INTRODUCTION
Phyllidia varicosa is a species of sea slug or nudibranch in the family Phyllidiidae that often contains defensive allomones. The animal is characterized by yellow and blue–grey color as tuberculate notal ridge and black longitudinal color as foot stripe. It is interesting to note that the color and shape of P. varicosa are adopted by the juvenile of sea cucumber Pearsonothuria graeffei as Batesian mimicry to survive predators. The nudibranch P. varicosa is ecologically known to biosynthesize a toxic compound by warning coloration or mucus secretion. The toxic compound is a result of sequestering specific sponge metabolites either as intact chemical structures or with transformations and then used as a chemical defense by the nudibranch.
A representative group of toxic compounds from P. varicosa was reported as nitrogenous sesquiterpenes [1] which can be obtained from different collection sites including Hawaii, Sri Lanka, the Philippines, Japan, and Indonesia. For example, Hawaiian specimens of P. varicosa consisted of 9-isocyanopupukeanane [2] and 2-isocyanopupukeanane [3], while Sri Lanka specimens contained 3-isocyanotheonellin [4]. Two molecules were isolated from Philippines specimens of P. varicosa as 4α-isocyanogorgon-11-ene and 4α-formamidogorgon-11-en [5]. Japanese and Indonesian specimens contained 10-isocyano-4-cadinene [6] and 9-thiocyanatopupukeanane [7], respectively. In contrast, only a few steroid compounds are distributed into six species of nudibranch including Dendrodoris fumata [8], Doriprismatica atromarginata [9], Phyllidiella pustolosa [10], Aldisa smaragdina [11], Diaulula sandiegensis [12], and Aldisa sanguinea cooperi [13] have been reported. The presence of steroids in the nudibranch may have a certain role in their metabolisms which are difficult to identify as special chemicals of ecological significance since they are common secondary metabolites in all types of living organisms [14]. However, dendrodoristerol isolated from the Vietnamese nudibranch D. fumata showed therapeutic potential against six cancer cell lines with IC50 21.63, 22.22, 24.53, 41.19, 25.34, and 21.59 μM, respectively [8].
In our quest for bioactive compounds from Indonesian marine organisms [15−19], we encountered an active hexane layer of P. varicosa with LC50 4.67 ± 0.91 μg/mL against brine shrimp lethality assay and purified the layer to give a merosteroid with a flexible side chain that we named varicosenone (1a). The structure includes stereochemical assignment which is the subject of this article.
MATERIAL AND METHODS
General
The 1H and 13C-NMR including correlated spectroscopy (COSY), heteronuclear single quantum coherence (HSQC), heteronuclear multiple bond correlation (HMBC), and nuclear Overhauser effect spectroscopy (NOESY) spectra were recorded on a Bruker Avance III-500 spectrometer. The 13C NMR chemical shifts were deduced by analyzing HSQC and HMBC spectra. The high-resolution electrospray ionization mass spectrometry (HRESIMS) data were recorded on a JEOL JMS-T1000GCV spectrometer with 2000 V as the default ionization voltage. The Shimadzu HPLC was used with Prominence LC-20AD, DGU-20A5, SPD-20A with UV detector λ 254 nm using HPLC column Cosmosil 5SL-II (normal silica, 20 mm I.D. × 250 mm). The column chromatography used a Merck silica gel 60 (0.063–0.20 mm), while the analytical thin layer chromatography (TLC) was performed on a Merck silica gel 60 F254 visualized with CeSO4. All chemical used were reagent grade as received.
Animal Material
Three specimens of P. varicosa (30 g) were collected from Jakarta, Banten, and South Sulawesi, Indonesia, while scuba diving (a depth of 10–15 m). It was then stored in EtOH. The sea slugs were recognized as P. varicosa sp. by one of us (Prof. Junichi Tanaka). The identification was also made based on the comparison of specimens with the characteristics of P. varicosa reported in Indo-Pacific nudibranchs book [20].
Extraction and Isolation
The newly collected sea slug specimens (wet weight, 30 g) stored in EtOH were extracted three times using MeOH (3 × 50 mL). The four solutions were pooled and concentrated under vacuum, and the resulting residue was partitioned between EtOAc and H2O. The EtOAc layer was then partitioned between hexane and 90% MeOHaq to give a stronger cytotoxic hexane layer. Purification of hexane layer (111 mg) by column chromatography [normal silica, n-Hexane:EtOAc (7:3)] followed by normal silica HPLC [n-Hexane:EtOAc (9:1)] gave varicosenone (1a) (0.96 mg) as a minor constituent, colorless oil; Rf : 0.71 n-hexane:EtOAc (7:3).
