INTRODUCTION
Herbal medicines are widely used in developed and developing countries to treat various diseases, including hypertension. This is attributable to the ease of obtaining herbal medicines, as well as their low costs and minimal side effects, and many herbal medicines contain phytochemicals that are effective against hypertension [1,2]. As such, herbal medicines are commonly used to improve health. Although herbal medicines are considered safe, they can have side effects that sometimes result in life-threatening consequences when used simultaneously with other medicines [3,4].
A study in a rural community of West Java, Indonesia, reported that hypertensive patients combined their pharmacological antihypertensive medication with Morinda citrifolia (14.1%) almost daily [5,6]. According to previous reviews on the communities of patients with hypertension in several countries, 80% of such patients use herbal medicines simultaneously with antihypertensive drugs, including the fruit of M. citrifolia [7].
Amlodipine (AML) is a widely used antihypertensive drug for treating hypertension [8]. Based on the results of previous studies, among the monotherapy categories, the various classes of prescribed antihypertensive medicines are calcium channel blockers (CCBs, 15%), followed by diuretics (8%), angiotensin receptor blockers (4%), and angiotensin-converting enzyme inhibitors (ACEIs, 2%), and this indicates that AML, either as monotherapy or in combination with other treatments, is the drug of choice for patients with hypertension [9].
The simultaneous use of herbal medicines and certain prescription drugs often leads to herb-drug interaction (HDI) with clinically significant outcomes, including mortalities. This largely depends on the nature of the herb, drug, and individual, as their synergistic or antagonistic interactions may reduce drug efficacy or cause organ toxicity [10]. Another research states that these interactions demonstrate the potential effects of herbal medicines on the bioavailability and pharmacokinetic (PK) parameters of antihypertensive drugs, synergistic or antagonistic effects may result from pharmacodynamic interactions [11,12]. In general, the severity of PK HDIs is determined by the toxicity of the co-administered drug when its plasma concentration exceeds the minimum toxic concentration or by the consequences when its therapeutic plasma concentration is not reached [13].
A study by Zhang et al. [14] reported that a multiherbal Chinese formula could reduce the pharmacological effects of AML when used simultaneously. Another study stated that Enantia chlorantha and lisinopril co-administration significantly increased hematocrit (HCT) levels in Wistar rats [15]. A study on the interaction of AML with herbs found that both rosella and ginger have lowered blood pressure better when used simultaneously with AML but had a significant impact on PK parameters of AML such as Cmax, AUC0-t, and Tmax [16]. In addition, based on previous studies, M. citrifolia can induce hepatotoxicity and kidney injury [17,18], and AML can cause liver enzyme elevations in idiosyncratic ways [19]. Consequently, simultaneously administered medications that regulate cytochrome (CYP) enzyme activity can lead to drug-herbs interactions [10,20]
To the best of our knowledge, no study has been performed on the simultaneous use effect of AML and Morinda citrifolia fruit extract (MCFE). Therefore, this study investigated the effects of simultaneous MCFE and AML treatment on antihypertensive activity and their toxic effect on hematologic, biochemistry parameters, and organs in rats.
MATERIAL AND METHODS
Materials
Morinda citrifolia extract is a standardized extract obtained from PT. Semarang Indo Plant (Central Java, Indonesia), batch number SLACG100, and the data analysis from the certificate of analysis received in Table 1.
We re-analyzed physicochemical parameters using the procedure in the subsection physicochemical analysis. AML besylate tablets were obtained from PT. Kimia Farma (Jakarta, Indonesia), and NaCl (Merck KGaA, Darmstadt, Germany). The phytochemical study used methanol, hydrochloric acid, sulfuric acid, and ferric chloride were purchased (Sigma–Aldrich, Germany), and all chemicals used are pro-analysis grade.
Animals
The Wistar rats were obtained from the animal house at Universitas Gadjahmada’s Integrated Research and Testing Laboratory, Yogyakarta, Indonesia. The rats were grouped and kept in acrylic cages under controlled conditions, including a 12-hour:12-hour light-dark cycle, constant temperature (23°C–25°C), and relative humidity (50%–55%). All animals were fed standard laboratory food and water ad libitum throughout the experiment and acclimatized for 2 weeks before experimentation [21]. At the end of the experiment, the animals were humanely euthanized through chamber delivery of carbon dioxide [22]. The experimental procedures were approved by the Research Ethics Committee, Universitas Padjadjaran, West Java, Indonesia (No. 565/UN6.KEP/EC/2020), and we followed the guidelines of the European Directive 2010/63/EU.
Physicochemical analysis
The various physicochemical parameters such as moisture content; ash values including total ash, acid-insoluble ash, and water-soluble ash levels; and ethanol-soluble extractive value were determined by the methods described in the World Health Organization (WHO) guidelines [23,24].
Determination of heavy metal content
Heavy metal analysis was conducted by atomic absorption spectroscopy (Perkin Elmer-400) using argon as the carrier gas, and the flow rate was maintained at 1 ml/2 minutes. 500 mg of air-dried powder (accurately weighed) was used to determine the major heavy metal content. The protocol added 5 ml of concentrated nitric acid, and the mixture was refluxed for 30 minutes at 60°C–80°C before cooling. Five milliliters of concentrated nitric acid were added, and the mixture was heated in a water bath. Two milliliters of 30% hydrogen peroxide solution were added to the mixture, which was warmed until a clear solution was obtained. The mixture was then cooled and filtered through Whatman 42 filter paper and diluted with deionized water to a volume of 100 ml in a volumetric flask [25,26].
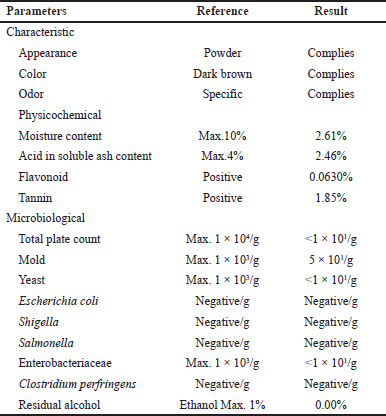 | Table 1. Analysis data from certificate of analysis of MCFE. [Click here to view] |
Phytochemical analysis
A qualitative phytochemical test of filtrates of MCFE was performed to clarify the presence of phytochemicals such as tannins, flavonoids, saponins, phenolic acid, alkaloids, and terpenoids using a standard procedure [27,28]. The qualitative phytochemical tests were carried out using Mayer’s test, Alkaline reagent test, foam layer test, Chloroform + Sulfuric acid test, and Ferric chloride test [29,28,30,31].
