INTRODUCTION
According to the World Health Organization [1], the term “campylobacteriosis” refers to an umbrella term for a group of infectious diseases that are caused by Campylobacters. These infections have been zoonotic diseases, meaning that they have been transferred to people via animals or animal products. Campylobacter is the most common pathogen that causes gastroenteritis in humans. This pathogen is responsible for an estimated 166 million instances of diarrhea along with 37,600 fatalities every calendar year [2]. The bacteria known as C. jejuni and C. coli are the most prevalent causes of illness in humans [3,4]. More than 80% of all human cases of campylobacteriosis are caused by eating raw or undercooked poultry meat, most frequently chicken [2]. The incidence of human campylobacteriosis and the prevalence of bacteria that are resistant to antibiotics both continue to rise worldwide. Campylobacter is becoming increasingly difficult to treat with antibiotics, and some strains have even evolved multidrug resistance (MDR) [5]. Major international health repercussions have been connected to the alarming rise of MDR Campylobacter strains that are resistant to quinolones and erythromycin [6]. The resistant bacteria were thought to be inherently more robust than their sensitive counterparts [7]. Therefore, to highlight these issues, this article focused on the pathogenicity, pathogenesis, and antimicrobial resistance of Campylobacter infections.
INCIDENCE OF CAMPYLOBACTER IN HUMAN
Campylobacteriosis is the most often reported zoonosis in the European Union, followed by salmonellosis and yersiniosis. In 2022, it was the most reported zoonosis in the EU, with 137,107 cases and a stable notification rate compared with 2021 [8]. The United States may exhibit one of two distinct campylobacteriosis epidemiology trends. A significant proportion of individuals will have clinical symptoms during the first pattern, describing an epidemic, whereas the second pattern reflects an individual case. Most cases of campylobacteriosis are regarded to be isolated incidents [9].
Campylobacter infections tend to be sporadic and more common in the warmer months of summer and autumn in developed nations [10], which may indicate a seasonal pattern related to ambient temperature. Western nations with temperate temperatures tend to have a seasonal rise, often between July and August [11], but Australia, New Zealand, and countries with tropical climates tend to see a less pronounced seasonal peak [11]. Campylobacter isolation rates in hens have been shown to be greater in the summer than in the winter, correlating with the seasonal increase in human illnesses [11]. Chickens and humans may not have gotten Campylobacter from the same place, although epidemic proportions in humans generally come before the high occurrence of poultry slaying waves [3]. Campylobacter infections are most frequent in children less than two years old in underdeveloped nations, and asymptomatic infections are also common in both children and adults. There are no clear seasonal trends associated with the disease as seen in developed countries [11]. Campylobacter infections seem to be more common among the elderly (>75), young adults (20–40), and small children (<4 years), perhaps because of various risk factors in these age groups [12].
The most prevalent cause of human campylobacteriosis is the ingestion of infected chicken, while there are other ways that Campylobacter spp. may spread to people [12]. In addition, cross-contamination has the potential to contaminate both raw and cooked meat [13]. Large campylobacteriosis outbreaks are often linked to polluted drinking water or raw or tainted milk; thus, this is not the sole dietary carrier for Campylobacter (Fig. 1) [13,14].
Finland, Norway, and Sweden are examples of countries with low colonization rates of chicken flocks, suggesting that factors other than chickens may have a significant influence, particularly for domestically acquired diseases. Campylobacter contagions throughout the globe will be impacted by both the growing popularity of international travel and the shifting international food trade, for instance, the rising popularity of imported chicken [15].
In addition, Deckert et al. [16] found that the likelihood of contracting campylobacteriosis varies between rural and urban areas. Although ruminant-associated genotypes have been detected more often in urban areas, many studies showed that chicken may play a larger role than previously assumed in transmitting Campylobacter to humans [17].
Abattoir workers may be at a higher risk of contracting Campylobacter due to their jobs, making them an appealing research population [18]. De Perio et al. [19] found in their study that laboratory-confirmed Campylobacter infections were most common among workers who had been in the slaughterhouse for less than a month (83%). The dangers to humans change with each type of animal and with each country, as well as with each person’s habits of food preparation and consumption [11].
PATHOGENICITY AND PATHOGENESIS OF CAMPYLOBACTER SPECIES
The term “pathogenicity” refers to a pathogen’s invasive potential or virulence, and its production, or both (the relative ability of a pathogen to overcome the body’s defenses and cause disease), while the term “pathogenesis” refers to the interaction between a pathogen and its host, with the outcome being either disease or carrier status depending on a wide range of factors including factors related to the invading strain (type, dose, and virulence of strain), others related to the host (age, sex, and host defense mechanisms), and environmental factors such as route of the entrance, and epidemiology of the region (distribution and frequency of a strain) [20].
