INTRODUCTION
Depression is a serious medical disorder that impairs mood, energy, sleep, and individual’s capacity to enjoy life. Stress state often disrupts homeostasis, wellbeing, and physiological balance, leading to neurodegenerative disorders and susceptibility to neuro-damage due to activation of the HPA axis, glucocorticoids, oxidative stress, and inflammation [1]. The vivid stress-operated mechanisms leading to depression have been depicted in Figure 1. Clinical research has shown that treating depression has poor therapeutic results [2]. Active ingredients in medicinal plants are crucial for clinical therapeutics due to their multi-pharmacological activities [3]. With regard to the adverse effects, plant-based bioactive compounds hold promise as an alternative to the current pharmaceutical therapies for depression. These molecules also have antioxidant and neuroprotective capabilities. The current review focuses on species from five selected genera (viz. Salvia, Mentha, Rosmarinus, Sideritis, and Scutellaria) of a vast yet unexplored family Lamiaceae in depressive disorders. The reports on antidepressant effects of reported plant species from these genera over the past 10 years of research have been presented.
MATERIALS AND METHODS
Using specific keywords for both whole plant products and plant extracts, such as “antidepressant plants lamiaceae,” “antidepressant extract lamiaceae,” and “antidepressant herbs lamiaceae,” the authors of this review searched internationally recognized databases, including Science Direct, PubMed, and Google scholar. Regarding a targeted search on antidepressant reports of particular plant species beneath each genus, a combination of keywords was employed. A graphical representation of the availability of articles on research done in the past 10 years has been represented in Figure 2.
SPECIES OF THE LAMIACEAE FAMILY WITH ANTIDEPRESSANTS EFFECTS
Salvia genus
Salvia, which means “to save,” is a Latin word that was used to name the genus in reference to its purported medical powers. With 1,000 species, Salvia is a rather varied genus [4]. Salvia genus belongs to the subfamily Nepetoideae in the Lamiaceae family. The existence of more than 100 active chemicals underlies the pharmacological actions of Salvia essential oils.
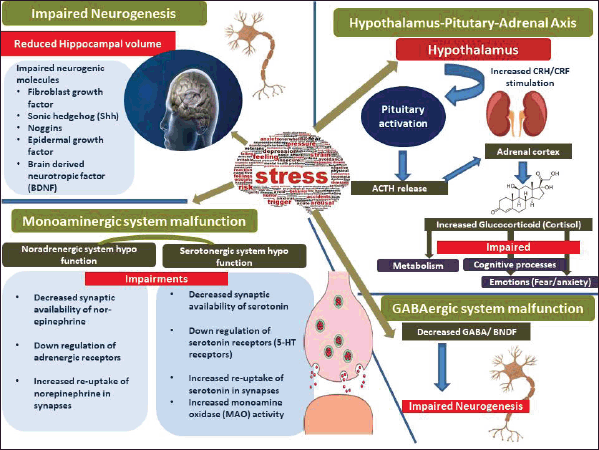 | Figure 1. Basic mechanisms of stress-induced impairments leading to depression. [Click here to view] |
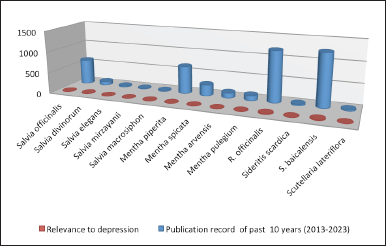 | Figure 2. Number of publications according to PubMed. [Click here to view] |
Salvia officinalis
In terms of active main contents, the species holds the highest value among other species. The aqueous leaf extract at an escalating dosage level of up to 1,000 mg/kg has been reported to dramatically lower the duration of mice immobility [5]. The methanolic extract of S. officinalis extract had effects against depression, exhibited anxiolytic action, and may have bioactive components (phenolic, flavonoid, and tannin) that boosted rat learning [6]. Apigenin, Hispidulin, and Cirsimaritin, as well as the diterpenes 7-methoxyrosmanol and galdosol, are among the components of S. officinalis that have been shown to affect benzodiazepine receptor activation [7]. At 300 mg/kg, both alcoholic and aqueous extracts delayed the start of sleep, and has been suggested that S. officinalis can be used to treat insomnia and anxiety [8].
Salvia divinorum
Recreational users of S. divinorum may experience subjective sensations partly due to Salvinorin A’s anxiolytic and antidepressant-like actions, mediated by both k-opioid and endocannabinoid systems [9]. In a study, anhedonia, which is frequent in depression, was reportedly reversed with SalvA [10].
Salvia elegans
Salvia elegans Vahl (Lamiaceae), sometimes referred to as “mirto” is a plant whose leaves and blooms are used to make an infusion that is used to treat CNS disorders in traditional Mexican medicine including insomnia, depression, and anxiety. Aerial fractions of S. elegans containing oleanolic acid (OA), rosifoliol, and agaraspirol have been reported to exhibit anxiolytic and antidepressant effects [11]. Salvia elegans leaf hydroalcoholic extract (60% ethanol) has been shown to provide sedative and antidepressant effects on mice [12,13]. According to a study ursolic acid and 5-O-(6-rhamnosylglucoside)-7-hydroxy-4’-methoxyflavanone extracted from S. elegans leaves, have antidepressant effects [14].