Cytotoxicity Assay
The cytotoxicity testing was conducted as previously reported [21]. Eggs of brine shrimp (Artemia salina) obtained from the commercial product were incubated in artificial seawater at 25 °C for 24 hours. A total of 10 hatched larvae were placed in a well of 24-well plate with brine (1 mL). The test sample was prepared with a series concentration of 2.5; 5; 25; 100 μg/mL and the plates were kept at 25 °C. DMSO was used as solvent to dissolve the material. The mobility of the larvae was observed for 24 and 48 hours to count number of live larvae. An individual without any motion during the observation was considered as dead. Samples were measured in triplicate. DMSO was used as control negative, while swinholide A, latrunculin A, laulimalide and paclitaxel were used as control positive. The LC50 was calculated using IBM SPSS 22 software and expressed in μg/mL together with its standard deviation (SD).
Calculation Study
The computational component was conducted with Spartan’20 software [22] utilizing the default NMR Spectrum protocol, which has shown success in aiding in structural assignment over a broad range of natural products [23]. A potential candidate stereoisomer was sketched in 2D, in this case, it was the structure 1a with C18 and C21 designated as R and S, respectively. Experimental 13C shifts were assigned based on NMR results. The remaining 3 possible stereo isomers (1b, 1c, and 1d) were generated, and the collection was submitted to the following computational NMR protocol, generating, refining, and establishing Boltzmann weighted NMR shifts for each candidate: (1) A conformational distribution was performed utilizing the MMFF molecular mechanics model and an initial conformer distribution was established, (2) Further equilibrium geometry calculations were performed using the HF/3-21G model, (3) and (4) energy, and equilibrium geometry calculations were made with the DFT model ωB97X-D/6-31G*, increasing the accuracy of the conformer distribution (and reducing the energy window of the distribution) with each step, culminating in a Boltzmann distribution with energies from (5) ωB97X-V/6-311+G(2df,2p)[6-311G*], and (6) NMR shifts determined from the same ωB97X-D/6-31G* model used in steps 3 and 4. Weighted 13C shifts for each of the 4 stereoisomers (1a, 1b, 1c, and 1d), were statistically compared to the experiment. Statistical data including mean and max absolute error, RMS error, and DP4 (%), indicated a preference for two of the four (1a and 1b). Experimental 1H shifts were added to the proton on C17 and the flexible side chain (C18 through C26), and the revised statistical comparison (against experimental shifts) reinforced the preference for 1a and 1b. DP4 results (Table 3) suggest 1a as the likely configuration.
 | Table 1. Cytotoxic activity against brine shrimp Artemia salina. [Click here to view] |
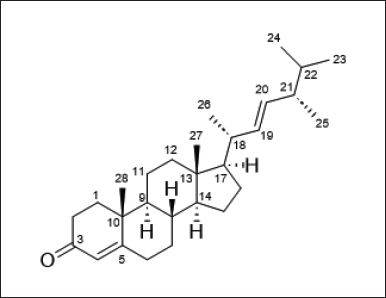 | Figure 1. Chemical structure of varicosenone (1a) [Click here to view] |
RESULTS AND DISCUSSION
Three specimens of P. varicosa were collected at Banten, Jakarta, and South Sulawesi and were exhaustively extracted with EtOH and MeOH. After concentration, the residue was partitioned between EtOAc and H2O. The EtOAc extract was then partitioned between hexane and 90% MeOH aqueous. The hexane layer showed significant toxicity against brine shrimp lethality assay with LC50 4.67±0.91 μg/mL, while the 90% MeOH aqueous showed LC50 > 10 μg/mL (Table 1). Thus, the hexane layer was purified on normal silica gel followed by HPLC using normal silica as a stationary phase to afford varicosenone, 1a (Fig. 1).