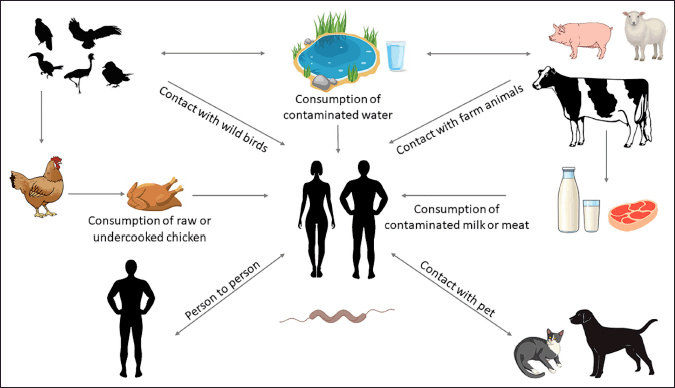 | Figure 1. Reservoirs and transmission pathways linked to Campylobacter species (Lopes et al. [14]). [Click here to view] |
Epidemiology
The etiology of animal diseases caused by Campylobacters has been known since 1909, but that of human diseases caused by Campylobacters has only been well acknowledged since around 1980 [21]. Due in large part to the difficulties of culturing and isolating these bacteria from fecal samples, the function of Campylobacter as an intestinal pathogen was not established until the 1970s. Campylobacter enteritis caused by C. jejuni or C. coli is the only type of campylobacteriosis of substantial public health relevance around the world. More than 90% of illnesses are caused by C. jejuni, while 5%–10% are caused by C. coli [22]. Sporadic illnesses and outbreaks have been linked to Campylobacter lari and Campylobacter upsaliensis. Most cases of campylobacteriosis in the European Union are linked to C. jejuni, followed by C. coli [23]. Because these thermophilic species invade the intestinal mucosa of many different birds and mammals, including those used for food production, they are often encountered as zoonotic food-borne illnesses [24]. At the consumer level, inadvertent intake of 1 drop of raw chicken juice can easily form an infectious dosage, which is as little as 500 organisms [11]. Infections caused by Campylobacter are usually not serious, but they can be fatal for infants, the elderly, and those with compromised immune systems [1]. Campylobacter infections occur more frequently annually than those caused by Salmonella species, Shigella species, or Escherichia coli O157:H7 [22]. Attachment to intestinal cells, colonization of the digestive system, invasion of specific cells, and toxin synthesis are the four basic steps in Campylobacter’s pathogenic process [25].
Virulence factors
Likely because of the dissimilarity in pathogenesis among Campylobacters along with other infections, specific virulence strategies of this pathogen have not yet been completely characterized [26]. Despite its widespread prevalence, little is understood about Campylobacter’s virulence factors or the mechanisms that allow such a weak organism to persist in the food chain, where it often acquires a more potent pathogenicity. Campylobacter’s motility, chemotaxis, adherence, and invasion mechanisms are all highly complicated multifactorial systems. Campylobacter has a number of mechanisms for responding to environmental stresses, such as antioxidant defense, heat shock, and the ability to transition into the viable but nonculturable (VBNC) state. Because of their genetic makeup, Campylobacter can endure food preparation, often emerging more virulent as a result [27]. Campylobacter spp. are adapted to survive in, colonize, and spread disease within this narrow ecological niche [28]. The high concentration of oxygen in the mucosa is likely responsible for their microaerophilic character [29]. Comparable to the avian gut in terms of optimal development temperature (42°C), the lining of the intestines in animals with temperatures below 37°C is also a suitable environment for these pathogens to thrive [30]. Virulence factors include flagella-mediated motility, adhesion to intestinal mucosa, invasive ability, and toxin production [11]. Although little is known about the pathogen’s mode of operation, it is known that colonization of the small intestine is a necessary step before the infection can reach its target organ, the colon [31]. Loss of colonization potential occurs when flagellin’s structural genes are inactivated, causing a disruption in motility [30]. In addition, Lipopolysaccharide (LPS) has been discovered in cell membranes, and its sticky properties are essential for mucosal cell penetration but not colonization [9]. Cellular inflammation, caused by invasion, is likely due to the creation of cytotoxins, which leads to decreased intestinal absorption [32]. There can be speculation that the acid resistance of this bacterium in the stomach and the salts in the bile plays a role in its capacity to invade the intestines [32]. Campylobacter jejuni and C. coli both produce a cytotonic toxin that, immunologically, is comparable to cholera toxin [2]. The sub mucosal edema and diarrhea seen in 3- and 4-day-old hens infected with C. jejuni isolated from patients with diarrhea are likely due to this toxin [11]. Dose-dependent and resistant to neutralization by Shiga-toxin immune serum, the cytotoxin generated by toxigenic strains of C. jejuni can be fatal. Aside from killing HeLa and Chinese hamster ovary (CHO) cells and chicken embryos, the toxin is widely recognized as a novel chemical [11]. Campylobacter jejuni isolates from surface water are less harmful than strains isolated from diarrheal patients, according to in vitro experiments including adhesion and cytotoxicity [33]. Some researchers believe that pathogenic isolates acquire the potential to colonize and produce toxins after being exposed to a susceptible host. Despite the fact that the severity of an illness may be affected by both the virulence of the strain and the host’s immunological status [34].
Flagella
The colonization of the small intestine relies on motility, which increases in very viscous circumstances. In addition, the function of flagella under a variety of chemotactic settings plays a crucial role in bacteria’s ability to adapt to their environment that may be found in the gastrointestinal tract [11]. Their ability to “corkscrew” through the mucosa is due to their peculiar motility, spiral form, and particularly length polar flagella. These anatomical features similarly aid in their ability to colonize while remaining mobile and stationary against the mucosal flow [11]. The attachment of C. jejuni to fibronectin on epithelial cells is thought to be the first step in the establishment of the bacterium after it has reached the gastrointestinal system. The outer membrane protein CadF on the bacterium is responsible for attachment promotion [29]. There are two flagellins that make up the C. coli flagellum, and they are quite similar to one another; FlaA is the chief flagellin, along FlaB is the slight flagellin [2]. The genes encoding these flagellin proteins are in tandem. Fla A is controlled by a promoter, and fla B by a dependent promoter [26]. Expression of adhesion, colonization of the gastrointestinal tract, and penetration of the host cells are all regulated by the flaA gene, which is located in the bacterium [2], thereby stopping the immune response.