Salvia mirzayanii and Salvia macrosiphon
Salvia mirzayanii and Salvia macrosiphon extracts showed similar antidepressant efficacy, with the hydroalcoholic extract being more efficient than the aqueous extract [15].
Mentha genus
The genus Mentha contains between 13 and 18 species of plants. The genus is widely distributed in North America, Europe, Africa, Asia, and Australia. Mentha piperita, Mentha spicata, Mentha rotundifolia, Mentha arvensis, and Mentha suaveolens are the most popular and widely grown mints for commercial cultivation.
Mentha piperita
Mentha piperita sometimes known as peppermint, is a hybrid species of Mentha aquatica and Mentha spicata L. The plant is one of the most extensively dispersed species in the Lamiaceae family and is found in Europe, Turkey, and a few areas of West Asia. Leaves contain phenolic acids, triterpenes, luteolin, rutin, hesperidin, and fatty acids, with menthol and menthone being the main components of essential oil [16]. Due to M. piperita’s potential as a monoamine oxidase inhibitor, peppermint may have antidepressant properties. The interactions between the dopaminergic, noradrenergic, and serotonergic receptors may be responsible for this effect [17,18]. Researchers suggest that the fraction containing polyphenols and its primary ingredients, rosmarinic acid, eriocitrin, and luteolin-7-O-rutinoside, may contribute to its biological activity [19].
Mentha spicata
The decoction of spearmint leaves is used to treat biliary diseases, menstrual cramps, stomach discomfort, constipation, gingivitis, and ondotalgies. Mentha spicata teas are commonly used by Colombian communities to alleviate stress and insomnia [20]. The Elevated Plus-Maze test showed increased open arm time and longer sleep time in mice treated with M. spicata extract and sodium pentobarbital [21]. The carvone moiety in the essential oil of M. spicata has been considered to contribute toward this activity through a serotonergic mechanism [22]. Recent research evaluated the impact of fresh peppermint leaves and infusions on university students’ mental health. The therapy group’s students reported improved memory, higher sleep quality, and less anxiety [23].
Mentha arvensis
The use of M. arvensis in traditional Korean medicine has been reported to have significant antidepressant potential [24]. Intraperitoneal injections of Japanese mint oil emulsion at dosages of 78, 1,56, and 2,25 l/kg of body weight increased struggle time and also reduced immobility in the Forced swim technique depicting considerable antidepressant and cytotoxic effects [25]. Citronellal, nerol, and linarin are the three primary essential oils extracted from the leaves and flowers of this species. According to in vitro experiments, the α-citronellal has been reported to possess inhibitive activities against the MAO-A enzyme [26].
Mentha pulegium
It is a fragrant plant that is native to America, a member of the Lamiaceae family, and grows in Asia, Ethiopia, Iran, and Western, Southern, and Central Europe. The bulk of the essential oil in this species is composed of a complex mixture of monoterpenes in oxygenated form (76.8%), including menthone, pulegone, neo-menthol, and 8-hydroxy-4(5)-p-menthen-3-one. The species has reportedly been effective in treating anxiety and anxiety symptoms [27]. The length of BALB/c mice immobility in the fast swim test was significantly reduced in a study after M. pulegium administration. The antidepressant effect, however, was not dose-dependent [28].
Rosmarinus genus
The Lamiaceae family’s woody, perennial Rosmarinus plant is native to the Mediterranean Basin and features fragrant, needle-like evergreen leaves.
Rosmarinus officinalis
Rosmarinus officinalis, L., a Mediterranean-native Lamiaceae plant, has a polyphenolic profile consisting of carnosic acid, carnosol, rosmarinic acid, and hesperidin [29]. The chronic administration of hydro-alcoholic extract has been proven to reduce hyperactivity and behavior in olfactory bulbectomized mice [30]. A pentacyclic triterpenoid named ursolic acid from this species has been reported to decrease mice’s immobility in the forced swimming and tail suspension method. In anxiety and depression models, it has been observed that the ethyl acetate, hexane, ethanolic, and essential oil-free fractions, as well as essential oil and the isolated chemicals Saligenin, rosmanol, cirsimaritin, carnosol, and betulinic acid, exhibit CNS effect [31–33]. The extract (distillation residue of essential oil) of R. officinalis, significantly improved depressive and anxiety-like behavior in mice, reversing the alterations in gene expressions caused due to stress [34]. Tyrosine hydroxylase (TH) and pyruvate carboxylase (PC), two important genes were significantly upregulated in PC12 cells treated with R. officinalis polyphenols [35].
Sideritisgenus
More than 150 plant species make up the Sideritis genus, which is mostly found in the Mediterranean region but also occurs in the Atlantic areas, North Africa, and even Norway.