Experimental procedure
Antihypertensive test activity
The antihypertensive activity was assessed in this study using a test developed by Wigati et al. [32] and Mulyati et al. [33]. Twenty-five Wistar rats (weight between 200 and 250 g) were randomly divided into five groups of five animals, each group as follows: normal, negative control (NaCl 8%), positive control [NaCl 8% + AML 1 mg/kg body weight (BW)], treatment-1 (NaCl 8% + MCFE 45 mg/kg BW), and treatment-2 (NaCl 8% + MCFE 45 mg/kg BW + AML 1 mg/kg BW). NaCl 8% 3 ml/kg BW induction was performed for 21 consecutive days and continued throughout the test. The AML dose was determined by converting the human dose to the rat dose and previous study [14]. The MCFE dose was determined by converting the human dose to the rat dose from The Indonesian herbal medicine formulary [34]. MCFE and AML were given orally from day 22 to 35. Blood pressure parameters, including systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial blood pressure (MABP), were measured using the CODA™ Non-Invasive Blood Pressure System version 4.1 (Kent Scientific Corporation, Torrington, CT) on days 0, 7, 14, 21, 28, and 35 [32,33].
Sub-chronic toxicity study
The sub-chronic oral toxicity study was conducted according to OECD Test Guideline 407: repeated dose 28-day oral toxicity test [35,36], with minor modification. Forty Wistar rats aged 8–10 weeks of both sexes were randomly divided into four groups (n = 20, 5 males and 5 females), one control group, and three treatment groups. The control group was given CMC-Na 0,5% as a vehicle, the positive control group was given AML 1 mg/kg BW, and the treatment groups were treatment-1 [MCFE 45 mg/kg BW (MCFE)], and treatment-2 [MCFE (45 mg/kg BW) + AML (1 mg/kg BW)]. An additional ten rats (five males and five females) were assigned to the satellite MCFE + AML group for the recovery period. The test substance was suspended in 0.5% CMC-Na and administered orally for 28 consecutive days. Every 5 days, the test material was blended with a vehicle. All animals were observed daily for clinical signs and food and water consumption. Every week, individual BWs were recorded. At day 28, rats in the control and dose groups were sacrificed. Throughout the recovery period of 14 days, satellite groups were continuously observed without treatment and then sacrificed. Rats were euthanized with an overdose of aesthetic ether. Kidney and liver tissues were removed to prepare hematoxylin and eosin-stained tissue sections.
Clinical observation and BW
Once per day, general clinical observations were made on all animals (morbidity and mortality). Behavioral patterns, physical appearance, and other toxicity-related symptoms were observed. Each rat was weighed once per week and immediately before the necropsy.
Hematological analysis
Blood samples were collected from retro-orbital sinus veins under light ether anesthesia, and the hematological analysis was conducted using whole blood collected in EDTA-treated tubes. The assessed hematological parameters included the WBC, differential leukocyte, RBC, and PLT counts; HB and HCT levels; MCV; MCH level; and MCHC. All analyses were performed using the BC-2800VET hematology analyzer (Mindray Bio-Medical Electronics, Shenzhen, China) [37,38].
Biochemical analysis
Blood samples were obtained in anticoagulant-free sterile tubes for biochemical testing (serum). Using spectrophotometric assay kits (DiaSys Diagnostic System, Germany), the levels of alanine transaminase (ALT), aspartate aminotransferase (AST), creatinine, and urea were determined.
Organ weight and histological analysis
The relative liver and kidney weights were determined by dividing organ weight by BW. To facilitate slicing, the organs were washed in an isotonic solution, fixed in 10% formalin, and embedded in paraffin. Hematoxylin–eosin dye was used to stain samples, which were then examined microscopically.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 9.5.1 statistical software (GraphPad Software, San Diego, CA). Antihypertensive outcomes were expressed as the mean, SD, and differences between treatment groups for each blood pressure were examined using two-way ANOVA followed by Tukey’s multiple comparison testing and Dunnett’s. All sub-chronic analysis data were reported as mean ± SD and analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons and Dunnett’s. Statistical significance was considered at p < 0.05.
RESULTS
Physicochemical analysis
Table 2 presents the results of the physicochemical analysis of MCFE. The ethanol-soluble, total, water-soluble, and acid-insoluble ash contents were 46.82, 9.67, 96.55, and 2.14% w/w, respectively. The moisture content was 4.07% w/w. The results of all physicochemical analyses are in accordance with the Herbal Pharmacopeia and Indonesian Materia Medica [39].
 | Table 2. Physicochemical parameters of MCFE. [Click here to view] |
 | Table 3. Concentrations of heavy metals in MCFE. [Click here to view] |
 | Table 4. Qualitative phytochemical analysis of MCFE. [Click here to view] |
Heavy metal analysis
Table 3 presents the heavy metal content in MCFE. The lead, cadmium, mercury, and arsenic concentrations were meager (0.01 μg/g). The concentrations of all analyzed heavy metals were within the permissible limits of the WHO and Joint FAO/WHO Expert Committee on Food Additives [40,41].
Qualitative phytochemical test
Table 4 presents the results of the qualitative phytochemical screening performed using MCFE. The results revealed the presence of flavonoids, alkaloids, tannins, saponins, steroids, terpenoids, and phenols.
Antihypertensive activity
Table 5 presents the antihypertensive activity of simultaneous use of MCFE and AML. On day 0, no significant difference in blood pressure was detected between the groups. After being administered NaCl for 21 days, the rats’ SBP, DBP, and MABP increased significantly in the intervention groups compared with the values in the normal group (all p < 0.05). On day 35, the treatment-1 and treatment-2 groups exhibited decreased SBP, DBP, and MABP. The reductions significantly differed from those in the negative control group (all p < 0.05). The most significant percentage decreases in blood pressure were recorded in the treatment-2 group, as SBP, DBP, and MABP decreased by −31.40 ± 2.39, −13.00 ± 2.55, and −25.20 ± 2.68 mmHg, respectively. However, these decreases were not significantly different from those in the positive control (p > 0.05) or treatment-1 group (p > 0.05).
Sub-chronic toxicity study
Clinical observation and BW
No mortality nor severe clinical signs were observed in any treated groups after 28 days of repeated oral administration. During the first 2 days after dosing, minor clinical signs (mild diarrhea) were observed in a few rats across all study groups. These minor symptoms did not affect the animals’ overall health and were considered typical for Wistar albino rats.
After 28 days of treatment, there were no significant differences (p > 0.05) in the BWs of the treated groups compared to the control group, as shown in Figure 1. At the oral doses administered, the positive control, treatment-1, and treatment-2 groups did not affect the normal growth of rats, as both the control and treatment groups appeared equally healthy.
Hematological parameters effect
Table 6 shows the hematological parameters after 28-day treatment. There were no significant differences in the hematological parameters of the positive control, treatment-1, and treatment-2 groups compared to the control group (p > 0.05). The results indicate that the positive control, treatment-1, and treatment-2 groups did not affect the production or circulation of blood cells.
Biochemical parameters effect
Figure 2 shows biochemical analysis results on male and female rats. There was a significant difference in both sexes between all group treatments and the control group (p < 0.05). In the male treatment-1 and treatment-2 groups, there were significant differences with the positive control male groups in AST and ALT levels (p < 0.05). Blood urea nitrogen (BUN) levels in the treatment-1 male group show no significant difference with the positive control male group, but there is a significant difference in the treatment-2 groups (p < 0.05). Meanwhile, there were no significant differences in creatinine levels in the treatment-1 and treatment-2 male groups compared with the positive control group (p > 0.05). Furthermore, in the female treatment-1 group, there was a significant difference in AST and ALT levels (p < 0.05) compared with the control and positive control group. In contrast, in the treatment-2 group, there was no significant difference in AST and ALT levels (p > 0.05). The BUN and creatinine levels in the treatment-1 and treatment-2 female groups significantly differ from the positive control group (p > 0.05). The results indicate that AML, MCFE, and MCFE + AML may affect liver and kidney function through increased AST, ALT, BUN, and creatinine levels.