Cytolethal distending toxin
Campylobacter spp. produce the most well-studied toxins include the Gram-negative bacteria-wide cytolethal distending toxin (CDT). It is been called a major contributor to the pathogen’s lethality [35]. It is related to the C. jejuni-induced local acute inflammation that causes enterocolitis [36]. CDT results from many different types of Gram-negative bacteria because of their genetic variety, but it acts quite similarly to other known genotoxins [37]. Cholesterol was discovered to be involved in C. jejuni CDT (Cj-CDT) poisoning of host cells through its interactions with membrane cholesterol-rich microdomains [38]. Eukaryotic cells are killed when they become stuck in the G2/M phase of the cell cycle due to CDT holotoxin, in which the genes cdt A, Cdt B, along Cdt C code for the three subunits [11]. Although CdtB’s function is well understood, CdtA and CdtC’s are not, and they need to be studied more thoroughly [2]. It has been hypothesized that this happens when the proteins in question combine with the outer membrane of the bacterium, resulting in contamination. CdtB transport into the host cell; however, is thought to need the presence of Cdt A along with Cdt C [2]. The CDT holotoxin is bound to the cell membrane by two proteins. In addition, host Deoxyribonucleic acid (DNA) is damaged because of the DNaseI-like activity of the CdtB active subunit [35]. Instead of attaching to the cell membrane, the DNase I activity of Cj-CdtB causes DNA double-strand breaks (DSBs), which ultimately lead to senescence or target-cell death through cell-cycle arrest during the G2/M phase, cell distention, and cell bloating [11]. The enzymatic subunit Aa-CdtB possesses DNase I activity and also has phosphatidylinositol 3-4-5 trisphosphate (PIP3) phosphatase activity, which triggers death in T cells [2]. Aa-CdtB was found to have homology with inositol polyphosphate 5-phosphatase based on structural and sequence analyses [2]. Cj-phosphatase CdtB’s activity, however, has not been documented as of yet [37]. Following lymphocyte poisoning with Aa-CDT, PIP3 levels drop, phosphatidylinositol-3-kinase (PI-3K)/PIP3/Akt gesturing is disrupted, and phosphorylation of glycogen synthase kinase 3 (GSK3) is reduced. In addition, blocking PI-3K signaling with Aa-CDT causes macrophages to generate more pro-inflammatory cytokines such as IL-1, TNF-, and IL-6. Based on these findings, CDT may function as an immune system modulator. Related research also found that Cj-CDT causes inflammation in the host intestines by stimulating IL-8 production and promoting chemotaxis by leukocytes [11].
Biofilm formation in Campylobacter
Biofilms are defined as communities of microorganisms that have formed an adhering film or matrix on a surface or interface [39]. Bacteria in biofilms are more resistant to being killed off because they are shielded from environmental stressors like drying out and being exposed to disinfectants and antibacterials. Food contamination and foodborne diseases may result from the formation of biofilms on surfaces used in food preparation, which protect bacteria from washing and sanitation efforts. Biofilm production is thought to be one of the processes that allows C. jejuni to persist in the environment [40]. Campylobacter jejuni (C. jejuni), a significant GI pathogen, has been found to live as three distinct monospecies biofilms in liquid culture. It agglomerates on glass, creating a floc, and produces a pellicle at the liquid–gas boundary. At room temperature and humidity, the microaerobic bacteria in flocs may persist for up to 24 days, whereas the survival rate for planktonic bacteria is just 12 days. Thus, it is possible that several biofilm formation mechanisms regulate biofilm types. It has been hypothesized that the prevalence of Campylobacter-associated food-borne diseases can be partially attributed to the importance of these poorly described modes of growth in the perseverance of C. jejuni in the surroundings [41]. Numerous human infections, including Pseudomonas aeruginosa, Staphylococcus epidermidis, Salmonella enteritidis, Vibrio cholerae, Streptococcus gordonii, and Burkholderia cepacia, form monospecies biofilms [11]. Auto agglutination of C. jejuni has been seen in a variety of media, including phosphate-buffered saline (PBS), minimal essential medium, and Mueller-Hinton broth [2]. However, it is unclear whether auto agglutination contributes to biofilm development in this species. Campylobacter jejuni was shown to be thriving in bacterial biofilms, C. jejuni presents serious threats to human health, so understanding how it may thrive in the wild and spread through the food web is essential. Bacteria that live in biofilms are resistant to treatments such as antibiotics and immune reactions from the host. That C. jejuni cells construct a biofilm to facilitate their transition across animal hosts is an intriguing concept. It has been shown that C. jejuni is capable of inhabiting spontaneous biofilms in bacteria; however, it has not yet been demonstrated that C. jejuni is capable of producing mono species biofilms. Although biofilms are commonly composed of polysaccharide, other than the gene cluster for the kps capsular polysaccharide, C. jejuni does not have any possibilities for a biofilm polysaccharide that is readily apparent. For this reason, the biofilm might be made up of anything from extracellular DNA to modified capsular polysaccharides to poly-amino acids [11].
SEQUELAE IN HUMANS
Human campylobacteriosis is usually mild and goes away on its own, but it can have severe consequences in rare cases [42]. Extraintestinal survival and growth are also possible in humans, especially individuals with impaired immune systems [30]. Hemorrhagic colitis, acute pancreatitis, peritonitis, and cholecystitis are all serious gastrointestinal conditions that possibly emerge as a consequence of Campylobacter infection that originate in the gut [22]. Meningitis, endocarditis, septic arthritis, reactive arthritis (ReA), and osteomyelitis are a few examples of the unique extraintestinal indications of infection. Other examples include neonatal sepsis. Less than 1% of campylobacteriosis patients have bacteremia; those at most risk include the immunocompromised, the very young, and the very old [9]. Guillain–Barré syndrome, ReA, and irritable bowel syndrome are the most well-known complications of campylobacteriosis. Preceding Campylobacter infection is also linked to the Guillain-Barré syndrome (GBS) variant Miller–Fisher syndrome. Inflammatory bowel disease (IBD) has been linked to gastroenteritis (not just Campylobacter), and there is mounting evidence that other functional gastrointestinal disorders (FGDs) are associated with the illness as well [43].