Sideritis scardica
Other names for S. scardica include “Greek tea” and “mountain tea.” The high concentration of flavonoid and phenolic chemicals in S. scardica is thought to be responsible for its pharmacological effects. According to research, Sideritis extract total phenolic content showed potential cognitive, anxiolytic, and antidepressant benefits [36]. Four flavonoids associated with genus Sideritis, include xanthomicrol and isoscutellarein 7-O-[6″′-O-acetyl--D-allopyranosyl-(12)]. Isoscutellarein 7-O-[6″′-O-acetyl--D-allopyranosyl-(12)]--D-glucopyranosideSaligenin and -6″-O-acetyl-D-glucopyranoside have been shown to specifically and permanently block hMAO-A [37]. Sideritis scardica extracts, known for triple monoamine reuptake inhibitors, have the potential for phytochemical therapy in treating mental disorders such as anxiety, depression, attention-deficit hyperactivity disorder, and neurodegenerative diseases [38].
Genus Scutellaria
Scutellaria, has between 360 and 469 recognized species. This genus is responsible for the identification of more than 295 compounds, including flavonoids and diterpenes. Flavonoids and neo-clerodane diterpenoids can be credited with the majority of the bioactivities [39]. Scutellaria is used in various therapeutic settings; however, there has not been much study done on this genus [40].
Scutellaria baicalensis
Flavonoid baicalein from dried roots of this species (Scutellariae radix) inhibits the inflammatory process through peripheral immunological response [41]. Baicalin and baicalein, two of S. baicalensis bioactive ingredients, block MAO A/B and facilitate the release of monoamines, particularly dopamine associated with neurological and psychiatric disorders [42]. Baicalein was found to have effective antidepressant effects which are probably attributed to the suppression of the HMGB1/TLR4/NF-B pathways [43]. It also significantly reduced TLR4 expression, decreased IL-1, IL-6, and TNF- levels in the hippocampus, and improved chronic mild stress-induced depressive-like symptoms [44]. In addition, baicalein has been shown to have antidepressant properties by promoting the differentiation of neurons, their maturation into mature neurons, and their survival [45]. Baicalin treatment significantly enhances hippocampus apoptosis which is thought to contribute to its potent antidepressant effects [46].
Scutellaria lateriflora
The extract of S. lateriflora has been reported to significantly improve global mood without affecting energy or cognition [47]. Baicalin and its aglycone baicalein among flavonoids are known to have anxiolytic activity because of their attachment to the benzodiazepine site of the GABA-A receptor [48].
EVALUATION OF ISOLATED COMPOUNDS IN-VIVO AGAINST DEPRESSION
Except for a few explored genera, there have been very less studies to explore a link between the antidepressant lead moieties and phytoconstituents in different species of the family Lamiaceae. The details of isolated moieties with recent reports on their molecular-level mechanisms against depression have been discussed in Table 1. The published literature from the studied genera indicates that most of the plant-based isolated components as potential candidates against depression fall under the categories of flavones, terpens, terpenoids, polyphenols, or their derivatives. Many of these components mentioned either in the whole plant extracts or in their essential oils have not been evaluated extensively in depression models and present a potential area for further research. For instance, linarin (a glycosylated flavonoid) and Carvone (terpene) were found to be an effective antidepressant principle but their antidepressant mechanism still remains unclear [49,50]. Hispidulin (flavonoid) reported to improve social withdrawal behavior in mice can be further explored for its efficacy and related mechanisms in depressive disorders [51].
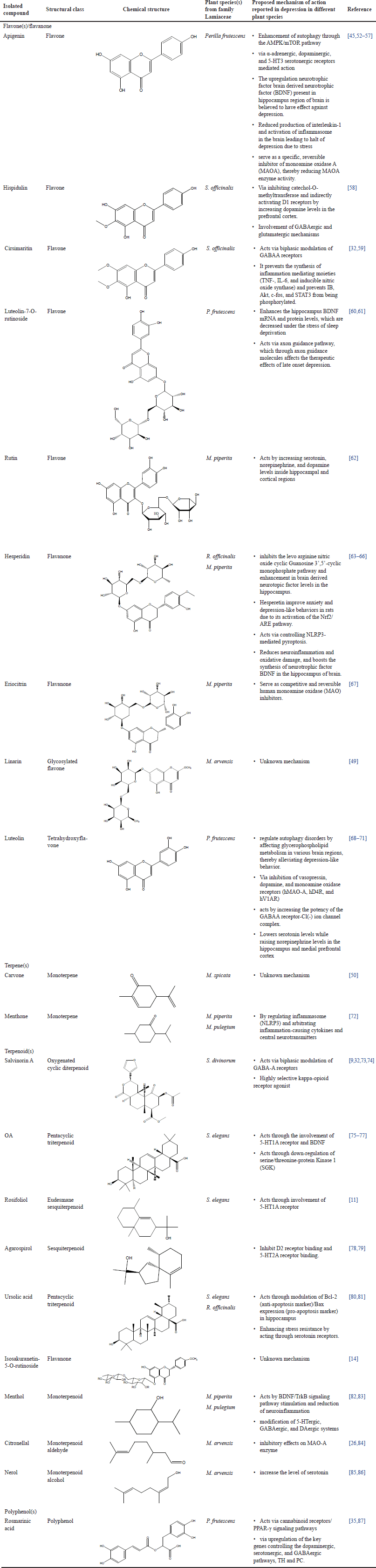 | Table 1. Details on isolated components from different plant species with proposed mechanism of action in depression. [Click here to view] |
FUTURE PERSPECTIVES AND CONCLUSION
This review explores the potential role of medicinal plants in depressive disorders, revealing promising therapeutic agents. Most extracts were nontoxic and comparable to synthetic drugs. However, the data is preliminary and lacks clear cellular and molecular mechanisms. Future studies should focus on detailed molecular mechanisms, dosages, clinical efficacy, and safety of these extracts and isolated compounds.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Sharma VK, Singh TG, Garg N, Dhiman S, Gupta S, Rahman MH, et al. Dysbiosis and Alzheimer’s disease: a role for chronic stress? Biomolecules. 2021 Apr 30;11(5):678. CrossRef