Varicosenone (1a) isolated as a minor constituent of P. varicosa had a molecular formula C28H44O by HRESIMS indicating seven degrees of unsaturation, m/z 397.3442 [M + H]+ (calcd. for C28H45O+ 397.3465). The planar structure of 1a was determined on the basis of spectral evidence including 1H, 13C, COSY, HSQC, and HMBC as well as by comparing with the synthetic molecule 24-methylcholesta-4,22-dien-3-one as reported by Wright et al. [24]. The NMR chemical shift assignment of varicosenone (1a) can be seen in Table 2. To confirm the relative configuration of 1a, especially in the rigid part, the NOESY spectrum of 1a was measured. The elucidation relative configuration of 1a especially for cyclic rings was revealed. The presence of two angular methyl groups in the steroid moiety was the same face and adopted trans ring junctions as in Figure 2.
The remaining task was to determine the relative configuration of 1a in the flexible side chain. Of the four 1a−1d possibilities (Fig. 3B), we were able to narrow into two possibilities of stereoisomers by comparing experimental results with calculated results using DFT-based NMR calculations and the NMR Spectrum protocol [23] in Spartan’20 software. The DP4 C (%) was 35.6 for 1a and 29.9 for 1b, respectively, while 1c and 1d gave DP4 C (%) 18.2 and 16.3, respectively. While not conclusive, there did appear a preference toward either 1a or 1b. Experimental 1H shifts were added for the proton on C17 and flexible portion from C18 to C26 which showed a high degree of uncertainty in relative configuration on the two chiral carbons (C18 and C21), and statistical analysis with DP4 scores were again performed. Results showed that while both 1a or 1b remained viable candidates, the likelihood of 1c and 1d was significantly reduced (statistically insignificant). Further, both DP4 H (%) and DP4 (H+C) (%) favored the same stereoisomer, 1a, by approximately 2 to 1, 36% and 32% for the 18S*, 21S* configuration and 64% and 68% for the 18R*, 21S* stereoisomer. The results of the four stereoisomers can be seen in Table 3. Because of the small amount of material, we were not able to confirm the cytotoxicity for the pure material.
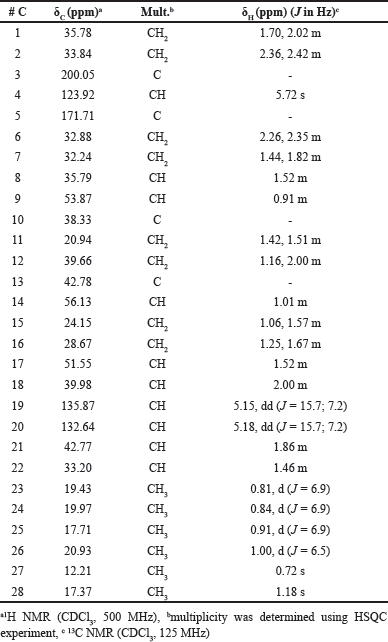 | Table 2. NMR Data for varicosenone (1a). [Click here to view] |
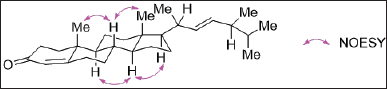 | Figure 2. Relative configuration of 1a especially in cyclic rings revealed by elucidation of NOESY spectrum. [Click here to view] |
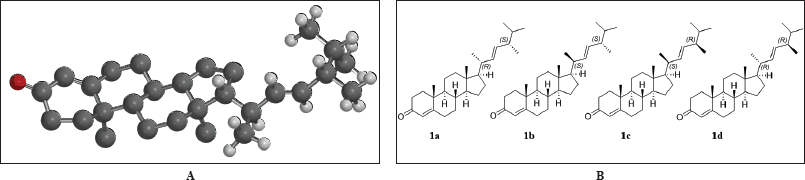 | Figure 3. 3D image of the system (hydrogens visible where experimental proton shifts were included (A). The four possibilities of stereoisomers 1a–d in the flexible side chain (B) [Click here to view] |
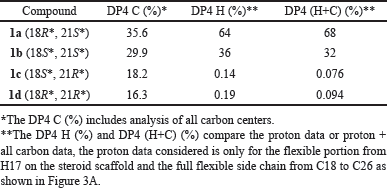 | Table 3. DP4 summary for C (%), H (%), H+C (%) for 1a, 1b, 1c, and 1d. [Click here to view] |
Although compound 1a has been reported as a commercial compound [24] and isolated from P. pustulosa [10]. The relative configuration of 1a remained to be established, especially in the flexible portion. Previous structure determination including stereochemical elucidation for this type of steroid has been done using chemical correlation of the related compounds [25]. There was no stereochemical determination for 1a. To the best of our knowledge, the stereochemical determination of the steroid class compound featured with a flexible side chain using DFT-based NMR calculations is a new approach. Biosynthetically, 1a is newly grouped as a merosteroid or more generally as a meroterpenoid because the compound has a mixed biosynthetic pathway [26]. Extra carbon atoms at C21 arose through the S-adenosylmethionine mechanism [27]. The side chain of 1a containing methyl group at C21 was unique providing significantly improved bioactive properties such as selectivity, solubility, half-life, and binding affinity of small molecule drugs [27]. In addition, the chemical metabolite 1a was discovered for the first time in the genus of Phyllidia mollusk.
