INTRODUCTION
Salcedoa mirabaliarum F. Jiménez Rodríguez et al. [1] (Asteraceae), is an endemic species to the island of Hispaniola. Continuing with the interest of our research group in the Asteraceae botanical family present on Hispaniola [2–4] a phytochemical study of the aqueous extract of the aerial parts of S. mirabaliarum was performed affording five guaianolide–type sesquiterpene lactones detected for the first time in this species: dihydroestafiatone, zaluzanin C, dihydroestafiatol, isoamberboin, and 4–epi–dihydroestafiatol.
MATERIALS AND METHODS
General experimental procedures
Nuclear magnetic resonance (NMR) spectra were obtained on a Bruker AVIII HD spectrometer with cryoprobe operating at 800 MHz in 1H and 200 MHz in 13C NMR, respectively. The chemical shift (δ) values are given in ppm. CDCl3 was used as a solvent. Column chromatography (CC) was performed on a Biotage Isolera One flash purification system (Biotage, Charlotte, North Carolina, USA) using a SNAP ULTRA SiO2 (100 g) cartridge. Analytical and preparative thin layer chromatography (TLC) was developed on silica gel 60 F254 plates (Merck KGaA, Darmstadt, Germany).
Plant material
The aerial parts of S. mirabaliarum were collected at Provincia Hermanas Mirabal, Municipio Tenares, Distrito Municipal Blanco Arriba, sección La Jíbara, paraje Mundo Nuevo (767 meters high) Dominican Republic, growing naturally with the following associated species: Tabebuia ricardii, Gesneria viridiflora, Pouteria domingensis, and Bombacopsis emarginata. The plant material was identified by Mr. Teodoro Clase, a botanist at Jardín Botánico Nacional “Dr. Rafael Ma. Moscoso”, Santo Domingo, Dominican Republic, where a voucher specimen (JBSD 121461) has been deposited.
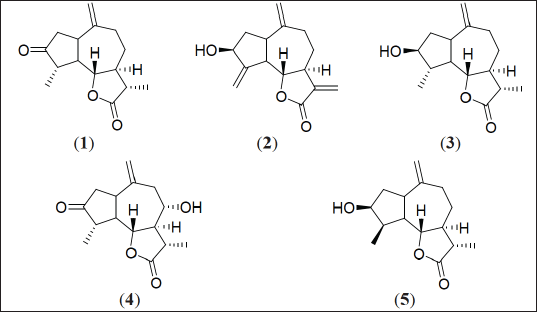 | Figure 1. Chemical structures of compounds 1-5. [Click here to view] |
Extraction and isolation
Dried and powdered aerial parts of S. mirabaliarum (300 g) were boiled with distilled water (4 L) for 2 hours, after which the resulting decoction was cooled, filtered, and extracted (3 × 3 L) with ethyl acetate (EA). After filtration, volume reduction and treatment successively with NaCl 1% and anhydrous Na2SO4, from the EA residue, it was possible to obtain 2.53 g of aqueous extract, which was subjected to CC (SiO2) eluting with increasing mixtures of acetone-hexane, affording 97 fractions. Preparative TLC over selected eluted fractions, allowed to isolate compounds 1–5 (Fig. 1).
RESULTS AND DISCUSSION
The EA residue (2.53 g) of the aqueous extract from S. mirabaliarum, afforded, after different chromatographic techniques, 10.9 mg of dihydroestafiatone (1), [5], 2.6 mg of zaluzanin C (2), 3.8 mg of dihydroestafiatol (3), 2.6 mg of isoamberboin (4), [6], and 1.8 mg of 4–epi–dihydroestafiatol (5), [7]. Their chemical structures were identified using 13C NMR by comparison with reported data.
Dihydroestafiatone (1)
13C NMR (200 MHz, CDCl3) δC = 219.2 (C–3), 178.2 (C–12), 149.1 (C–10), 112.6 (C–14), 88.5 (C–6), 50.9 (C–5), 48.6 (C–7), 47.3 (C–4), 44.0 (C–2), 41.8 (C–11), 39.8 (C–1), 39.1 (C–9), 32.9 (C–8), 14.0 (C–15), 13.4 (C–13).