2. Cui R. Editorial: a systematic review of depression. Curr Neuropharmacol. 2015;13(4):480. CrossRef
3. Behl T, Kumar K, Brisc C, Rus M, Nistor-Cseppento DC, Bustea C, et al. Exploring the multifocal role of phytochemicals as immunomodulators. Biomed Pharmacother. 2021 Jan;133:110959. CrossRef
4. Etminan A, Pour-Aboughadareh A, Noori A, Ahmadi-Rad A, Shooshtari L, Mahdavian Z, et al. Genetic relationships and diversity among wild Salvia accessions revealed by ISSR and SCoT markers. Biotechnol Biotechnol Equip. 2018 May 4;32(3):610–7. CrossRef
5. Maliki I, Es-Safi I, El Moussaoui A, Mechchate H, El Majdoub YO, Bouymajane A, et al. Salvia officinalis and Lippia triphylla: chemical characterization and evaluation of antidepressant-like activity. J Pharm Biomed Anal. 2021 Sep 5;203:114207. CrossRef
6. El Gabbas Z, Bezza K, Laadraoui J, Makbal R, Aboufatima R, Chait A. Salvia officinalis induces antidepressant-like effect, anxiolytic activity and learning improvement in hippocampal lesioned and intact adult rats. Bangladesh J Pharmacol. 2018 Dec 21;13(4):367–78. CrossRef
7. Lopresti AL. Salvia (Sage): a review of its potential cognitive-enhancing and protective effects. Drugs R D. 2017 Mar;17(1):53–64. CrossRef
8. Motaghi S, Teimouri M. Investigation of anxiolytic and hypnotic effects of aqueous and hydroalcoholic extracts of Salvia officinalis in adult mice. Iran J Physiol Pharmacol. 2018 Oct 10;2(3):151–44.
9. Braida D, Capurro V, Zani A, Rubino T, Viganò D, Parolaro D, et al. Potential anxiolytic- and antidepressant-like effects of salvinorin A, the main active ingredient of Salvia divinorum, in rodents. Br J Pharmacol. 2009 Jul;157(5):844–53. CrossRef
10. Harden MT, Smith SE, Niehoff JA, McCurdy CR, Taylor GT. Antidepressive effects of the κ-opioid receptor agonist salvinorin A in a rat model of anhedonia. Behav Pharmacol. 2012 Oct;23(7):710–5. CrossRef
11. Martínez-Hernández GB, Jiménez-Ferrer E, González-Cortazar M, Román-Ramos R, Tortoriello J, Vargas-Villa G, et al. Antidepressant and anxiolytic compounds isolated from Salvia elegans interact with serotonergic drugs. Naunyn Schmiedebergs Arch Pharmacol. 2021 Dec;394(12):2419–28. CrossRef
12. Mora S, Millán R, Lungenstrass H, Díaz-Véliz G, Morán JA, Herrera-Ruiz M, et al. The hydroalcoholic extract of Salvia elegans induces anxiolytic- and antidepressant-like effects in rats. J Ethnopharmacol. 2006 Jun 15;106(1):76–81. CrossRef
13. Herrera-Ruiz M, García-Beltrán Y, Mora S, Díaz-Véliz G, Viana GS, Tortoriello J, et al. Antidepressant and anxiolytic effects of hydroalcoholic extract from Salvia elegans. J Ethnopharmacol. 2006;107(1):53–8. CrossRef
14. González-Cortazar M, Maldonado-Abarca AM, Jiménez-Ferrer E, Marquina S, Ventura-Zapata E, Zamilpa A, et al. Isosakuranetin-5-O-rutinoside: a new flavanone with antidepressant activity isolated from Salvia elegans Vahl. Molecules. 2013 Oct 25;18(11):13260–70. CrossRef
15. Sarkoohi P, Fathalipour M, Ghasemi F, Javidnia K, Emamghoreishi M. Antidepressant effects of the aqueous and hydroalcoholic extracts of Salvia mirzayanii and Salvia macrosiphon in male mice. Shiraz E-Med J. 2020 Feb 29;21(2). CrossRef
16. Saharkhiz MJ, Motamedi M, Zomorodian K, Pakshir K, Miri R, Hemyari K. Chemical composition, antifungal and antibiofilm activities of the essential oil of Mentha piperita L. ISRN Pharm. 2012;2012:718645. CrossRef
17. Abbasi-Maleki S, Bakhtiarian A, Nikoui V. Involvement of the monoaminergic system in the antidepressant-like effect of the crude extract of Mentha piperita (Lamiaceae) in the forced swimming test in mice. Synergy. 2017;5:21–8. CrossRef
18. Andisa E, Fitri LL. Peppermint (Mentha piperita) as Antidepressant on male Wistar rat (Rattus norvegicus) exposed to blue light. J Mat Sains. 2019 Sep 30;23(1):1–6. CrossRef
19. Bodalska A, Kowalczyk A, Wlodarczyk M, Fecka I. Analysis of polyphenolic composition of a herbal medicinal product-peppermint tincture. Molecules. 2019 Dec 24;25(1):69. CrossRef
20. Fatiha B, Khodir M, Farid D, Tiziri R, Karima B, Sonia O, et al. Research article optimisation of solvent extraction of antioxidants (phenolic compounds) from Algerian mint (Mentha spicata L.). Pharmacogn Commun. 2012 Oct;2(4):78.