CONCLUSION
In conclusion, our effort to isolate a minor constituent of P. varicosa gave varicosenone 1a, a merosteroid in a minute amount. The planar structure was known, while the stereochemical determination was new. The relative configurations of cyclic portion 1a were determined using the NOESY spectrum, while the flexible portion was elucidated using DFT-based NMR calculations for the possible stereoisomers for the two-chiral centers in the flexible portion as 18R*, 21S*. Therefore, the relative configuration of 1a was 8S*, 9S*, 10R*, 13R*, 14S*, 17R*, 18R*, 21S*. Varicosenone (1a) was discovered for the first time in the genus of Phyllidia.
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments: NH and JT. Performed the experiments: NH, BSN, WSO, TAT, HDY, FFD, DTR, AS, AM, and JT. Analyzed the data: NH, BSN, WSO, HDY, FFD, AM, and JT. Wrote the paper: NH, WSO, and JT.
FINANCIAL SUPPORT
This work was supported by the Ministry of Education, Culture, Research and Technology of the Republic of Indonesia.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Chan W, Shaughnessy AE, van den Berg C, Garson MJ, Cheney KL. The validity of brine shrimp (Artemia sp.) to assess the ecological function of marine natural products. J Chem Ecol. 2021;47:83446. CrossRef
2. Burreson BJ, Scheuer PJ, Finer J, Clardy J. 9-Isocyanopupukeanane, a marine invetebrate allomone with a new sesquiterpene skeleton. J Am Chem Soc. 1975;97:4763−64. CrossRef
3. Hagadone MR, Burreson BJ, Scheuer PJ, Finer JS, Clardy J. Defense allomones of the nudibranch Phyllidia varicosa Lamarch 1801. Helv Chim Acta. 1979;62:2848−94. CrossRef
4. Gulavita NK, de Silva ED, Hagadone MR, Karuso P, Scheuer PJ, van Duyne GD, Clardy J. Nitrogenous bisabolene sesquiterpenes from marine invetebrates. J Org Chem. 1986;51:5136−39. CrossRef
5. Kassühlke KE, Potts BCM, Faulkner DJ. New nitrogenous sesquiterpenes from two phillippine nudibranchs, Phyllidia pustulosa and P. varicosa, and from a Palauan sponge, Halichondria cf. lendenfeldi. J Org Chem. 1991;56:3747−50. CrossRef
6. Okino T, Yoshimura E, Hirota H, Fusetani N. New antifouling sesquiterpenes from four nudibranch of the family Phyllididae. Tetrahedron. 1996;52:9447−54. CrossRef
7. Yasman, Edrada RA, Wray V, Proksch P. New 9-thiocyanatopupukeanane sesquiterpenes from the nudibranch Phyllidia varicosa and its sponge-prey Axinyssa aculeata. J Nat Prod. 2003;66: 151214. CrossRef
8. Huong PTM, Phong NV, Thao NP, Binh PT, Thao DT, Thanh NV, Cuong NX, Nam NH, Thung DC, Minh CV. Dendrodoristerol, a cytotoxic C20 steroid from the Vietnamese nudibranch mollusk Dendrodoris fumata. J Asian Nat Prod Res. 2020;22:193−200. CrossRef
9. Kolesnikova SA, Lyakhova EG, Diep ChN, Tu VA, Huong PhT, Kalinovskii AI, Dmitrenok PS, Nam NH, Stonik VA. Stereoidal metabolites from the Vietnamese nudibranch mollusk Doriprismatica atromarginata. Chem Nat Compd. 2017;53:194−95. CrossRef
10. Lyakhova EG, Kolesnikova SA, Kalinovskii AI, Stonik VA. Secondary metabolites of the Vietnamese nudibranch mollusk Phyllidiella pustulosa. Chem Nat Compd. 2010;46:534−38. CrossRef