TREATMENT
Treatment of Campylobacter infections
Campylobacteriosis is a common bacterial infection that often resolves on its own with only supportive care. Enteritis that is particularly severe may benefit from antibiotic treatment. Campylobacteriosis treatment may vary according to the antibiotic class used to treat the infection and the location where it was contracted [44]. Considering a patient’s travel history is important if empiric antibiotic therapy is required due to observed variations in Campylobacter susceptibility around the world [44]. In severe or persistent cases, antibiotics may be used, but their effectiveness for minor intestinal infections is still debatable [45]. Antibiotic treatment is warranted in those who are immune weakened, including those with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS). Antibiotic therapy can minimize the shedding of infectious organisms [46]. Instances of C. jejuni and C. coli that were isolated from retail meats, slaughterhouses, and processing factories that were examined by the federal government, as well as human clinical cases are regularly compiled by the National Antimicrobial Resistance Monitoring System (NARMS) in the United States due to the threat to human health posed by these bacteria [47]. Macrolides (often erythromycin) and fluoroquinolones (mostly ciprofloxacin) are the typical antibiotics used to treat campylobacteriosis [44]. The prevalence of erythromycin-resistant organisms is still quite low [11]. Among the fluoroquinolones, ciprofloxacin (CIP) is among the most widely prescribed preventative medications for international travelers [48]. Human infections caused by strains of Campylobacter that are resistant to macrolides and fluoroquinolones have been on the rise in a number of nations [49]. Although tetracyclines are not commonly used, they are an option for adults with resistant infections [50]. Aminoglycosides like gentamicin, when given intravenously, are the standard of care for treating severe bacteremia and other systemic infections [50]. Azithromycin (macrolide), clindamycin (lincosamide), and chloramphenicol are examples of antibiotics that are widely used to treat campylobacteriosis, but strains of Campylobacter that are resistant to these drugs have been described [51]. When compared to patients with infections caused by drug-susceptible isolates, those who have campylobacteriosis caused by antibiotic-resistant strains may be at a larger risk of experiencing unfavorable consequences, such as the development of an invasive disease or death [52]. Whether for therapeutic or recreational use, numerous resistant clones may be selected and maintained with relative ease [52]. The development in addition to the spread of infectious diseases that are resistant to treatment, commensal bacteria, and infections affecting humans in animals used for food production, is all made possible by selective pressure. Due to this stress, these diseases are now found in animals that are used for human consumption [53–67]. Growing data suggests that people may be exposed to resistant Campylobacter via ingestion of animal products. Scientists have proven that they can separate sick humans and livestock into distinct serotypes and genotypes [68,69]. Treatment of human Campylobacter infections is complicated by antibiotic resistance, which occurs in the same multidrug-resistant fashion as it does in animals. Resistance to fluoroquinolones (like ciprofloxacin) and tetracycline is the most frequent form of antimicrobial resistance in the world. There are also reports of a rise in resistance to macrolides [70].
ANTIBIOTIC RESISTANCE IN CAMPYLOBACTER
To prevent disease, cure existing illnesses, and promote animal growth, antibiotics have been used carelessly in the animal production industry for decades [53–67,71]. Antibiotic resistance has become more common and widespread among enteric bacteria as a result of the widespread use of these drugs, which has also boosted the survival and spread of antibiotic-resistance genes in the genomes of microorganisms [56]. Pathogens’ rising antibiotic resistance and potential for MDR are cause for concern. Antibiotic-resistant emerged as a result of extensive and unrestricted use of antimicrobial drugs in food animal production, Campylobacter species have evolved and spread across the world. After fluoroquinolones were approved for use in poultry in Europe and the United States, strains of Campylobacter spp. identified from animals and human patients showed rising levels of resistance to the drugs [72]. The use of antimicrobials in animals can lead to the development of drug-resistant bacteria, which poses a risk to humans in the case of zoonotic pathogens like Campylobacters. Clinical difficulty in controlling a disease is not caused by gene transfer or the development of drug resistance if the organism has already acquired antibiotic resistance [57]. Antibiotic resistance can be spread through the consumption of contaminated food. Antibiotic residues in food may play a role in this phenomenon, in addition to the transmission of drug-resistant food-borne pathogens and the ingestion of resistant strains of the original food microflora, which may subsequently transmit their resistance to pathogenic bacteria [73]. The existence of antibiotic-resistant strains of Campylobacter in the food chain has raised concerns about the efficacy of the limited treatment options available for human infections [50], since contaminated food is a major source of Campylobacter infection in people. While certain types of resistance are built into a person from the moment they are born, other forms of resistance may be learned. Numerous bacterial species share the quality of having resistance mechanisms ingrained in their DNA from the beginning of their existence. Bacteria that are resistant to antibiotics can withstand treatment because they either do not share a target with the antibiotic, their cell walls effectively block antibiotic molecules from entering the cell, or the microorganisms generate enzymes that degrade the antibiotic. Other