Organ weight and histological analysis
Figure 3 shows the organ liver and kidney weight of positive control, treatment-1, and treatment-2 male and female groups. After 28 days of treatment, a macroscopic examination revealed no organ abnormalities in any treatment or control group, regardless of gender. The liver and kidney’s absolute and relative organ weights in all treatment groups did not differ significantly (p > 0.05) from the control group among males and females. These findings indicated that administering AML, MCFE, and MCFE + AML once daily for 28 days did not alter the liver’s and kidneys’ absolute or relative weight.
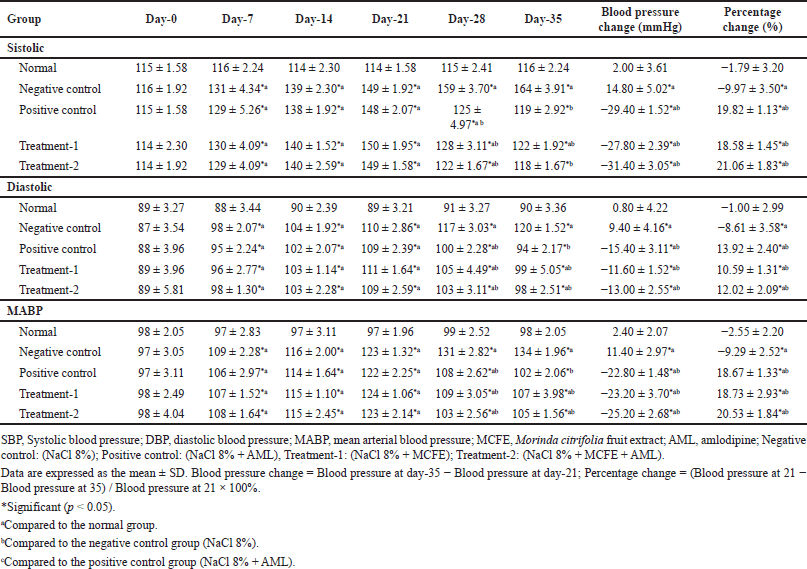 | Table 5. Antihypertensive activity of simultaneous use of MCFE and AML. [Click here to view] |
 | Figure 1. In the sub-chronic toxicity study, the BW of male and female rats after AML, MCFE, and AML + MCFE administration. Values are expressed as mean ± SD; p < 0.05, the significant difference compared to the control group using the one-way ANOVA continued by Tukey’s multiple comparison test. Abbreviations: MCFE, Morinda citrifolia fruit extract; AML, amlodipine; Positive control: (AML); Treatment-1: (MCFE); Treatment-2: (MCFE + AML). [Click here to view] |
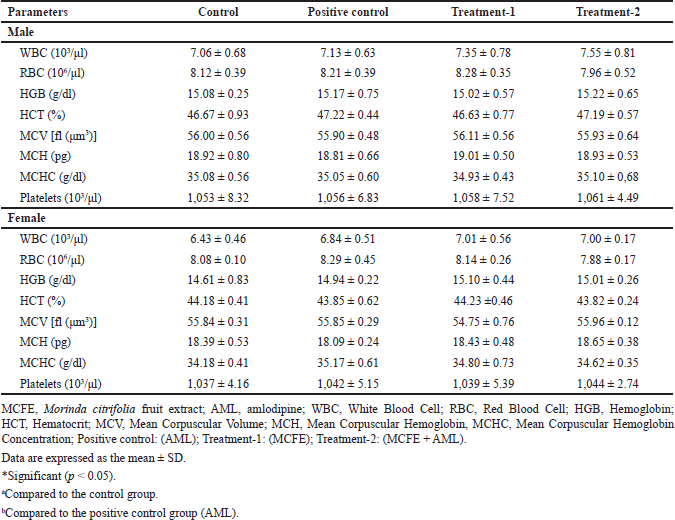 | Table 6. Hematological parameters of male and female rats in sub-chronic toxicity. [Click here to view] |
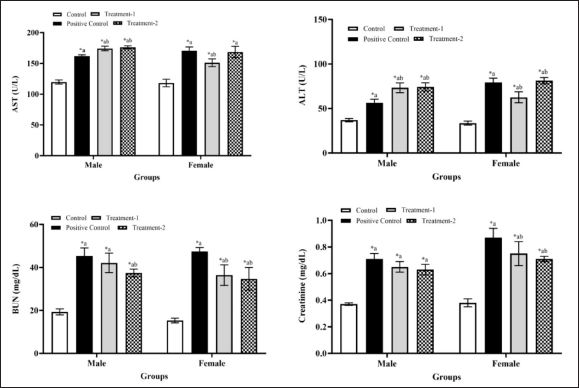 | Figure 2. Biochemical parameters of male and female rats in sub-chronic toxicity. Values are expressed as the mean + SD. Compared to the control group; Compared to the AML group; *significant (p < 0.05). Abbreviations: MCFE, Morinda citrifolia fruit extract; AML, amlodipine; Positive control: (AML); Treatment-1: (MCFE); Treatment-2: (MCFE + AML); AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; BUN, Blood urea nitrogen. [Click here to view] |
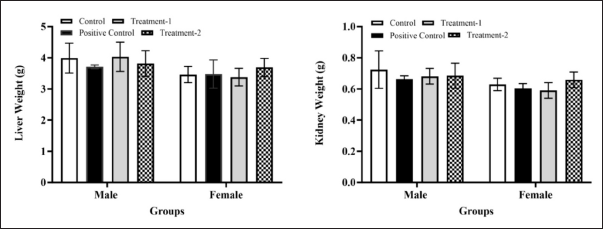 | Figure 3. The relative organ weight (liver and kidney) of male and female rats in the sub-chronic toxicity study. Values are expressed as the mean ± SD. Compared to the control group; Compared to AML group; *significant (p < 0.05). Abbreviations: MCFE, Morinda citrifolia fruit extract; AML, amlodipine; Positive control: (AML); Treatment-1: (MCFE); Treatment-2: (MCFE + AML). [Click here to view] |
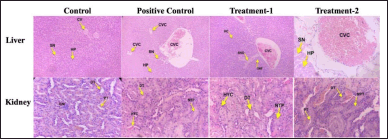 | Figure 4. Histopathology of the male liver and kidney in sub-chronic toxicity study (Hematoxylin and eosin stained). Normal histology showed on the liver and kidney of a control group. The liver of the posotive control group showed slight congestion of the central vein. Mild central vein congestion, sinusoidal dilatation, and inflammation in the treatmen-1 group were seen. The treatment-2 group showed moderate central vein congestion. The kidney positive control, treatment-1, and treatment-2 groups showed narrowing of proximal tubules, epithelium necrosis of proximal tubules, and hyalin cast. CV, Central vein; SN, Sinusoidal; HP, Hepatocyte; CVC, Central Vein Congestion, INF, Inflammation; GM, Glomerulus; DT, Distal Tubules; PT, Proximal Tubules; NPT, Narrowing, and Necrosis Proximal Tubules, HYC, Hyaline Cast; Positive control: (AML); Treatment-1: (MCFE); Treatment-2: (MCFE + AML). [Click here to view] |
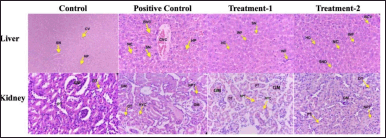 | Figure 5. Histopathology of the female liver and kidney in sub-chronic toxicity study (Hematoxylin and eosin stained). Normal histology showed on the liver and kidney of the control group. The liver of the positive control group showed moderate congestion of the central vein, bleeding around of central vein, and necrosis of hepatocytes. Mild inflammatory cell infiltration in the treatment-1 group was seen. The treatment-2 group showed moderate central vein congestion, bleeding around the central vein, inflammation cell infiltration, and necrosis of hepatocytes. In the kidney of positive control and treatment-1, groups showed moderate to severe narrowing of proximal tubules, epithelium necrosis of proximal tubules, and hyalin cast. Mild necrosis of epithelium and narrowing proximal tubules were seen in the treatmen-2 group. CV, Central vein; SN, Sinusoidal; HP, Hepatocyte; CVC, Central Vein Congestion; BVC, Bleeding around Central Vein; INF, Inflammation; GM, Glomerulus; DT, Distal Tubules; PT, Proximal Tubules; NPT, Narrowing, and Necrosis Proximal Tubules, HYC, Hyaline Cast; NC, Necrosis Hepatocyte; Positive control: (AML); Treatment-1: (MCFE); Treatment-2: (MCFE + AML). [Click here to view] |
The histological analysis of the positive control, treatment-1, and treatment-2 groups confirmed the treatment-related changes in the liver and kidney (male) of rats compared to the control group (Fig. 4). In the liver, mild to moderate central venous congestion was observed in 3/5 of males in the positive control, treatment-1, and treatment-2 groups. The treatment-2 group showed moderate central vein congestion. At the same time, sinusoidal dilatation and infiltration of inflammatory cells in the MCFE male group were also seen. In the kidney, hyaline cast material in the lumen of the proximal tubule and necrosis of the proximal tubular epithelium was observed in 3/5 males in positive control, treatment-1, and treatment-2 groups with percentage damage of 6%, 5%, and 4%, respectively.