21. Caro DC, Rivera DE, Ocampo Y, Franco LA, Salas RD. Pharmacological evaluation of Mentha spicata L. and Plantago major L., medicinal plants used to treat anxiety and insomnia in Colombian Caribbean Coast. Evid Based Complement Alternat Med. 2018 Aug 7;2018:5921514. CrossRef
22. Jedi-Behnia B, Abbasi Maleki S, Mousavi E. The antidepressant-like effect of Mentha spicata essential oil in animal models of depression in male mice. J Adv Biomed Sci. 2017 May 10;7(1):141–9.
23. Abdelhalim AR. The effect of Mentha piperita L. on the mental health issues of university students: a pilot study. J Pharm Pharmacogn Res. 2021;9:49–57. CrossRef
24. Park B-K, Kim NS, Kim YR, Yang C, Jung IC, Jang I-S, et al. Antidepressant and anti-neuroinflammatory effects of bangpungtongsung-san. Front Pharmacol. 11:958. CrossRef
25. Yousuf T, Akter R, Ahmed J, Mazumdar S, Talukder D, Nandi NC, et al. Evaluation of acute oral toxicity, cytotoxicity, antidepressant and antioxidant activities of Japanese mint (Mentha arvensis L.) oil. Phytomedic Plus. 2021 Nov 1;1(4):100140. CrossRef
26. Kukula-Koch W, Koch W, Czernicka L, Glowniak K, Asakawa Y, Umeyama A, et al. MAO-A inhibitory potential of terpene constituents from ginger rhizomes-a bioactivity guided fractionation. Molecules. 2018 May 29;23(6):1301. CrossRef
27. Sánchez M, González-Burgos E, Iglesias I, Lozano R, Gómez-Serranillos MP. Current uses and knowledge of medicinal plants in the Autonomous Community of Madrid (Spain): a descriptive cross-sectional study. BMC Complement Med Ther. 2020 Oct 14;20(1):306. CrossRef
28. Rabiei Z, Gholami M, Rafieian-Kopaei M. Antidepressant effects of Mentha pulegium in mice. Bangladesh J Pharmacol. 2016 Aug 7;11(3):711–5. CrossRef
29. Tai J, Cheung S, Wu M, Hasman D. Antiproliferation effect of Rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomedicine. 2012 Mar 15;19(5):436–43. CrossRef
30. Farr SA, Niehoff ML, Ceddia MA, Herrlinger KA, Lewis BJ, Feng S, et al. Effect of botanical extracts containing carnosic acid or rosmarinic acid on learning and memory in SAMP8 mice. Physiol Behav. 2016 Oct 15;165:328–38. CrossRef
31. Ghasemzadeh Rahbardar M, Hosseinzadeh H. Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders. Iran J Basic Med Sci. 2020 Sep;23(9):1100–12.
