INTRODUCTION
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by elevated blood glucose levels. This complex disease results due to either a defect in the secretion of insulin or insulin action or both [1]. The prevalence of DM is further anticipated to increase by 51% by the year 2045 [2]. The two major factors attributed to DM’s pathogenesis are environmental and genetic factors [3]. Diabetic nephropathy (DN), neuropathy, and retinopathy are microvascular complications while cardiovascular disease, peripheral artery disease, and stroke are manifestations of macrovascular complications [4].
DN is one of the secondary microvascular complications of type 2 diabetic mellitus, a leading cause of end-stage renal disease (ESRD) worldwide [5]. Clinically DN patients present with persistent microalbuminuria (≥30 to 299 mg/g creatinine) which may gradually progress to macroalbuminuria (≥300 mg/g creatinine) and this leads to a fall in estimated glomerular filtration rate (eGFR) causing renal impairment and finally ESRD. As nephropathy advances, the patients depend on renal replacement therapy [6]. The onset of DN is related to abnormal changes in renal hemodynamics, due to the activation of pathways such as protein kinase C, hexosamine biosynthesis, and aldose reductase [7]. It is challenging to understand the etiology of DN as the factors contributing to its beginning are not completely understood to date [8]. The etiology of the onset of DN is associated with gene polymorphism and abnormal pathways that cause abnormal changes in renal hemodynamics [9].
Angiotensin converting enzyme (ACE) gene
The renin-angiotensin-aldosterone system (RAAS) is involved in the pathophysiology of DN by causing the constriction of the glomerular efferent arteriole and increasing the intraglomerular pressure. Angiotensin II is a vasoconstrictor that promotes kidney damage, fibrosis, proliferation, and hypertrophy of renal parenchyma by increasing the renal hydrostatic pressure and ultrafiltration of renal proteins by constriction of post-glomerular arteriole leading to the onset and progression of DN [10]. One of the widely studied gene polymorphisms within RAAS is the ACE gene which is considered to be one of the candidate genes responsible for the genetic predisposition of DN [11]. The ACE gene is located on the 17q23 chromosome containing 26 exons and 25 introns and contains 21 kb base [12]. Studies have shown that single nucleotide polymorphism occurs frequently in the ACE gene and six polymorphism markers have been identified. The most investigated ones are Alu insertion/deletion (I/D) in the 16th intron of the ACE gene [13]. It was Rigat et al. [14] and Rahimi [15] reported the ACE I/D polymorphism of 287–bp sequence of DNA in intron 16 of the gene.
ACE enzyme
The activation of RAAS including angiotensinogen, angiotensin 1, angiotensin 2, angiotensin-converting enzyme, renin, and angiotensin receptor are the factors associated with the onset of DN [16]. The key role of ACE in the enzymatic cascade of generating vasoactive peptides not only influences systemic blood pressure but also influences intrarenal hemodynamics [17].
Diabetic nephropathy
DN is characterized by an increase in renal artery pressure, thickening of the basement membrane, glomerular hyperplasia, and renal dysfunction [18]. Due to renal malfunction, there is an increase in urine protein, an increase in blood pressure, and progressive renal damage leading to the cause of chronic kidney disease and end-stage- renal disease [19]. The early stages of DN are characterized by hyperfiltration and albuminuria that result in a progressive decline in renal function and a decline in the glomerular filtration rate [20]). The risk of DN is strongly attributed to polygenetic factors and there is ongoing research to identify the genetic loci for DN susceptibility. There are many studies that suggest that the double-deletion (DD) polymorphism of the ACE genotype is responsible for the DN disease progression. However, these findings have not been confirmed [21]. There is an increase in the serum ACE levels in patients with DN than the type-2 DM patients which may be a new biomarker to predict DN progression [15]. There is a need to fill the knowledge gap in understanding ACE gene polymorphism and its serum levels in diabetic patients and patients with DN.
Objectives
To compare serum ACE levels in patients with type-2 diabetes with or without nephropathy.
To evaluate the pattern of ACE single nucleotide polymorphism (SNP) rs267604983 gene polymorphism in patients with type-2 diabetes with or without nephropathy.
To find the association between ACE gene polymorphism and various stages of DN.
To find the association between serum ACE levels and various stages of DN.
MATERIAL AND METHODS
In this analytical cross-sectional study, we have enrolled 108 patients who are diagnosed with type-2 diabetes as the control group as per American Diabetic Association criteria without microalbuminuria and 108 DN patients as the case group as per spot microalbuminuria test (Microalbumin assay kit). The study was carried out at the medicine department, of a tertiary care teaching hospital and the Central Research Laboratory of attached medical college. Mangalore, India, from May 2021 to April 2023. Data collection was started after obtaining the Central Ethics Committee’s approval and written informed consent was taken from the study participants. Patients with malignancies and albuminuria due to other causes (nephrotic syndrome, nephritic syndrome, urinary tract infection, clinically diagnosed chronic kidney disease, lupus nephritis, and acute kidney injury) were excluded from the study.