Figure 5 presented the histological analysis of the positive control, treatment-1, and treatment-2 female group showing the change in the liver and kidney compared to the control group. The liver of the positive control group showed hepatocyte necrosis, central venous congestion, and bleeding around the central veins, with mild damage. The mild infiltration of inflammation cells was indicated in the treatment-1 and treatment-2 groups, while hepatocyte necrosis, central vein congestion, and bleeding around the central veins in the treatment-2 group were also seen. In the female kidney, hyaline cast material in the lumen of the proximal tubule and necrosis of the proximal tubular epithelium with percentage damage of 80%, 47%, and 20%, respectively, were observed in 2/5 females in positive control, treatment-1, and treatment-2 group. Toxic effects on the liver and kidneys were found in both male and female groups, and gender differences did not affect the apparent toxic results.
DISCUSSION
This study demonstrated that the simultaneous use of MCFE and AML or MCFE alone could decrease SBP, DBP, and MABP in NaCl-induced rats. The percent decrease in blood pressure was higher in the treatment-2 (MCFE + AML) group than in the other groups. Still, the change was not significantly different from that in rats treated with treatment-1 (MCFE) or positive control (AML). We may assume that simultaneous treatment with AML and MCFE has the same effect on blood pressure as either AML or MCFE alone. Our study also demonstrated that the simultaneous use of AML of MCFE could cause toxic effects on liver and kidney function through AST, ALT, BUN, Creatinine levels, and histology organs of rats.
We used the dose for AML 1 mg/Kg BW in our study based on Zhang et al. [14] study in 2019 and based on converting human dose to rat dose calculation. The Zhang study investigated the effects of the simultaneous use of danshen tablets, a traditional Chinese herbal medicine, and AML tablets in rat’s PKs. The result showed HDIs between danshen tablets and AML, whereas the dose of MCFE used in this study was based on the dose listed on the Indonesian herbal medicine formulary (500 mg/kg BW), which was then converted into a rat dose (45 mg/kg BW) [32].
Morinda citrifolia fruit exerts hypotensive effects through a vasodilatory mechanism involving its smooth muscle relaxant activity, acting as a nonspecific spasmolytic agent, and it could have an angiotensin-I converting enzyme-inhibitory effect [34]. Meanwhile, AML is an effective first-line option among several available antihypertensive medications and long-acting CCB inhibiting calcium entry into vascular smooth muscle cells and cardiac cells, decreasing peripheral vascular resistance [8]. A study revealed that AML could lower blood pressure by reducing malondialdehyde levels and increasing Na+ K+ ATPase, superoxide dismutase, and endothelial nitric oxide (NO) levels [42,43]. According to other research, M. citrifolia juice administration increased vasodilation in patients with hypertension and induced vasorelaxation in the aorta via NO generation by endothelial cells [44] Based on these findings, M. citrifolia could have the same antioxidant action as AML, and it may have the same activity as AML, in line with the results of this study.
The higher percent decrease in blood pressure in the MCFE + AML (treatment-2) group was caused by their combined antioxidant activities. This is in line with the results of previous studies stating that the combination of Carthamus tinctorius extract and captopril could reduce blood pressure by increasing NO bioavailability and reducing oxidative stress [45]. Another study reported that combining garlic or garlic oil and carvedilol exerts antihypertensive and cardioprotective effects by increasing lactate dehydrogenase, creatinine phosphokinase, superoxide dismutase, and catalase activities [46,47]. In addition, the combination of AML and ACEIs is more effective than AML alone in controlling blood pressure [48].
Combining herbal medicine and prescription drugs may provide a more desirable effect than either treatment alone [49]. When herbal and prescription drugs are used together, drug–herbal interactions may occur. Drug–herbal interactions are considered additive if the combined effect is the same as the sum of the effect of each drug alone [50]. The simultaneous use of herbal medicines and drugs can cause pharmacodynamic interactions such as additive, synergistic, or antagonistic effects [49,51]. Previous studies stated that simultaneously administering Zingiber officinale or Hibiscus sabdariffa with AML improves its pharmacodynamic response [16]. On the other hand, HDIs have traditionally focused on metabolic enzyme and/or transporter-mediated PK changes in a drug induced by concomitant herbal products because PK changes of a drug can result in the alternation of efficacy and toxicity [52,53]
According to sub-chronic toxicity (28 days) in the current study, clinical observation and the rat’s BW showed no abnormal animal behavior, nor did mortality appear in the positive control, treatment-1, and treatment-2 groups until the end of the study. The average BW of male and female rats between the first and fourth week increased in all group treatments. This indicates that neither AML, MCFE nor the combination of MCFE + AML reduced the BW of the test animals after 28 days of administration. An increase in BW is one of the indicators of the health status of the experimental animals [54,55] because BW changes can be attributed to adverse drug effects [21,56]. In this study, both male and female rats in the treatment and control groups gained BW because they were in a growing phase.