32. Abdelhalim A, Karim N, Chebib M, Aburjai T, Khan I, Johnston GA, et al. Antidepressant, anxiolytic and antinociceptive activities of constituents from Rosmarinus officinalis. J Pharm Sci. 2015;18(4):448–59. CrossRef
33. Machado DG, Cunha MP, Neis VB, Balen GO, Colla A, Bettio LE, et al. Antidepressant-like effects of fractions, essential oil, carnosol and betulinic acid isolated from Rosmarinus officinalis L. Food Chem. 2013 Jan 15;136(2):999–1005. CrossRef
34. Sasaki K, Ferdousi F, Fukumitsu S, Kuwata H, Isoda H. Antidepressant- and anxiolytic-like activities of Rosmarinus officinalis extract in rodent models: involvement of oxytocinergic system. Biomed Pharmacother. 2021 Dec;144:112291. CrossRef
35. Sasaki K, El Omri A, Kondo S, Han J, Isoda H. Rosmarinus officinalis polyphenols produce anti-depressant like effect through monoaminergic and cholinergic functions modulation. Behav Brain Res. 2013 Feb 1;238:86–94. CrossRef
36. Kokras N, Poulogiannopoulou E, Sotiropoulos MG, Paravatou R, Goudani E, Dimitriadou M, et al. Behavioral and neurochemical effects of extra virgin olive oil total phenolic content and sideritis extract in female mice. Molecules. 2020 Oct 28;25(21):5000. CrossRef
37. Turkmenoglu FP, Baysal I, Ciftci-Yabanoglu S, Yelekci K, Temel H, Pasa S, et al. Flavonoids from Sideritis species: human monoamine oxidase (hMAO) inhibitory activities, molecular docking studies and crystal structure of xanthomicrol. Molecules. 2015 Apr 23;20(5):7454–73. CrossRef
38. Knörle R. Extracts of Sideritis scardica as triple monoamine reuptake inhibitors. J Neural Transm (Vienna). 2012 Dec;119(12):1477–82. CrossRef
39. Zehravi M, Karthika C, Azad AK, Ahmad Z, Khan FS, Rahman MS, et al. A background search on the potential role of Scutellaria and its essential oils. Biomed Res Int. 2022 Jul 27;2022:7265445. CrossRef
40. Shen J, Li P, Liu S, Liu Q, Li Y, Sun Y, et al. Traditional uses, ten-years research progress on phytochemistry and pharmacology, and clinical studies of the genus Scutellaria. J Ethnopharmacol. 2021 Jan 30;265:113198. CrossRef
41. Liu HT, Lin YN, Tsai MC, Wu YC, Lee MC. Baicalein exerts therapeutic effects against endotoxin-induced depression-like behavior in mice by decreasing inflammatory cytokines and increasing brain-derived neurotrophic factor levels. Antioxidants (Basel). 2022 May 11;11(5):947. CrossRef
42. Limanaqi F, Biagioni F, Busceti CL, Polzella M, Fabrizi C, Fornai F. Potential Antidepressant effects of Scutellaria baicalensis, Hericium erinaceus and Rhodiola rosea. Antioxidants (Basel). 2020 Mar 12;9(3):234. CrossRef
43. Liu L, Dong Y, Shan X, Li L, Xia B, Wang H. Anti-depressive effectiveness of baicalin in vitro and in vivo. Molecules. 2019 Jan 17;24(2):326. CrossRef
44. Guo LT, Wang SQ, Su J, Xu LX, Ji ZY, Zhang RY, et al. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J Neuroinflammation. 2019 May 8;16(1):95. CrossRef
45. Zhang R, Ma Z, Liu K, Li Y, Liu D, Xu L, et al. Baicalin exerts antidepressant effects through Akt/FOXG1 pathway promoting neuronal differentiation and survival. Life Sci. 2019 Mar 15;221:241–8. CrossRef
46. Yu HY, Yin ZJ, Yang SJ, Ma SP, Qu R. Baicalin reverses depressive-like behaviours and regulates apoptotic signalling induced by olfactory bulbectomy. Phytother Res. 2016 Mar;30(3):469–75. CrossRef
47. Brock C, Whitehouse J, Tewfik I, Towell T. American skullcap (Scutellaria lateriflora): a randomised, double-blind placebo-controlled crossover study of its effects on mood in healthy volunteers. Phytother Res. 2014 May;28(5):692–8. CrossRef
48. Awad R, Arnason JT, Trudeau V, Bergeron C, Budzinski JW, Foster BC, et al. Phytochemical and biological analysis of skullcap (Scutellaria lateriflora L.): a medicinal plant with anxiolytic properties. Phytomedicine. 2003 Nov;10(8):640–9. CrossRef
49. Guzmán-Gutiérrez SL, Reyes-Chilpa R, González-Diego LR, Silva-Miranda M, López-Caamal A, García-Cruz KP, et al. Five centuries of Cirsium ehrenbergii Sch Bip. (Asteraceae) in Mexico, from Huitzquilitl to Cardo Santo: history, ethnomedicine, pharmacology and chemistry. J Ethnopharmacol. 2023 Jan 30;301:115778. CrossRef