Zaluzanin C (2)
13C NMR (200 MHz, CDCl3) δC = 170.0 (C–12), 153.1 (C–4), 147.9 (C–10), 139.7 (C–11), 120.2 (C–13), 114.4 (C–14), 111.3 (C–15), 83.9 (C–6), 73.6 (C–3), 50.0 (C–7), 45.6 (C–5), 44.2 (C–1), 39.1 (C–2), 34.3 (C–9), 30.6 (C–8).
Dihydroestafiatol (3)
13C NMR (200 MHz, CDCl3) δC = 178.6 (C–12), 149.3 (C–10), 112.5 (C–14), 86.0 (C–6), 78.4 (C–3), 52.9 (C–7), 50.6 (C–5), 47.0 (C–4), 42.2 (C–11), 42.1 (C–1), 38.4 (C–2), 37.0 (C–9), 32.8 (C–8), 18.1 (C–15), 13.1 (C–13).
Isoamberboin (4)
13C NMR (200 MHz, CDCl3) δC = 218.9 (C–3), 178.4 (C–12), 143.7 (C–10), 115.0 (C–14), 83.1 (C–6), 75.8 (C–8), 54.0 (C–7), 51.4 (C–5), 49.2 (C–9), 47.3 (C–4), 43.6 (C–2), 41.1 (C–11), 39.6 (C–1), 16.4 (C–13), 14.4 (C–15).
4–epi–dihydroestafiatol (5)
13C NMR (200 MHz, CDCl3) δC = 178.8 (C–12), 148.4 (C–10), 111.8 (C–14), 83.0 (C–6), 73.8 (C–3), 51.5 (C–7), 47.2 (C–5), 42.1 (C–11), 41.3 (C–1), 40.4 (C–4), 39.3 (C–2), 34.7 (C–9), 32.8 (C–8), 13.3 (C–13), 8.1 (C–15).
CONCLUSION
In summary, we have reported the isolation of five guaianolide–type sesquiterpene lactones (1–5) from the aqueous extract of S. mirabaliarum. All compounds are reported for the first time in this species.
ACKNOWLEDGMENT
This research received financial support from the Ministerio de Educación Superior, Ciencia y Tecnología, Dominican Republic, under grant FONDOCYT 2022–2C1–092.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Jiménez Rodríguez F, Katinas L, Tellería MC, Crisci JV. Salcedoa gen. nov., a Biogeographic Enigma in the Caribbean Mutisieae (Asteraceae). Syst Bot. 2004;29(4):987–1002. CrossRef
2. Castillo QA, Padrón JM, Wojtas L, Keramane M, Germosén EA. Koanolide A, antiproliferative germacrane–type sesquiterpene lactone from Koanophyllon gibbosum. Tetrahedron Lett. 2019;60(25):1640–2. CrossRef
3. Castillo QA, Padrón JM, Keramane M. Koanolides B–D, new sesquiterpene lactones from Koanophyllon gibbosum. Phytochem Lett. 2022;47:63–6. CrossRef
4. Eiroa JL, Triana J, Pérez FJ, Castillo QA, Brouard I, Quintana J, et al. Secondary metabolites from two Hispaniola Ageratina species and their cytotoxic activity. Med Chem Res. 2018;27(7):1792–9. CrossRef
5. Krishna Kumari GN, Masilamani S, Ganesh MR, Aravind S. Microbial transformation of zaluzanin–D. Phytochemistry. 2003;62(7):1101–4. CrossRef
6. Ha TJ, Lee KD, Lee JR, Lee J, Park KH, Yang MS. Studies on the constituents from the flowers of Hemisteptia lyrata (Bunge) (I). Kor J Pharmacogn. 2001;32(3):238–41.
7. Zhu Y, Hu P–Z, He Z–W, Wu Q–X, Li J, Wu W–S. Sesquiterpene lactones from Scorzonera austriaca. J Nat Prod. 2010;73(2):237–41. CrossRef