The most vital tissue is the blood, which may reflect changes in metabolic processes. As a result, significant changes in blood parameters are markers of pharmacological, chemical, and disease-related toxicity [57,58]. In this study, the simultaneous use of MCFE and AML did not significantly affect rats’ hematological variables (WBC, RBC, HB, HCT, MCV, MCH, MCHC, and platelets), in line with previously reported findings that MCFE administration in rats at a dose of 2,000–5,000 mg/kg BW for 13 weeks had no adverse effect on the hematological profile of rats [59].
In the present study, AST, ALT, BUN, and creatinine levels were significantly elevated (p < 0.05) in positive control, treatment-1, and treatment-2 groups compared to the control group of both sexes. Organ anatomic-pathological alterations frequently accompany the biochemical alterations of the serum. Since injured organs discharge their contents into circulation, this may alter the normal biomarker concentrations in the blood plasma [60,61]. The liver and kidney, which are involved in eliminating xenobiotics, are sensitive organs susceptible to alteration by substances, such as plants and drugs [62].
AST and ALT are serum enzymes that indicate hepatocellular toxicity and liver injury [63]. In this study, the levels of AST and ALT were significantly elevated (p < 0.05) in the positive control, treatment-1, and treatment-2 groups when compared with the values of the control group in both sexes. An increase in serum AST and ALT, the effects of using AML have been reported. Although the mechanism of the hepatotoxic effect is not widely known, this must still be considered [19]. Furthermore, there is a potential risk of HDIs leading to adverse side effects, including adverse hepatic reactions such as hepatotoxicity or liver injury, which is an increase in serum aminotransferase (ALT/AST) levels by at least two times the normal upper limit (2N) or increase over 2–4× normal/control limits [64,63]. Increased levels of AST and ALT in the group of male and female rats that were given MCFE + AML simultaneously showed an interaction between AML and MCFE. This aligns with previous research stating that herbal-drug interactions can induce hepatotoxicity [10,50]. Several plant secondary metabolites, including terpenes, alkaloids, and anthraquinone, can affect liver enzymes [65,66]; these compounds are present in MCFE and, when combined with pharmaceuticals, can modulate various cytochrome P450 enzymes, particularly CYP3A4 [67,68]. AML was metabolized by cytochrome P450 CYP3A4 isoenzymes; modulators of CYP3A4 could enhance the intrinsic hepatotoxicity of other substances by increasing their conversion to toxic metabolites [69,70]. Therefore, plant metabolites with a given pharmacological property/metabolizing enzyme should not be combined with drugs that have the same pharmacological property/metabolizing enzyme [71].
Furthermore, kidney dysfunction was evaluated by measuring creatinine and BUN. Creatinine and urea are nitrogenous nonprotein byproducts of protein metabolism that must be continuously eliminated. Therefore, an increase in these kidney function indices indicates that kidney dysfunction is predominantly caused by injury [72]. This study showed a significant (p < 0.05) increase in serum creatinine and BUN levels in all group treatments compared to the control group in both sexes. The simultaneous use of MCFE + AML (treatment-2 group) on male rats shows significantly elevated BUN levels compared to the AML (positive control) group. Meanwhile, in creatinine levels, there is no significant difference. In the female group of MCFE + AML (treatment-2), there was a significant increase in creatinine and BUN levels. Due to their inherent properties, plant extracts can have renal toxicity, and this effect is not only related to the presence of contaminants in the extract; herbal-drug interactions with a compound present in the herb must also be considered [73,74]. The kidney can be regarded as an important target organ for exogenous toxins. Nephrotoxicity is a kidney-specific condition in which toxic chemicals, herbs, or drugs disrupt excretion [75,76]. In general, nephrotoxicity is associated with numerous factors, such as direct nephrotoxic effects of the herbal product or its compound, herbal-drug interactions that enhance nephrotoxicity, the insolubility of substances and their metabolites in urine, and protracted exposure at high doses [77].
This study examined the liver and kidney sections for histopathological alterations. For liver histological changes in male rats caused by AML, MCFE, and the simultaneous use of MCFE + AML, we found mild damage, such as central venous congestion, sinusoidal dilatation, and inflammation. Meanwhile, in female positive control, treatment-1, and treatment-2 group livers showed histological changes, including necrosis hepatocyte, sinusoidal dilatation, congestion of central veins, bleeding around central veins, and infiltration of inflammation cells. The xenobiotic-induced hepatic lesions are diverse and heterogeneous. All liver cells (hepatocytes and endothelial cells) are potentially implicated. Consequently, xenobiotics can be responsible for all liver lesions, affecting all liver vascular system levels. Conventional medications, medicinal plants, and industrial agents are involved in vascular lesions. This lesion can be identified by sinusoidal dilatation without obstruction of the liver’s efferent vessels [78–80]. Sinusoids regulate hepatic microcirculation, transporting oxygen, nutrients, and toxins between the vascular space and hepatocytes. Sinusoidal dilatation and congestion are common due to obstructed hepatic venous outflow and elevated venous pressure [79].
We also observed histological changes in male and female positive control, treatment-1, and treatment-2 kidney groups through narrowing of the proximal tubule, necrosis of the proximal tubule epithelium, and hyaline casts. The kidney’s proximal tubules play a vital role in the reabsorption and excretion of substrates from the body into the urine, resulting in elevated local concentrations of xenobiotics. Impaired endocytosis, receptor/transporter inhibition, lysosome disruption, oxidative stress and depletion of antioxidant defenses, and mitochondrial dysfunction are the xenobiotic-induced causes of proximal tubule dysfunction [73,81]. The nephrotoxicity of xenobiotics depends on the drug’s intrinsic reactivity with subcellular or molecular targets. Both cytochrome P450 and cysteine conjugate-lyase are almost exclusively localized in the proximal tubule, and bioactivation contributes at least partially to proximal tubular lesions. Finally, proximal tubular cells appear more susceptible to ischemic damage than distal tubular cells [82,83]. Hyaline casts are the most common cast encountered in renal biopsy specimens [84]. They are associated with increased glomerular permeability and granular (necrotic cellular debris) casts indicative of previous tubule cell necrosis associated with chemicals that induce α2u-globulin nephropathy [85]. In this study, the satellite rats of both sexes showed the same histological results as the treatment group, indicating that organ damage can be irreversible. Therefore, more studies with a prolonged exposure time (90 days) are required to determine whether organ recovery or repair is delayed after drug administration.
The limitations of this study included the inability of the study design to provide specific information about the mechanisms behind the interactions. In addition, mechanism-based research can be conducted, such as in-vitro microsomal studies to confirm the function of particular drug-metabolizing enzymes and the PK profiles of simultaneous use of these herbs with antihypertensive drugs.
