplies.org/uploads/tx_rhscpublications/DFID_Access to essential medicines-UK policy %26 plans_2004.pdf
7. WHO. Children: improving survival and well-being [Internet]. Geneva, Switzerland: WHO; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/children-reducing-mortality
8. Margaret C. Ten years in public health. Geneva, Switzerland: World Health Organization; 2017. p 81–3.
9. WHO. Access to medicines: making market forces serve the poor. In: ten years In Public Health [Internet]. Geneva, Switzerland: WHO; 2017. p 13–25. Available from: https://www.who.int/publications/m/item/access-to-medicines-making-market-forces-serve-the-poor
10. WHO. WHO Medicines Starategy, Countries. Geneva, Switzerland: World Health Organisation; 2004 [Internet]. Available from: https://apps.who.int/iris/bitstream/handle/10665/84307/WHO_EDM_2004.5_eng.pdf
11. WHO. Public health, innovation and intellectual property rights: unfinished business. Bull World Health Organ. 2006;84(5):1–6.
12. WHO and HAI. Measuring medicine prices, availability, affordability and price components [Internet]. 2nd ed. Geneva, Switzerland: WHO; 2008. 2 p. Available from: https://www.who.int/publications/i/item/WHO-PSM-PAR-2008.3
13. Latifah E, Kristina SA, Suryawati S, Satibi. Overview of drug availability and influencing factors in several low, lower and upper-middle countries: a systematic review. Syst Rev Pharm. 2019;10(1):67–72.
14. Ahmad NS, Islahudin F. Affordability of essential medicine prices in Malaysia’s private health sector. Patient prefer adherence [Internet]. 2018 Jul;12:1231–7. Available from: https://www.dovepress.com/affordability-of-essential-medicine-prices-in-malaysias-private-health-peer-reviewed-article-PPA
15. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc [Internet]. 2015 Sep;13(3):147–53. Available from: https://journals.lww.com/01787381-201509000-00006
16. Wilairatana P, Masangkay FR, Kotepui KU, Milanez GDJ, Kotepui M. Prevalence and characteristics of malaria among COVID-19 individuals: a systematic review, meta-analysis, and analysis of case reports. PLoS Negl Trop Dis [Internet]. 2021 Oct 1;15(10):e0009766. Available from: https://dx.plos.org/10.1371/journal.pntd.0009766
17. Dong Z, Tao Q, Yan B, Sun G. Availability, prices and affordability of essential medicines in Zhejiang Province, China. PLoS One [Internet]. 2020;15(11 November):1–11. Available from: http://dx.doi.org/10.1371/journal.pone.0241761
18. Alefan Q, Amairi R, Tawalbeh S. Availability, prices and affordability of selected essential medicines in Jordan: a national survey. BMC Health Serv Res. 2018;18(1):1–12.
19. Saeed A, Saeed H, Saleem Z, Fang Y, Babar ZUD. Evaluation of prices, availability and affordability of essential medicines in Lahore Division, Pakistan: a cross-sectional survey using WHO/HAI methodology. PLoS One. 2019;14(4):1–10.
20. Kristina SA, Aditama H, Endarti D, Widayanti AW. Evaluating accessibility of essential medicines in indonesia: a survey on availability and prices in public and private health sectors. Int J Pharm Res. 2020;12(2):692–9.
21. Nguyen HTT, Dinh DX, Nguyen TD, Nguyen VM. Availability, prices and affordability of essential medicines: a cross-sectional survey in Hanam province, Vietnam. PLoS One. 2021;16(11 November):e0260142.
22. Kasonde L, Tordrup D, Naheed A, Zeng W, Ahmed S, Babar ZUD. Evaluating medicine prices, availability and affordability in Bangladesh using World Health Organisation and Health Action International methodology. BMC Health Serv Res. 2019;19(1):1–12.
23. Mohamed Ibrahim MI, Alshakka M, Al-Abd N, Bahattab A, Badulla W. Availability of essential medicines in a country in conflict: a quantitative insight from Yemen. Int J Environ Res Public Health. 2021;18(1):1–13.
24. WHO. Global action plan for the prevention and control of noncommunicable diseases 2013-2020 [Internet]. Geneva, Switzerland: WHO Press; 2013. 37 p. Available from: https://www.who.int/publications/i/item/9789241506236
25. WHO. Medicines, vaccines and health products. Geneva, Switzerland: WHO Press; 2018. Vol. 19, pp 2019–23.
26. Dong Z, Tao Q, Sun G. Survey and analysis of the availability and affordability of essential drugs in Hefei based on WHO / HAI standard survey methods. BMC Public Health. 2020;20(1):1–10.
27. Mendis S. The availability and affordability of selected essential medicines for chronicl diseases in six low- and middle-income countries. Bull World Health Organ [Internet]. 2007 Apr 1;85(4):279–88. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2636320/pdf/06-033647.pdf/
28. Dong Z, Zhang S, Wu S, Xie X, Sun G, Yu X. Study on the accessibility and affordability of 50 drugs in Wuhan based on the WHO/HAI standardization method. Front Public Heal. 2023;11:1108007.
29. Gong S, Cai H, Ding Y, Li W, Juan X, Peng J, et al. The availability, price and affordability of antidiabetic drugs in Hubei province, China. Health Policy Plan [Internet]. 2018 Oct 1;33(8):937–47. Available from: https://academic.oup.com/heapol/article/33/8/937/5095198
30. Cameron A, Ewen M, Ross-Degnan D, Ball D, Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet [Internet]. 2009 Jan;373(9659):240–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673608617626
31. Acosta A, Ciapponi A, Aaserud M, Vietto V, Austvoll-Dahlgren A, Kösters JP, et al. Pharmaceutical policies: effects of reference pricing, other pricing, and purchasing policies. Cochrane Database Syst Rev [Internet]. 2014 Oct 16;2019(8):1–13. Available from: http://doi.wiley.com/10.1002/14651858.CD005979.pub2