types of antibiotic-resistant bacteria include those that have cell walls that prevent antibiotic molecules from entering the cell [53]. Acquired resistance occurs when a resistant bacterial strain changes over time or gets its resistance genes from another strain through horizontal gene transfer [53]. Several processes work together to facilitate horizontal gene transfer. Transmissible resistance genes often originate from one of three basic sources. Conjugation permits the transfer of large plasmids (non-chromosomal DNA molecules that may replicate independently from the chromosome DNA) from one bacteria to another, which can facilitate the transmission of several resistance genes [9]. Transposons are DNA sequences that may transfer between plasmids or between the chromosome and plasmids, and they can transport several resistance genes. Some resistance elements are possibly encoded by intergons, which are genetic units discovered in genomes, plasmids, and transposons that contain proteins that capture, remove, transfer, and splice additional genes or cassettes of genes onto chromosomes [49]. Co-selection of antibiotic resistance and infectiousness could take place amongst bacterial strains of the same or separate species via the passing on of mobile genetic traits through conjugation, transformation, and transduction. Extra-chromosomal components called plasmids may help bacteria adapt to new habitats and environments by bestowing traits such as resistance, pathogenicity, and persistence [74]. Indirectly and eventually, environmental variables affect the regulation of both virulence-encoding genes and genes responsible for antimicrobial resistance [75]. Regulation of virulence and antibiotic resistance genes: mechanisms and determinants are shown in detail in Figure 2. Antibiotics are divided into several groups based on their shared structural properties, bacterial cell targets, and modes of action [49]. According to Taylor [9], resistance to one class of antibiotics may lead to resistance to all of those drugs. When the target is shared (as with macrolides and lincosamides), or when the resistance mechanism is not very specific (as with efflux pumps), there may be cross-resistance between seemingly unrelated classes [49]. Macrolide-resistant Campylobacter isolates may be more harmful than non-resistant bacteria, according to the results of many investigations done in the United States, Thailand, and Denmark. Possible mechanisms include up-regulation of virulence, enhanced fitness in resistant isolates, and co-selection for virulence [66]. Several genes in Campylobacter (including several efflux pumps) confer antibiotic resistance, and several of these genes also contribute to the bacterium’s pathogenicity [2]. MDR is characterized as resistance to three or more antibiotic classes [76]. Campylobacter species that are resistant to various medicines are becoming more common, limiting available treatment choices [50]. It has been shown that the emergence of Campylobacter strains resistant to these medications in the food supply is largely attributable to the extensive use of antibiotics as growth promoters, treatments, and prophylaxis in food animals, particularly fluoroquinolones [77]. Compared to human isolates, those from animals and livestock are more likely to have (multi-)resistant strains [77]. The spread and introduction of antibiotic-resistant genes (ARGs) may pose a greater threat than the antibiotics themselves, and ARGs can be found in a wide variety of organisms including humans (Fig. 3). The widespread emergence of antibiotic-resistant strains of both pathogenic and commensal bacteria can be directly attributed to the widespread use and abuse of these drugs. Selection for antibiotic resistance is influenced by both the quantity and method with which antibiotics are used. It is important to note that the frequency with which resistance arises is not directly proportional to the amount of use; other social, ecological, and genetic factors also play a role. Emerging and adapting in the presence of antibiotics, resistant bacteria appear to take on a “life of their own.” They spread and keep the resistance characteristics alive even when antibiotics are not present, putting at risk efforts to reverse bacterial resistance through reduced antibiotic use alone. To overcome resistance, it is necessary to bring back the previously vulnerable flora in both humans and their natural environments [58].
 | Figure 2. Several processes and variables influence the regulation of genes expressing virulence and antibiotic resistance. (A) Conjugation. (B) Transfection. (C) Modification of the antibiotic target. (D) Increased mutation rates. (E) Transformation. (F) Antibiotic inactivation. (G) Efflux pump; with (H) environmental variables having a secondary and ultimate influence on all the others (Bunduru? et al. [40]). [Click here to view] |
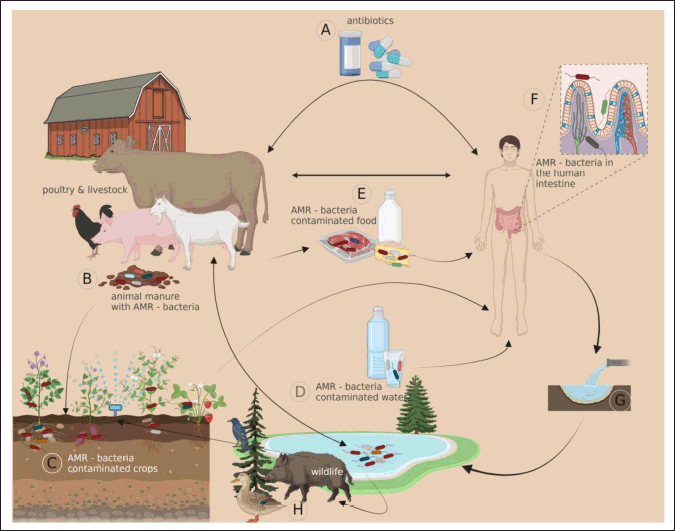 | Figure 3. The significance of animals in antibiotic resistance propagation in the environment (Bunduru? et al. [40]). [Click here to view] |
CONCLUSION
Campylobacter would be a severe threat to public health. This review provided risk managers with knowledge regarding thermotolerant Campylobacter spp. for them to develop ways to limit the risk of human campylobacteriosis through a vigilant approach at all levels. As a result, appropriate control and intervention methods are critical to limiting the risk of this pathogen and the establishment of resistance issues.
AUTHOR CONTRIBUTIONS
MHGK, FAM, and SSA: Conceptualized and designed the study. MHGK: Drafted the manuscript. FAM and SSA: Data collection. FAM: Edited the manuscript. All authors have read, reviewed, and approved the final manuscript.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. World Health Organization (WHO). The global view of campylobacteriosis: report of an expert consultation, Utrecht, Netherlands, 2013;9–11 July 2012.
2. Kanaan MHG, Abdullah SS. Emerging threats by campylobacter on public health. Germany: LAP LAMBERT Academic Publisher; ISBN: 978-620-4-74990-7; 2022.