CONCLUSION
The simultaneous use of MCFE and AML may have the same effect in reducing high blood pressure as AML or MCFE alone. The treatment of MCFE and simultaneous use of MCFE + AML did not affect the hematological parameters of rats but affected AST, ALT, BUN, and creatinine levels. This treatment also caused histological changes in the rat’s organs, including the liver and kidneys, that led to toxic effects. Therefore, the simultaneous use of M. citrifolia and AML to treat hypertension should not be considered.
ACKNOWLEDGMENT
The authors thank The Directorate of Research and Community Engagement Universitas Padjadjaran for APC Funding.
AUTHOR CONTRIBUTIONS
Conceptualization, NA, IMP, and ANH; methodology, NA; validation, NA, ANH, IMP, and EH; formal analysis, NA; investigation, NA; writing—original draft preparation, NA, IMP, and ANH.; writing—review and editing, NA, IMP, ANH; supervision, EH, IMP, and ANH.; All authors have read and agreed to the published version of the manuscript
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
The experimental procedures were approved by the Research Ethics Committee, Universitas Padjadjaran, West Java, Indonesia (No. 565/UN6.KEP/EC/2020), and we followed the guidelines of the European Directive 2010/63/EU.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Landazuri P, Chamorro NL, Cortes BR. Medicinal plants used in the management hypertension. J Anal Pharm Res. 2017;5(2):5–7. CrossRef
2. Chrysant SG, Chrysant GS. Herbs used for the treatment of hypertension and their mechanism of action. Curr Hypertens Rep. 2017 Sep 18;19(9):77. CrossRef
3. Kafeel H. Possibility of potential herbal-drug interactions in elderly population. J. Phytopharmacol. 2016;5(3):128–30. Available from: www.phytopharmajournal.com CrossRef
4. Agbabiaka T, Wider B, Watson LK, Goodman C. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review protocol. Syst Rev. 2016;5(1):1–5. CrossRef
5. Basuki B, Siagian M, Ilyas EI, Amri Z. Combined traditional medicine and pharmacological antihypertensive drugs in a rural community of West Java, Indonesia. Med J Indones. 2004 Nov 1;13(4):246. CrossRef
6. Rahmawati R, Bajorek B. The use of traditional medicines to lower blood pressure?: a survey in rural areas in Yogyakarta province , Indonesia. Aust Med J. 2018;11(3):153–62. CrossRef
7. Azizah N, Halimah E, Puspitasari IM, Hasanah AN. Simultaneous use of herbal medicines and antihypertensive drugs among hypertensive patients in the community: a review. J Multidiscip Healthc. 2021 Feb;14:259–70. CrossRef
8. Fares H, DiNicolantonio JJ, O’Keefe JH, Lavie CJ. Amlodipine in hypertension: a first-line agent with efficacy for improving blood pressure and patient outcomes. Open Heart. 2016 Sep 28;3(2):1–7. CrossRef
9. Malpani AK, Waggi M, Panja P, Christien TM. Study of prescribing pattern of antihypertensive drugs and evaluation of the prescription with JNC 8 guidelines in North Karnataka Hospital. Indian J Pharm Pract. 2018 Dec 3;11(4):193–7. CrossRef
10. Parvez MK, Rishi V. Herb-drug interactions and hepatotoxicity. Curr Drug Metab. 2019 Mar 27;20(4):275–82. CrossRef
11. Chhabra A, Singh G, Upadhyay Y. A review on herbal drug interaction. Asian J Pharm Res Dev. 2020 Feb 14;8(1):94–9. CrossRef
12. Sharma V, Madaan R, Bala R, Goyal AK, Sindhu R. Pharmacodynamic and pharmacokinetic interactions of herbs with prescribed drugs: a review. Plant Arch. 2021 Jan 15;21(Suppliment-1):185–98. CrossRef
13. Gouws C, Hamman JH. What are the dangers of drug interactions with herbal medicines? Expert Opin Drug Metab Toxicol. 2020;16:165–7. CrossRef
14. Zhang H, Han X, Li Y, Li H, Guo X. Effects of Danshen tablets on pharmacokinetics of amlodipine in rats. Pharm Biol. 2019;57(1):306–9. CrossRef
15. Ajani EO, Ibrahim LB. Toxicological evaluations of combined administration of ethanolic stem bark extract of Enantia chlorantha and lisinopril in experimental type 2 diabetes. Clin Phytosci. 2020;6(1):1–9. CrossRef
16. Alam MA, Bin Jardan YA, Alzenaidy B, Raish M, Al-Mohizea AM, Ahad A, et al. Effect of Hibiscus sabdariffa and Zingiber officinale on pharmacokinetics and pharmacodynamics of amlodipine. J Pharm Pharmacol. 2021 Sep 1;73(9):1151–60. CrossRef
17. Mohamad Shalan NAA, Mustapha NM, Mohamed S. Chronic toxicity evaluation of Morinda citrifolia fruit and leaf in mice. Regul Toxicol Pharmacol. 2016;83:46–53. CrossRef
18. Haslan H, Suhaimi H, Das S. Herbal supplements and hepatotoxicity: a short review. Nat Prod Commun. 2015;10(10):1779–84. CrossRef
19. Varghese G, Madi L, Ghannam M, Saad R. A possible increase in liver enzymes due to amlodipine: a case report. SAGE Open Med Case Rep. 2020 Jan;8:1–5. CrossRef
20. Borse SP, Singh DP, Nivsarkar M. Understanding the relevance of herb–drug interaction studies with special focus on interplays: a prerequisite for integrative medicine. Porto Biomed J. 2019 Mar;4(2):e15. CrossRef
21. Kang S, Johnston TV, Ku S, Ji GE. Acute and sub-chronic (28-day) oral toxicity profiles of newly synthesized prebiotic butyl-fructooligosaccharide in ICR mouse and Wistar rat models. Toxicol Res (Camb). 2020;9(4):484–92. CrossRef
22. Derelanko MJ. The toxicologist’s pocket handbook. 3rd ed. Boca Raton, FL: CRC Press; 2017.
23. Balasubramaniam G, Sekar M, Badami S. Pharmacognostical, physicochemical and phytochemical evaluation of Strobilanthes kunthianus (Acanthaceae). Pharmacogn J. 2020 Jun 1;12(4):731–41. CrossRef
24. Balekundri A, Mannur V. Quality control of the traditional herbs and herbal products: a review. Futur J Pharm Sci. 2020 Dec;6(1):1–9. CrossRef
25. Uddin AH, Khalid RS, Alaama M, Abdualkader AM, Kasmuri A, Abbas SA. Comparative study of three digestion methods for elemental analysis in traditional medicine products using atomic absorption spectrometry. J Anal Sci Technol. 2016 Dec 1;7(1):1–7. CrossRef
26. Chumbhale D, Upasani C. Pharmacognostic standardization of stems of Thespesia lampas (Cav.) Dalz &Gibs. Asian Pac J Trop Biomed. 2012 May;2(5):357–63. CrossRef
27. Shaikh JR, Patil M. Qualitative tests for preliminary phytochemical screening: an overview. Int J Chem Stud. 2020 Mar 1;8(2):603–8. CrossRef
28. Adusei S, Otchere JK, Oteng P, Mensah RQ, Tei-Mensah E. Phytochemical analysis, antioxidant and metal chelating capacity of Tetrapleura tetraptera. Heliyon. 2019;5(11):1–5. CrossRef
29. Gul R, Jan SU, Faridullah S, Sherani S, Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia Indigenous to Balochistan. Sci World J. 2017:1–7. CrossRef
30. Rahman N, Dewi NU, Bohari. Phytochemical and antioxidant activity of avocado leaf extract (Persea americana Mill.). Asian J Sci Res. 2018;11(3):357–63. CrossRef
31. Sorescu AA, Nuta A, Ion RM, Iancu L. Qualitative analysis of phytochemicals from sea buckthorn and gooseberry. In: Phytochemicals—source of antioxidants and role in disease prevention. London, United Kingdom: InTechOpen; 2018. CrossRef
32. Indonesia Ministry of Health. Indonesian herbal medicine formulary. Jakarta, Indonesia: Indonesia Ministry of Health; 2016.