Sample collection
Five milliliters of whole blood were collected with aseptic precautions from the study participants. The sample collected was divided into three parts: 2 ml of whole blood in the plain vial was centrifuged for 10 minutes at 25°C. The serum obtained was stored at −80°C to analyze the serum ACE levels. 2 ml of EDTA whole blood were used for analyzing the genetic polymorphism.
Genotyping
The extraction of genomic DNA from the whole blood was done by phenol-chloroform method and ethanol precipitation method. The polymorphism was analyzed by the polymerase chain reaction and DNA sequencing methods. The SNP for ACE gene polymorphism selected was rs267604983. The primer sequence used was upstream primer 5′AACATCACTGGCACTTGGGT3′ and downstream primer 5′TGGTCCGTCTTTACCCTCCT3′. The initial step of polymerase chain reaction (PCR) was denaturation which was performed at 95°C for 5 minutes, followed by the amplification for 35 cycles at 95°C for 30 seconds. The annealing temperature was set for 30 seconds at 72°C. The final step of PCR was an extension which was done for 30 seconds at 72°C. The PCR product was then subjected for DNA sequencing results has been shown (Fig. 1).
Measurement of serum ACE levels
The serum ACE levels of the samples were measured by using an ELISA kit (Fine Test, Wuhan Fine Biotech Co., Ltd) according to the manufacturer’s protocol. The standards and the serum samples were added to a 96-well plate. Biotinylated ACE antibody was added to sample wells and streptavidin: horse radish peroxidase conjugate was added to all the wells and the plate was incubated for 60 minutes at 37°C. After washing the plates thoroughly for four times with diluted buffer, tetra methyl benzidine substrate was added to all the wells. After adding the stop solution, the intensity of the color in each well was measured by a microplate reader.
Sample size calculation
Based on previous studies and precision taken as 0.6, with the effect size of 0.386, α–error of 5%, and power being 80%, the sample size was calculated. The calculation was done by N-master version 2 software. The sample size calculated was 108 cases and 108 controls. The total sample size was 216.
Statistical analysis
The demographic details of the patients were compared between the cases and the controls. Data were expressed in mean ± SD, for continuous variables which are normally distributed while median and inter quartile range (IQR) for data which are skewed. An unpaired t-test was used to compare age, body mass index (BMI), glycated hemoglobin (HbA1c), fasting blood sugar (FBS), albumin, cholesterol, triglycerides, high density lipoprotein (HDL), and low density lipoprotein (LDL). Mann–Whitney U test was used for the duration, blood urea, serum creatinine, and serum levels of ACE. The qualitative data collected was reported as frequency and percentage. The chi-square test was used to find the association between the gene polymorphism and serum levels with the different stages of DN. The p-value less than 0.05 was considered statistically significant.
RESULTS
The demographic profile of the study participants (Table 1), and biochemical parameters (Table 2) are compared and depicted respectively.
The number of male patients was higher in both the control and case groups. However, the gender ratio did not show any statistically significant results. The duration of diabetes and smoking history showed a significance of p < 0.0001 and 0.025, respectively.
Out of the nine biochemical parameters, blood urea, serum creatinine, and albumin showed a significant difference between the cases and control. Mann–Whitney U test (Duration of diabetes, Blood urea and serum creatinine), p < 0.0001 highly significant.
Unpaired T test (Smoking status, Serum Albumin, and HDL) p < 0.05, statistically significant.
The mean serum ACE levels were 51.53 (40.54, 63.20) ng/ml in the DN patients whereas control patients had an ACE level of 47.27 ng/ml (37.37, 87.27). However, the difference was not statistically significant (Table 3).
Receiver operating diagnostic curve (ROC) curve was plotted by taking the serum levels of ACE of case and control groups (Fig. 2). By taking the sensitivity and specificity values, the cut-off value found was 60.28 ng/ml. Taking this cut-off value, we found the association of genotypes with the different stages of DN. The area under the curve value was estimated to be 0.485 which also clearly demonstrated that serum ACE level is not a good biomarker in predicting DN in the control group.
We analyzed the serum ACE levels in different stages of DN as per the spot albuminuria test (Table 4) and eGFR, respectively (Table 5). There was no statistically significant difference in the serum ACE levels between the stages as shown in Tables 4 and 5. This indicates that serum ACE levels might not vary significantly with the severity of DN.