50. Fonseca ECM, Ferreira LR, Figueiredo PLB, Maia CDSF, Setzer WN, Da Silva JKR. Antidepressant effects of essential oils: a review of the past decade (2012-2022) and molecular docking study of their major chemical components. Int J Mol Sci. 2023 May 25;24(11):9244. CrossRef
51. Mouri A, Lee HJ, Mamiya T, Aoyama Y, Matsumoto Y, Kubota H, et al. Hispidulin attenuates the social withdrawal in isolated disrupted-in-schizophrenia-1 mutant and chronic phencyclidine-treated mice. Br J Pharmacol. 2020 Jul;177(14):3210–24. CrossRef
52. Zhang L, Lu RR, Xu RH, Wang HH, Feng WS, Zheng XK. Naringenin and apigenin ameliorates corticosterone-induced depressive behaviors. Heliyon. 2023 Apr 20;9(5):e15618. CrossRef
53. Olayinka JN, Akawa OB, Ogbu EK, Eduviere AT, Ozolua RI, Soliman M. Apigenin attenuates depressive-like behavior via modulating monoamine oxidase a enzyme activity in chronically stressed mice. Curr Res Pharmacol Drug Discov. 2023 Jul 11;5:100161. CrossRef
54. Al-Yamani MJ, Mohammed Basheeruddin Asdaq S, Alamri AS, Alsanie WF, Alhomrani M, Alsalman AJ, et al. The role of serotonergic and catecholaminergic systems for possible antidepressant activity of apigenin. Saudi J Biol Sci. 2022 Jan;29(1):11–7. CrossRef
55. Kalivarathan J, Kalaivanan K, Chandrasekaran SP, Nanda D, Ramachandran V, Venkatraman AC. Apigenin modulates hippocampal CREB-BDNF signaling in high fat, high fructose diet-fed rats. J Funct Foods. 2020 May 1;68:103898. CrossRef
56. Weng L, Guo X, Li Y, Yang X, Han Y. Apigenin reverses depression-like behavior induced by chronic corticosterone treatment in mice. Eur J Pharmacol. 2016 Mar 5;774:50–4. CrossRef
57. Li X, Han Y, Zhou Q, Jie H, He Y, Han J, et al. Apigenin, a potent suppressor of dendritic cell maturation and migration, protects against collagen-induced arthritis. J Cell Mol Med. 2016 Jan;20(1):170–80. CrossRef
58. Abdelhalim, Abeer, Imran Khan, Nasiara Karim. The contribution of ionotropic gabaergic and N-methyl-D-Aspartic acid receptors in the antidepressant-like effects of hispidulin. Pharmacogn Mag. 2019;15(62):62.
59. Cai Y, Zheng Q, Sun R, Wu J, Li X, Liu R. Recent progress in the study of Artemisiae scopariae Herba (Yin Chen), a promising medicinal herb for liver diseases. Biomed Pharmacother. 2020 Oct;130:110513. CrossRef
60. Ryu D, Jee HJ, Kim SY, Hwang SH, Pil GB, Jung YS. Luteolin-7-O-glucuronide improves depression-like and stress coping behaviors in sleep deprivation stress model by activation of the BDNF signaling. Nutrients. 2022 Aug 12;14(16):3314. CrossRef
61. Liu K, Li H, Zeng N, Li B, Yao G, Wu X, et al. Exploration of the core pathways and potential targets of luteolin treatment on late-onset depression based on cerebrospinal fluid proteomics. Int J Mol Sci. 2023 Feb 9;24(4):3485. CrossRef
62. Foudah AI, Alqarni MH, Alam A, Devi S, Salkini MA, Alam P. Rutin improves anxiety and reserpine-induced depression in rats. Molecules. 2022 Oct 27;27(21):7313. CrossRef
63. Cao H, Yang D, Nie K, Lin R, Peng L, Zhou X, et al. Hesperidin may improve depressive symptoms by binding NLRP3 and influencing the pyroptosis pathway in a rat model. Eur J Pharmacol. 2023 Aug 5;952:175670. CrossRef
64. Li S, Zhu J, Pan L, Wan P, Qin Q, Luo D, et al. Potential protective effect of hesperidin on hypoxia/reoxygenation?induced hepatocyte injury. Exp Ther Med. 2021;22(1):1–9. CrossRef
65. Kosari-Nasab M, Shokouhi G, Ghorbanihaghjo A, Abbasi MM, Salari AA. Hesperidin attenuates depression-related symptoms in mice with mild traumatic brain injury. Life Sci. 2018 Nov 15;213:198–205. CrossRef
66. Donato F, de Gomes MG, Goes AT, Filho CB, Del Fabbro L, Antunes MS, et al. Hesperidin exerts antidepressant-like effects in acute and chronic treatments in mice: possible role of l-arginine-NO-cGMP pathway and BDNF levels. Brain Res Bull. 2014 May;104:19–26. CrossRef
67. Carradori S, Gidaro MC, Petzer A, Costa G, Guglielmi P, Chimenti P, et al. Inhibition of human monoamine oxidase: biological and molecular M deling studies on selected natural flavonoids. J Agric Food Chem. 2016 Nov 30;64(47):9004–11. CrossRef
68. Wu X, Xu H, Zeng N, Li H, Yao G, Liu K, et al. Luteolin alleviates depression-like behavior by modulating glycerophospholipid metabolism in the hippocampus and prefrontal cortex of LOD rats. CNS Neurosci Ther. 2023 Sep 16;00:1–16. CrossRef