3. Bahrndorff S, Rangstrup-Christensen L, Nordentoft S, Hald B. Foodborne disease prevention and broiler chickens with reduced Campylobacter infection. Emerg. Infect. Dis. 2013;19(3):425. CrossRef
4. Mikulic M, Humski A, Njari B, Ostovic M, Duvnjak S, Cvetnic Z. Prevalence of thermotolerant campylobacter spp. in chicken meat in croatia and multilocus sequence typing of a small subset of Campylobacter jejuni and Campylobacter coli isolates. Food Technol Biotechnol. 2016;54(4):475–81. CrossRef
5. Tillotson, GS, Theriault N. New and alternative approaches to tackling antibiotic resistance. F1000prime reports. 2013;5(15). CrossRef
6. Iovine NM. Resistance mechanisms in Campylobacter jejuni. Virulence. 2013;4(3):230–40. CrossRef
7. Chai LC, Fatimah AB, Ghazali FM, Lee HY, Tunung R, Shamsinar AT et al. Biosafety of Campylobacter jejuni from raw vegetables consumed as Ulam with reference to their resistance to antibiotics. Int. Food Res. 2008;15(2):125–135.
8. European Food Safety Authority (EFSA). Campylobacteriosis and salmonellosis still below pre-pandemic levels; West Nile virus infections on the rise. 12 December 2023. Available from https://www.efsa.europa.eu/en/news/campylobacteriosis-and-salmonellosis-still-below-pre-pandemic-levels-west-nile-virus
9. Taylor WJ. Isolation, antibiotic resistance, and molecular characterization of Campylobacter from poultry, swine and dairy cows. Doctoral Dissertation University of Tennessee, Knoxville. 2012.
10. Hadi Ghaffoori Kanaan M, Jebur Obayes Al-Isawi A, Ahmad Mohamme F. Antimicrobial resistance and antibiogram of thermotolerant campylobacter recovered from poultry meat in Baghdad Markets, Iraq. Arch. Razi Inst. 2022;77(1):231–37. CrossRef
11. Kanaan, MHG 2017. Antimicrobial efficiency of ozonated water as an intervention against food-borne pathogen Campylobacter jejuni contaminating chicken meat Doctoral Dissertation, University of Baghdad, Iraq. 2017.
12. Kanaan MH, Khashan HT. Prevalence of multidrug resistant thermotolerant species of Campylobacter in retail frozen chicken meat in Baghdad Province. Curr Res Microbiol Biotechnol. 2018;6(1):1431–40.
13. Kanaan MHG, Abdulwahid MT. Prevalence rate, antibiotic resistance and biotyping of thermotolerant campylobacter isolated from poultry products vended in Wasit Markets. Curr. Res. Nutr. Food Sci. 2019;7(3):905–17. CrossRef
14. Lopes GV, Ramires T, Kleinubing NR, Scheik LK, Fiorentini ÂM, da Silva WP. Virulence factors of foodborne pathogen Campylobacter jejuni. Microb. Pathog. 2021;161(Pt A):105265. CrossRef
15. Skarp CPA, Hänninen ML, Rautelin HIK. Campylobacteriosis: the role of poultry meat. Clin. Microbiol. Infect. 2016;22(2):103–108. CrossRef
16. Deckert AE, Taboada E, Mutschall S, Poljak Z, Reid-Smith RJ, Tamblyn S et al. Molecular epidemiology of Campylobacter jejuni human and chicken isolates from two health units. Foodborne Pathog Dis. 2014;11(2),150–55. CrossRef
17. Bessell PR, Rotariu O, Innocent GT, Smith-Palmer A, Strachan NJ, Forbes KJ et al. Using sequence data to identify alternative routes and risk of infection: a case-study of campylobacter in Scotland. BMC Infect. Dis. 2012;12(1):80. CrossRef
18. Ellström P, Hansson I, Söderström C, Engvall EO, Rautelin H. A prospective follow-up study on transmission of Campylobacter from poultry to abattoir workers. Foodborne pathogens and disease. 2014;11(9):684–8. Doi: https://doi.org/10.1089/fpd.2014.1753.
19. de Perio MA, Niemeier RT, Levine SJ, Gruszynski K, Gibbins JD. Campylobacter infection in poultry-processing workers, Virginia, USA, 2008–2011. Emerg. Infect. Dis. 2013;19(2):286–8. CrossRef
20. Kanaan MHG. Isolation and identification of methicillin resistant staphylococcus aureus (MRSA) from locally produced raw milk and soft cheese from Some Regions in Baghdad. Doctoral Dissertation, College of Veterinary Medicine, University of Baghdad, Iraq. 2013.
21. Silva J, Leite D, Fernandes Mena C, Gibbs PA, Teixeira P. Campylobacter spp.as a foodborne pathogen: a Review. Front. Microbiol. 2011;2(200):1–12. CrossRef
22. Allos BM, Blaser MJ. Campylobacter jejuni and related species. In: Mandell GL, Bennett JE and Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 7th ed. Philadelphia, PA: Churchill Livingstone. 2010. pp. 2793–802.
23. Whiley H, van den Akker B, Giglio S, Bentham R. The role of environmental reservoirs in human campylobacteriosis. Int. J. Environ. Res. Public Health. 2013;10(11):5886–907. CrossRef
24. Nichols GL, Richardson JF, Sheppard SK, Lane C, Sarran C. Campylobacter epidemiology: a descriptive study reviewing 1 million cases in England and Wales between 1989 and 2011. BMJ Open. 2012;2(4):1179. CrossRef
25. Haddad N, Marcé C, Magras C, Cappelier JM. An overview of methods used to clarify pathogenesis mechanisms of Campylobacter jejuni. J. Food Prot. 2010;73(4):786–802. CrossRef
26. Dastia JI, Tareena AM, Lugerta R, Zautnera AE, Gro U. Campylobacter jejuni: a brief overview on pathogenicity associated factors and disease mediating mechanisms. Int. J. Med. Microbiol. 2010;300(4):205–11. CrossRef
27. Bolton DJ. Campylobacter virulence and survival factors. Food Microbiol. 2015;48(5):99–108. CrossRef
28. Murphy C, Carroll C, Jordan N. Environmental survival mechanisms of the foodborne pathogen Campylobacter jejuni. J Appl. Microbiol. 2006;100(4):623–32. CrossRef
29. Park SF. The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. Int. J. Food Microbiol. 2002;74(3):177–88. CrossRef
30. Wassenaar T, Newell D. The Genus Campylobacter. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E editors. 2006; The Prokaryotes. New York: Springer, pp. 119–38.