ACE gene polymorphism
We looked into the ACE gene polymorphism rs267604983 by gene sequencing. The frequency distribution of the wild genotype (GG) was higher in the control group (95.4%) while it was 47.2% in cases. The frequency of the mutant allele (GA + AA) was comparatively higher in DN patients (52.8%) whereas it was 4.6% in the control group (Table 6). This clearly showed the predominance of mutant alleles in the DN group compared to the control group.
The frequency distribution of the GG genotype (homozygous) was seen in 71.3%, followed by the GA genotype in 28.2% (heterozygous recessive) and the AA genotype (mutant) in 0.5% of patients. The distribution was consistent with Hardy Weinberg’s equilibrium (Table 7).
We looked for the association between the ACE gene polymorphism and the serum ACE levels in the DN patients and we found that serum ACE levels did not vary much in different genotypes as given in Table 8. However, the statistical p-value was 0.222 which was not significant.
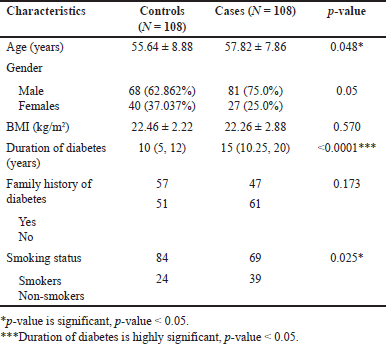 | Table 1. Showing the demographic characteristics between the type-2 diabetic patients with or without nephropathy. [Click here to view] |
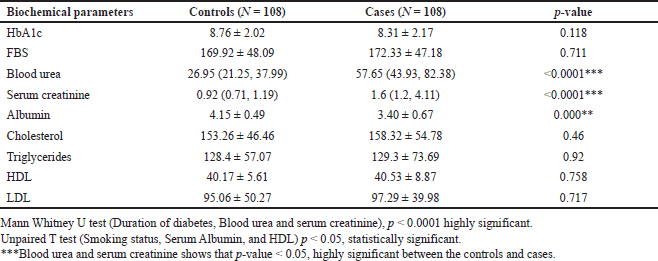 | Table 2. Showing the biochemical parameters between the type-2 diabetic patients with or without nephropath. [Click here to view] |
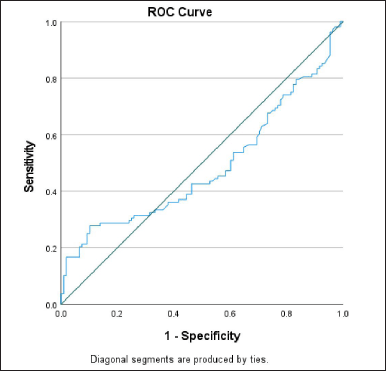 | Figure 1. Showing the ROC curve of ACE. The area under the curve is 0.485. [Click here to view] |
 | Table 3. The serum ACE levels among the patients with or without nephropathy. [Click here to view] |
 | Table 4. Showing the association between serum ACE levels with the stages of albuminuria. [Click here to view] |
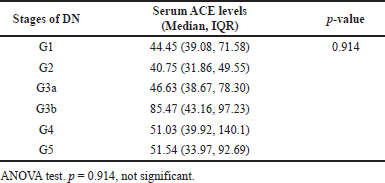 | Table 5. Association between serum ACE levels with the stages CKD as per eGFR. [Click here to view] |
The pattern of genotype distribution in different stages of chronic kidney disease as per eGFR is given in Table 9.
We compared the different stages of albuminuria in different genotypes. It was found that in the case group, there were 52.5% GG and 47.5% GA + AA genotype patients with moderately increased albuminuria (30–300 mg/g) and 32.1% GG and 67.9% GA + AA genotype patients with severely increased albuminuria (>300 mg/g). The statistical analysis did not show a significant difference in albuminuria stages. The p-value was 0.063 (Table 10). However, we could appreciate that the patients with wild genotype showed slight protection in developing albuminuria while we could see the preponderance of carrier patients having severe albuminuria. This implies that while the ACE gene polymorphism might be associated with DN susceptibility, it might not have a strong influence on albuminuria severity.