69. Park SE, Paudel P, Wagle A, Seong SH, Kim HR, Fauzi FM, et al. Luteolin, a potent human monoamine oxidase-a inhibitor and dopamine D4 and vasopressin V1A receptor antagonist. J Agric Food Chem. 2020 Sep 30;68(39):10719–29. CrossRef
70. Huang L, Kim MY, Cho JY. Immunopharmacological activities of luteolin in chronic diseases. Int J Mol Sci. 2023 Jan 21;24(3):2136. CrossRef
71. De la Peña JB, Kim CA, Lee HL, Yoon SY, Kim HJ, Hong EY, et al. Luteolin mediates the antidepressant-like effects of Cirsium japonicum in mice, possibly through modulation of the GABAA receptor. Arch Pharm Res. 2014 Feb;37(2):263–9. CrossRef
72. Xue J, Li H, Deng X, Ma Z, Fu Q, Ma S. L-Menthone confers antidepressant-like effects in an unpredictable chronic mild stress mouse model via NLRP3 inflammasome-mediated inflammatory cytokines and central neurotransmitters. Pharmacol Biochem Behav. 2015 Jul;134:42–8. CrossRef
73. Cruz A, Domingos S, Gallardo E, Martinho A. A unique natural selective kappa-opioid receptor agonist, salvinorin A, and its roles in human therapeutics. Phytochemistry. 2017 May;137:9–14. CrossRef
74. Beguin C, Potter DN, DiNieri JA, Munro TA, Richards MR, Paine TA, et al. N-methylacetamide analog of salvinorin A: a highly potent and selective κ-opioid receptor agonist with oral efficacy. J Pharmacol Exp Ther. 2008 Jan 1;324(1):188–95. CrossRef
75. Fajemiroye JO, Polepally PR, Chaurasiya ND, Tekwani BL, Zjawiony JK, Costa EA. Oleanolic acid acrylate elicits antidepressant-like effect mediated by 5-HT1A receptor. Sci Rep. 2015 Jul 22;5:11582. CrossRef
76. Yi LT, Li J, Liu Q, Geng D, Zhou YF, Ke XQ, et al. Antidepressant-like effect of oleanolic acid in mice exposed to the repeated forced swimming test. J Psychopharmacol. 2013 May;27(5):459–68. CrossRef
77. Dong SQ, Wang SS, Zhu JX, Mu RH, Li CF, Geng D, et al. Oleanolic acid decreases SGK1 in the hippocampus in corticosterone-induced mice. Steroids. 2019 Sep;149:108419. CrossRef
78. Wang S, Yu Z, Wang C, Wu C, Guo P, Wei J. Chemical constituents and pharmacological activity of agarwood and Aquilaria plants. Molecules. 2018 Feb 7;23(2):342. CrossRef
79. Okugawa H, Ueda R, Matsumoto K, Kawanishi K, Kato K. Effects of sesquiterpenoids from “Oriental incenses” on acetic acid-induced writhing and D2 and 5-HT2A receptors in rat brain. Phytomedicine. 2000 Oct;7(5):417–22. CrossRef
80. Colla ARS, Pazini FL, Lieberknecht V, Camargo A, Rodrigues ALS. Ursolic acid abrogates depressive-like behavior and hippocampal pro-apoptotic imbalance induced by chronic unpredictable stress. Metab Brain Dis. 2021 Mar;36(3):437–46. CrossRef
81. Naß J, Abdelfatah S, Efferth T. Ursolic acid enhances stress resistance, reduces ROS accumulation and prolongs life span in C. elegans serotonin-deficient mutants. Food Funct. 2021 Mar 7;12(5):2242–56. CrossRef
82. Zhu SM, Xue R, Chen YF, Zhang Y, Du J, Luo FY, et al. Antidepressant-like effects of L-menthol mediated by alleviating neuroinflammation and upregulating the BDNF/TrkB signaling pathway in subchronically lipopolysaccharide-exposed mice. Brain Res. 2023 Oct 1;1816:148472. CrossRef
83. Wang W, Jiang Y, Cai E, Li B, Zhao Y, Zhu H, et al. L-menthol exhibits antidepressive-like effects mediated by the modification of 5-HTergic, GABAergic and DAergic systems. Cogn Neurodyn. 2019 Apr;13(2):191–200. CrossRef
84. Victoria FN, Anversa R, Penteado F, Castro M, Lenardão EJ, Savegnago L. Antioxidant and antidepressant-like activities of semi-synthetic α-phenylseleno citronellal. Eur J Pharmacol. 2014 Nov 5;742:131–8. CrossRef
85. Zhang Y, Long Y, Yu S, Li D, Yang M, Guan Y, et al. Natural volatile oils derived from herbal medicines: a promising therapy way for treating depressive disorder. Pharmacol Res. 2021 Feb;164:105376. CrossRef
86. Lei G, Gao G, Zhou M, Guo J, Chen Y. Water-soluble essential oil components of flowers of Paeonia × suffruticosa cultivars and in silico analysis with antidepressant targets. Nat Prod Res. 2023 May 31:1–4. CrossRef
87. Lataliza AAB, de Assis PM, da Rocha Laurindo L, Gonçalves ECD, Raposo NRB, Dutra RC. Antidepressant-like effect of rosmarinic acid during LPS-induced neuroinflammatory model: the potential role of cannabinoid receptors/PPAR-γ signaling pathway. Phytother Res. 2021 Dec;35(12):6974–89. CrossRef