31. Poly F, Guerry P. Pathogenesis of Campylobacter. Curr. Opin. Gastroenterol. 2008;24(1):27–31. CrossRef
32. Van Deun K, Haesebrouck F, Hendrickx M, Favoreel H, Dewulf J, Ceelen L, Dumez L et al. Virulence properties of Campylobacter jejuni isolates of poultry and human origin. J. Med. Microbiol.2007; 56(Pt 10):1284–9. CrossRef
33. Newell DG, Fearnley C. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 2003; 69 (8): 4343–51. CrossRef
34. Zilbauer M, Dorrell N, Wren BW, Bajaj-Elliott M. Campylobacter jejuni mediated disease pathogenesis: an update. Trans.R. Soc. Trop. Med. Hyg. 2008; 102(2):123–9. CrossRef
35. Ge Z, Schauer DB, Foz JG. In vivo virulence properties of bacterial cytolethal-distending toxin. Cell. Microbi.ol. 2008;10(8):1599–607. doi: 10.1111/j.1462-5822.2008.01173.x
36. Zheng J, Meng J, Zhao S, Singh R, Song W. Campylobacter-induced interleukin-8 secretion in polarized human intestinal epithelial cells requires Campylobacter-secreted Cytolethal distending toxin- and toll-like receptor-mediated activation of NF-kappa B. Infect. Immun. 2008;76(10):4498–508. CrossRef
37. Gargi A, Reno M, Blanke SR. Bacterial toxin modulation of the eukaryotic cell cycle: are all Cytolethal distending toxins created equally? Front. Cell. Infect. Microbiol. 2012;2(124):124. CrossRef
38. Lin J. Novel approaches for Campylobacter control in poultry. Foodborne Pathog. Dis. 2009;6(7):755–65. CrossRef
39. Piccirillo A, Dotto G, Salata C, Giacomelli M. Absence of class 1 and class 2 integrons among Campylobacter jejuni and Campylobacter coli isolated from poultry in Italy. J. Antimicrob. Chemother. 2013;68(11):2683–5. CrossRef
40. Bunduru? IA, Balta I, ?tef L, Ahmadi M, Pe? I, McCleery D et al. Overview of virulence and antibiotic resistance in campylobacter spp. livestock isolates. Antibiotics. 2023;12(2):402. CrossRef
41. Kanaan MHG. Effect of biofilm formation in a hostile oxidative stress environment on the survival of Campylobacter jejuni recovered from poultry in Iraqi markets. Vet. World. 2024;17(1):136–42. CrossRef
42. Wagenaar JA, Van Bergen MA, Mueller MA, Wassenaar TM, Carlton RM. Phage-therapy reduces Campylobacter jejuni colonization in broilers. Vet. Microbiol. 2005;109(3–4):275–83. CrossRef
43. Havelaar AH, Haagsma JA, Mangen MJ, Kemmeren JM, Verhoef LP, Vijgen SM. Disease burden of foodborne pathogens in the Netherlands. Int. J Food Microbiol. 2009;156(3):231–8. CrossRef
44. Alfredson DA, Korolik V. Antibiotic resistance and resistance mechanisms in Campylobacter jejuni and Campylobacter coli. FEMS Microbiol. Lett. 2008;277(2):123–32. CrossRef
45. Ge B, Wang F, Sj?lund-Karlsson M, McDermott PF. Antimicrobial resistance in Campylobacter: susceptibility testing methods and resistance trends. J Microbiol Methods. 2013;95(1):57–67. CrossRef.
46. Centers for Diseases Control and Prevention (CDC). United States: Diagnosis and Treatment. 2019: Available from https://www.cdc.gov/campylobacter/diagnosis.html
47. Jara M, Lanzas C. Early evaluation of the Food and Drug Administration (FDA) guidance on antimicrobial use in food animals on antimicrobial resistance trends reported by the National Antimicrobial Resistance Monitoring System (2012–2019). One Health (Amsterdam, Netherlands), 17(1):100580–100580. CrossRef
48. Moore JE, Corcoran D, Dooley JS, Fanning S, Lucey B, Matsuda M et al. Campylobacter: review article. Vet. Res. 2005;36(3):351–382. CrossRef:2005012
49. European Food Safety Authority (EFSA). Foodborne antimicrobial resistance as a biological hazard—Scientific Opinion of the Panel on Biological Hazards. EFSA J. 2008;6(8):765. CrossRef
50. Engberg J, Keelan M, Gerner-Smidt P. Antimicrobial resistance in Campylobacter. In: Aarestrup F (ed). Antimicrobial resistance in bacteria of animal origin. Washington, DC: ASM Press. 2006; 269–291.