11. Gavagnin M, Ungur N, Mollo E, Templado J, Cimino G. Structure and synthesis of a progesterone homologue from the skin of the dorid nudibranch Aldisa smaragdina. Eur J Org Chem. 2002;9:1500−504. CrossRef
12. Williams DE, Ayer SW, Andersen RJ. Diaulusterols A and B from the skin extracts of the dorid nudibranch Diaulula sandiegensis. Can J Chem. 1986;64:1527−29. CrossRef
13. Ayer SW, Andersen RJ. Steroidal antifeedants from the dorid nudibrach Aldisa sanguinena cooperi. Tetrahedron Lett. 1982;23:1039−1042. CrossRef
14. Chen ZH, Guo, YW, Li, XW. Recent advances on marine mollusk-derived natural products: chemistry, chemical ecology, and therapeutical potential. Nat Prod Rep. 2023; 40:509−56. CrossRef
15. Hanif N, Murni A, Tanaka C, Tanaka J. Marine natural products from Indonesian waters. Mar Drugs. 2019;17:364. CrossRef
16. Hanif N, Murni A, Tanaka J. Sangiangols A and B, two new dolabellanes from an Indonesian marine soft coral, Anthelia sp. Molecules. 2020;25:3803. CrossRef
17. Hanif N, Dinelsa FF, Yanti HD, Murni A, Tanaka J. Stereochemical determination of NMR chemical shifts in marine terpenoids, antheliol and sangiangol B, using DFT calculations. Nat Prod Res. 2023;37:3170−76. CrossRef
18. Hanif N, Ardianti R, Ahmadi P, Setiawan A, Mohamad K, de Voogd NJ, Murni A, Tanaka J. Ichthyotoxic principles against zebrafish embryos from the Indonesian marine sponge Neopetrosia chaliniformis. J App Pharm Sci. 2018;8:044−048. CrossRef.
19. Hanif N, Ardan MS, Tohir D, Setiawan A, de Voogd NJ, Farid M, Murni A, Tanaka J. Polybrominated diphenyl ethers with broad spectrum antibacterial activity from the Indonesian marine sponge Lamellodysidea herbacea. J App Pharm Sci. 2019;9:001−006. CrossRef.
20. Gosliner TM, Valdés Á, Behrens DW. Nudibranch and sea slug identification Indo-Pacific. Jacksonville, FL: New World Publications, Inc.; 2015.
21. Sakai R, Tanano K, Ono T, Kitano M, Iida Y, Nakano K, Jimbo M. Soritesidine, a novel proteinous toxin from the Okinawan marine sponge Spongosorites sp. Mar Drugs. 2019;17:216. CrossRef
22. Wavefunction, Inc. Spartan’20 version 1.1.4 (Irvine, CA), Wavefunction, Inc.
23. Hehre W, Klunzinger P, Deppmeier B, Driessen A, Uchida N, Hashimoto M, Fukushi E, Takata Y. Efficient protocol; for accurately calculating 13C chemical shifts of conformationally flexible natural products: scope, asessment, and limitations. J Nat Prod. 2019;82:2299. CrossRef
24. Wright JLC, McInnes AG, Shimizu S, Smith DG, Walter JA, Idler D, Khalil W. Identification of C-24 alkyl epimers of marine sterols by 13C nuclear magnetic resonance spectroscopy. Can J Chem. 1978;56:1898−903. CrossRef
25. Ha TBT, Kokke WCMC, Djerassi C. Minor sterols of marine invertebrates 37. Isolation of novel coprostanol and 4α-methyl sterol from the tunicate Ascidia nigra. Steroids, 1982;40:433−53. CrossRef
26. Goyer E, Lavaud C, Massiot G. Meroterpenoids? A historical and critical review of this biogenetic determinant. Nat Prod Rep. 2023; 40:1071−77. CrossRef
27. Aberle B, Kowalczyk D, Massini S, Egler-Kemmerer A.-N, Gergel S, Hammer SC, Hauer B. Methylation of unactivated alkenes with engineered methyltransferases to generate non-natural terpenoids. Angew Chem Int Ed. 2023; 62:e202301601. CrossRef