33. Wigati D, Anwar K, Sudarsono, Nugroho AE. Hypotensive activity of ethanolic extracts of Morinda citrifolia L. leaves and fruit in dexamethasone-induced hypertensive rat. J Evid Based Complementary Altern Med. 2017;22(1):107–13. CrossRef
34. Mulyati AH, Sulaeman A, Marliyati SA, Rafi M, Fikri AM. Preclinical trial of propolis extract in prevention of high salt diet-induced hypertension. Pharmacogn J. 2021 Jan 1;13(1):89–96. CrossRef
35. Baig MW, Majid M, Nasir B, Hassan SS, Bungau S, Haq IU. Toxicity evaluation induced by single and 28-days repeated exposure of withametelin and daturaolone in Sprague Dawley rats. Front Pharmacol. 2022 Sep 26;13:999078. CrossRef
36. OECD. Test no. 407: repeated dose 28-day oral toxicity study in rodents. OECD; 2008. French: OECD.org; 2008:1–30.
37. Ramadan KS, Alshamrani SA. Effects of Salvadora persica extract on the hematological and biochemical alterations against immobilization-induced rats. Scientifica (Cairo). 2015;2015:1–5. CrossRef
38. Olayode OA, Daniyan MO, Olayiwola G. Biochemical, hematological and histopathological evaluation of the toxicity potential of the leaf extract of Stachytarpheta cayennensis in rats. J Tradit Complement Med. 2020;10(6):544–54. CrossRef
39. Ministry of Health of Indonesia. Indonesian herbal pharmacopeia II: Directorate General of Pharmaceuticals and Medical Devices, Ministry of Health of Indonesia; Jakarta. Indonesia; 2017:1–561.
40. Joint FAO/WHO Expert Committee on Food Additives, Food and Agriculture Organization of the United Nations, World Health Organization. Evaluation of certain contaminants in food: seventy-second report of the Joint FAO/WHO Expert Committee on Food Additives. Rome, Italy: Joint FAO/WHO Expert Committee on Food Additives; 2010.
41. Shelar M, Gawade V, Bhujbal S. A review on heavy metal contamination in herbals. J Pharm Res Int. 2021 May 15;33:7–16. CrossRef
42. Sorriento D, De Luca N, Trimarco B, Iaccarino G. The antioxidant therapy: new insights in the treatment of hypertension. Front Physiol. 2018;9:258. CrossRef
43. Mason RP, Jacob RF, Dawoud H, Wagner MR, Mahmud FJ, Malinski T. Amlodipine increases nitric oxicide realase in endothelial cells collected from donors of different races and enos gene variants. J Am Coll Cardiol. 2016 Apr;67(13):2321. CrossRef
44. Yoshitomi H, Zhou J, Nishigaki T, Li W, Liu T, Wu L, et al. Morinda citrifolia (Noni) fruit juice promotes vascular endothelium function in hypertension via glucagon-like peptide-1 receptor-CaMKKβ-AMPK-eNOS pathway. Phytother Res. 2020 Sep 1;34(9):2341–50. CrossRef
45. Maneesai P, Prasarttong P, Bunbupha S, Kukongviriyapan U, Kukongviriyapan V, Tangsucharit P, et al. Synergistic antihypertensive effect of Carthamus tinctorius L. extract and captopril in l-NAME-induced hypertensive rats via restoration of eNOS and AT1R expression. Nutrients. 2016 Feb 29;8(3):122. CrossRef
46. Asdaq SMB, Challa O, Alamri AS, Alsanie WF, Alhomrani M, Almutiri AH, et al. Cytoprotective potential of aged garlic extract (Age) and its active constituent, s-allyl-l-cysteine, in presence of carvedilol during isoproterenol-induced myocardial disturbance and metabolic derangements in rats. Molecules. 2021 Jun 1;26(11):3203. CrossRef
47. Asdaq SMB, Challa O, Alamri AS, Alsanie WF, Alhomrani M, Asad M. The potential benefits of using garlic oil and its active constituent, dially disulphide, in combination with carvedilol in ameliorating isoprenaline-induced cardiac damage in rats. Front Pharmacol. 2021 Sep 27;12:739758. CrossRef
48. Salam A, Atkins ER, Hsu B, Webster R, Patel A, Rodgers A. Efficacy and safety of triple versus dual combination blood pressure-lowering drug therapy: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2019 Aug 1;37(8):1567–73. CrossRef
49. Iqbal SMF, Ahmad S, Parray SA. Herb-drug interaction and role of pharmacovigilance. Int J Adv Pharm Med Bioall Sci. 2015;3(1):67–74.
50. Kahraman C, Arituluk ZC, Irem I, Cankaya T. The clinical importance of herb-drug interactions and toxicological risks of plants and herbal products. In: Erkekoglu P, Ogawa T, editors. Medical toxicology. London, United Kingdom: InTechOpen; 2021:3–31. CrossRef
51. Oga EF, Sekine S, Shitara Y, Horie T. Pharmacokinetic herb-drug interactions: insight into mechanisms and consequences. Eur J Drug Metab Pharmacokinet. 2016;41(2):93–108. CrossRef
52. Choi YH, Chin YW. Multifaceted factors causing conflicting outcomes in herb-drug interactions. Pharmaceutics. 2021;13:1–13. CrossRef
53. Choi YH. Interpretation of drug interaction using systemic and local tissue exposure changes. Pharmaceutics. 2020;12(5):417. CrossRef