51. Gibreel A, Taylor D. Macrolide resistance in Campylobacter jejuni and Campylobacter coli. J. Antimicrob. Chemother. 2006; 58(2):243–55. CrossRef
52. Humphrey T, O’Brien S, Madsen M. Campylobacters as zoonotic pathogens: a food production perspective. Int. J. Food Microbiol. 2007; 117(3):237–57. CrossRef
53. Kanaan MH. Antibacterial effect of ozonated water against methicillin-resistant Staphylococcus aureus contaminating chicken meat in Wasit Province, Iraq. Vet World. 2018;11(10):1445–53. CrossRef
54. Kanaan MH. Prevalence, resistance to antimicrobials, and antibiotypes of Arcobacter species recovered from retail meat in Wasit marketplaces in Iraq. Int. J. One Health. 2021;7(1):142–50. CrossRef
55. Kanaan MHG. Meat bio preservation as a promising method in food technology, EC Vet. Sci. 2019;4(3):1.
56. Kanaan MHG, Al-Shadeedi SM, Al-Massody AJ, Ghasemian A. Drug resistance and virulence traits of Acinetobacter baumannii from Turkey and chicken raw meat. Comp. Immunol. Microbiol. Infect. Dis. 2020;70(1):101451. CrossRef
57. Kanaan MHG, Mohammed FA. Antimicrobial resistance of Campylobacter jejuni from Poultry meat in local markets of Iraq. Plant Arch. 2020;20(1):410–15.
58. Kanaan MH. Prevalence and antimicrobial resistance of Salmonella enterica serovars Enteritidis and Typhimurium isolated from retail chicken meat in Wasit markets, Iraq. Vet World. 2023;16(3):455–463. CrossRef
59. Kanaan MHG, Abdullah SS. 2019. Methicillin-resistant Staphylococcus Aureus as a Superbug foodborne pathogen. Germany: LAP LAMBERT Academic Publisher; ISBN: 978-613-9-94418-7; 2019.
60. Kanaan MHG, Khashan HT. Molecular typing, virulence traits and risk factors of pandrug-resistant Acinetobacter baumannii spread in intensive care unit centers of Baghdad city, Iraq. Rev. Med. Microbiol. 2022;33(1):51–5. CrossRef
61. Kanaan MHG, Tarek AM. Innovative modern bio-preservation module of meat by lytic bacteriophages against emergent contaminants. Open Vet. J. 2022;12(6):1018–26. CrossRef
62. Kanaan MHG, Khalil ZK, Khashan HT, Ghasemian A. Occurrence of virulence factors and carbapenemase genes in Salmonella enterica serovar Enteritidis isolated from chicken meat and egg samples in Iraq. BMC Microbiol. 2022;22(1):1–8. CrossRef
63. Kanaan MHG, Salim ID, Tarek AM, Abdullah SS. Assessment of the knowledge, attitude, and practice related to visceral leishmaniasis among residents of Al-Suwaira city, Wasit Governorate, Middle East of Iraq. J Prev Med Hyg. 2022;63(3):E429–E434.
64. Kanaan MHG, Tarek AM, Abdullah SS. Knowledge and attitude among samples from community members, pharmacists and health care providers about antibiotic resistance in Al-Suwaria city/Wassit province/Iraq. In IOP Conf. Ser.: Earth Environ. Sci. 2021;790(1):012059.
65. Kanaan MHG, Tarek AM, Abdullah SS. Prevalence and antimicrobial resistance of Staphylococcus aureus in some types of dairy products in Baghdad Markets. J Dis Global Health. 2023;16(1),1–7. CrossRef
66. Kanaan MH, Anah G, Jasim GA, Ghasemian A. In-vitro protoscolicidal and immunomodulatory effects of Cinnamomum camphora and Ziziphora tenuior against Echinococcus granulosus protoscolices. Rev Med Microbiol. 2020;32(1):45–50.
67. Kanaan M, Abdullah S. Evaluation of aqueous Ozone as a method to combat multidrug-resistant Staphylococcus aureus tainting cattle meat sold in Wasit marketplaces. Mansoura Vet. Med. J. 2021; 22(3):117-123. CrossRef
68. Engberg J, Aarestrup FM, Taylor DE, Gerner- Smidt P, Nachamkin I. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates, Emerg. Infect. Dis. 2001;7(1):24–34. CrossRef
69. Kanaan MH, Salim ID, Tarek AM, Abdullah SS. Knowledge, attitude, and hygiene practices of food handlers related to food safety in Al-Suwaira City, Wasit Province in Iraq. Int. J. One Health. 2023;9(2):150–58.
70. Centers for Diseases Control and Prevention (CDC). United States: Antibiotic resistance threats in the United States. 2010; Available from https://www.cdc.gov/drugresistance/biggest-threats.html
71. Mudenda S, Nsofu E, Chisha P, Daka V, Chabalenge B, Mufwambi W et al. Prescribing patterns of antibiotics according to the WHO AWaRe classification during the COVID-19 pandemic at a teaching hospital in Lusaka, Zambia: implications for strengthening of antimicrobial stewardship programmes. Pharmacoepidemiology. 2023;2(1):42–53. CrossRef
72. Smith JL, Fratamico PM. Fluoroquinolone resistance in Campylobacter. J Food Prot. 2010; 73(6):1141–52. CrossRef
73. Pesavento G, Ducci B, Comodo N, Nostro AL. Antimicrobial resistance profile of Staphylococcus aureus isolated from raw meat: a research for methicillin resistant Staphylococcus aureus (MRSA). Food Control. 2007;18(3):196–200. CrossRef
74. Johnson TJ, Nolan LK. Pathogenomics of the virulence plasmids of Escherichia coli. Microbiol. Mol. Biol. Rev. 2009;73(4):750–74. CrossRef
75. Schroeder M, Brooks BD, Brooks AE. The complex relationship between virulence and antibiotic resistance. 2017;Genes. 8(1):39. CrossRef
76. Krueger N, Anderson R, Krueger W. Prevalence and concentration of Campylobacter in rumen contents and feces in pasture and feedlot-fed cattle. 2008; Foodborne Pathog. Dis. 5(5):571–77. CrossRef
77. European Food Safety Authority (EFSA). European Union: The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2019-2020. EFSA J. 2020;20(3). CrossRef