54. Ghasemi A, Jeddi S, Kashfi K. The laboratory rat: age and body weight matter. EXCLI J. 2021;20:1431–45.
55. Cho H, Jeon S, Lee M, Kang K, Kang H, Park E, et al. Analysis of the factors influencing body weight variation in hanwoo steers using an automated weighing system. Animals. 2020 Aug 1;10(8):1–8. CrossRef
56. de Oliveira AM, da Silva WAV, Ferreira MRA, Paiva PMG, de Medeiros PL, Soares LAL, et al. Assessment of 28-day oral toxicity and antipyretic activity of the saline extract from Pilosocereus gounellei (Cactaceae) stem in mice. J Ethnopharmacol. 2019 Apr 24;234:96–105. CrossRef
57. Shirsat P, Ziyaurrahman AR, Kashikar R, Athavale M, Athavale T, Taware P, et al. Subacute toxicity study of the ethanolic extract of Mesua ferrea (L.) flowers in rats. Drug Chem Toxicol. 2022;45(4):1570–7. CrossRef
58. Raina P, Chandrasekaran CV, Deepak M, Agarwal A, Ruchika KG. Evaluation of subacute toxicity of methanolic/aqueous preparation of aerial parts of O. sanctum in Wistar rats: clinical, haematological, biochemical and histopathological studies. J Ethnopharmacol. 2015 Dec 4;175:509–17. CrossRef
59. Chaiyasut C, Sivamaruthi BS, Duangjitcharoen Y, Kesika P, Sirilun S, Chaiyasut K, et al. Evaluation of subchronic toxicity of Lactobacillus paracasei HII03 fermented Morinda citrifolia (Noni) fruit juice. Asian J Pharm Clin Res. 2018;11(11):381–4. CrossRef
60. Everds NE. Evaluation of clinical pathology data:correlating changes with other study data. Toxicol Pathol. 2015;43(1):90–7. CrossRef
61. Hasan KMM, Tamanna N, Haque MA. Biochemical and histopathological profiling of Wistar rat treated with Brassica napus as a supplementary feed. Food Sci Hum Wellness. 2018 Mar 1;7(1):77–82. CrossRef
62. Li W, Jiang H, Ablat N, Wang C, Guo Y, Sun Y, et al. Evaluation of the acute and sub-chronic oral toxicity of the herbal formula Xiaoer Chaigui Tuire Oral Liquid. J Ethnopharmacol. 2016 Aug 2;189:290–9. CrossRef
63. Singh D, Cho WC, Upadhyay G. Drug-induced liver toxicity and prevention by herbal antioxidants: an overview. Front Physiol. 2016;6:363. CrossRef
64. Kalas MA, Chavez L, Leon M, Taweesedt PT, Surani S. Abnormal liver enzymes: a review for clinicians. World J Hepatol. 2021 Nov 27;13(11):1688–98. CrossRef
65. Brewer CT, Chen T. Hepatotoxicity of herbal supplements mediated by modulation of cytochrome P450. Int J Mol Sci. 2017;18:2353. CrossRef
66. Kang L, Li D, Jiang X, Zhang Y, Pan M, Hu Y, et al. Hepatotoxicity of the major anthraquinones derived from polygoni multiflori radix based on bile acid homeostasis. Front Pharmacol. 2022 May 18;13:878817. CrossRef
67. Dorofeeva MN, Shikh EV, Sizova ZM, Tarasenko AV, Denisenko NP, Smirnov VV, et al. Antihypertensive effect of amlodipine in co-administration with omeprazole in patients with hypertension and acid-related disorders: cytochrome P450-associated aspects. Pharmgenomics Pers Med. 2019;12:329–39. CrossRef
68. Wang YK, Li WQ, Xia S, Guo L, Miao Y, Zhang BK. Metabolic activation of the toxic natural products from herbal and dietary supplements leading to toxicities. Front Pharmacol. 2021;12:758468. CrossRef
69. Zhang C, Gao Z, Niu L, Chen X. Effects of triptolide on pharmacokinetics of amlodipine in rats by using LC-MS/MS. Pharm Biol. 2018 Jan 1;56(1):132–7. CrossRef
70. Cappelleri G. A case of serial liver injury induced by plant food supplements in a young healthy man. J Clin Gastroenterol Treat. 2017 Sep 30;3(3):1–4. CrossRef
71. Suroowan S, Abdallah HH, Mahomoodally MF. Herb-drug interactions and toxicity: underscoring potential mechanisms and forecasting clinically relevant interactions induced by common phytoconstituents via data mining and computational approaches. Food Chem Toxicol. 2021 Oct 1;156:112432. CrossRef
72. Njinga NS, Kola-Mustapha AT, Quadri AL, Atolani O, Ayanniyi RO, Buhari MO, et al. Toxicity assessment of sub-acute and sub-chronic oral administration and diuretic potential of aqueous extract of Hibiscus sabdariffa calyces. Heliyon. 2020 Sep 1;6(9). CrossRef
73. Pearson A, Gafner S, Rider CV, Embry MR, Ferguson SS, Mitchell CA. Plant vs. kidney: evaluating nephrotoxicity of botanicals with the latest toxicological tools. Curr Opin Toxicol. 2022;32:100371. CrossRef
74. Touiti N, Houssaini TS, Achour S. Overview on pharmacovigilance of nephrotoxic herbal medicines used worldwide. Clin Phytosci. 2021 Dec;7(1). CrossRef
75. Griffin BR, Faubel S, Edelstein CL. Biomarkers of drug-induced kidney toxicity. Ther Drug Monit. 2019;41:213–26. CrossRef
76. Xu X, Zhu R, Ying J, Zhao M, Wu X, Cao G, et al. Nephrotoxicity of herbal medicine and its prevention. Front Pharmacol. 2020;11:569551. CrossRef
77. Kili?-Pstrusi?ska K, Wiela-Hoje?ska A. Nephrotoxicity of herbal products in europe—a review of an underestimated problem of nephrotoxicity of herbal products. Int J Mol Sci. 2021;22(8):4132. CrossRef
78. Brancatelli G, Furlan A, Calandra A, Dioguardi Burgio M. Hepatic sinusoidal dilatation. Abdom Radiol. 2018 Aug 1;43(8):2011–22. CrossRef
79. Furlan A, Minervini MI, Borhani AA, Dioguardi Burgio M, Tublin ME, Brancatelli G. Hepatic sinusoidal dilatation: a review of causes with imaging-pathologic correlation. Semin Ultrasound CT MRI. 2016 Dec 1;37(6):525–32. CrossRef
80. Larrey D, Meunier L, Valla D, Hillaire S, Hernandez-Gea V, Dutheil D, et al. Drug induced liver injury and vascular liver disease. Clin Res Hepatol Gastroenterol. 2020 Sep 1;44(4):471–9. CrossRef
81. Vormann MK, Gijzen L, Hutter S, Boot L, Nicolas A, van den Heuvel A, et al. Nephrotoxicity and kidney transport assessment on 3D perfused proximal tubules. AAPS J. 2018 Sep 1;20(5):1–1. CrossRef
82. Al-Naimi M, Rasheed H, Hussien N, Al-Kuraishy H, Al-Gareeb A. Nephrotoxicity: role and significance of renal biomarkers in the early detection of acute renal injury. J Adv Pharm Technol Res. 2019;10:95–9. CrossRef
83. Barnett LMA, Cummings BS. Nephrotoxicity and renal pathophysiology: a contemporary perspective. Toxicol Sci. 2018;164:379–90. CrossRef
84. Dvanajscak Z, Cossey LN, Larsen CP. A practical approach to the pathology of renal intratubular casts. Semin Diagn Pathol. 2020;37:127–34. CrossRef
85. Curtis Seely J, Senior Pathologist D, Brix A. Kidney, renal tubule—cast [Internet]. Research Triangle Park, NC: NTP; 2023.