We compared the duration of diabetes with the serum ACE levels, albuminuria, and eGFR in DN patients. We grouped the duration as the patients having DM <5 years, between 5 to 10 years, 11 to 15 years and >16 years Tables 11–13. However, the serum ACE levels did not show statistically significant value, (p = 0.315) with respect to the duration of diabetes among the diabetic nephropathy patient (Table 11). Fischer’s exact test was used to find the association between the duration of diabetes and albuminuria levels. However, this did not show any statistically significant result (p = 0.138) (Table 12). Chi-square test was used to find the association of duration of diabetes with stages of eGFR which showed statistically significant difference (p < 0.027) (Table 13)
 | Table 6. The pattern of ACE genotype between the patients with or without nephropathy in the study population. [Click here to view] |
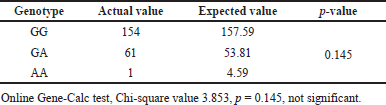 | Table 7. Hardy Weinberg equilibrium test for ACE gene. [Click here to view] |
 | Table 8. Showing the association between ACE gene polymorphism and serum sirtuin level of cases taking the cut-off value (chi-square or Fischer’s exact test). [Click here to view] |
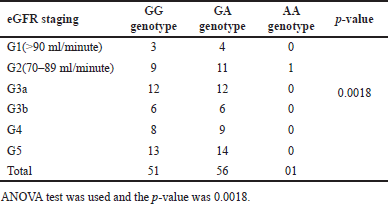 | Table 9. Frequency distribution of ACE genotypes in different stages of eGFR. [Click here to view] |
 | Table 10. Distribution of genotype in different stages of albuminuria. [Click here to view] |
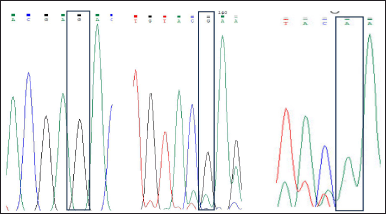 | Figure 2. Showing the GG, GA, and AA genotypes. [Click here to view] |
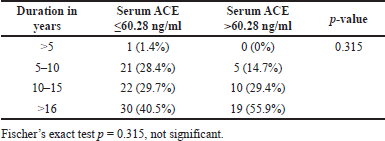 | Table 11. Shows the association of duration with serum ACE levels. [Click here to view] |
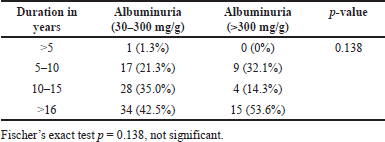 | Table 12. Shows the association of duration with albuminuria. [Click here to view] |
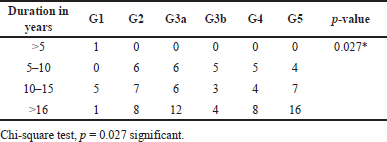 | Table 13. Shows the association of duration with eGFR stages. [Click here to view] |
DISCUSSION
Angiotensin II which is a powerful vasoconstrictor is synthesized from angiotensin-I by the action of ACE enzyme. This enzyme also inactivates the bradykinin. Angiotensin-II can raise blood pressure after binding to the AT-I receptor. The rise in blood pressure is brought about by multiple mechanisms such as the excretion of aldosterone and increased sodium retention; there is the excretion of arginine, vasopressin, and adrenocorticotropic hormone in the pituitary, and the release of norepinephrine of the sympathetic nerve [7]. With this background, we investigated the role of ACE gene polymorphism and the serum ACE levels in type-2 diabetic patients with or without nephropathy. Many researchers have studied ACE I/D polymorphism in DN patients [8,11]. However, there is a very limited study on ACE rs267604983gene polymorphism in DN patients. There are no studies focusing on the association of ACE gene polymorphism with the serum levels of ACE in DN patients. Therefore, we explored the relationship of ACE rs267604983 on the serum levels of ACE in DN patients.
The frequency of the wild-type genotype (GG) was higher in controls, while the mutant allele (GA + AA) was more prevalent in DN patients. However, the ACE gene polymorphism did not significantly influence serum ACE levels in DN patients.
The study done by Ma et al. [7], in ACE rs267604983 in 80 DN patients and 78 patients with type-2 diabetes as controls in the Chinese population. This study suggested that the AA genotype and A allele were significantly associated with the onset of DN. The frequency of the GG genotype was more in the control group than in the cases. The AG genotype frequency was higher in the case group than in the control group. AA genotype was more in the case group than in the control group. In our study, the GG genotype frequency was higher in controls than in cases. In our study population, the GA genotype was more in cases than in controls which supports the Ma et al. [7] study.
Taha et al. [22] studied the association of ACE I/D and G2350A genotype in DN patients. They found that the frequency of the GG genotype was more in cases than in controls. GA and AA genotypes were more in the case group than in the control. However, the association was not significant. This result is in contrast to our study findings.
The study by Ruggenenti et al. [23] investigated the association between ACE gene polymorphism and DN in both type 1 and type 2 diabetes patients. The researchers found that the ACE gene polymorphism was associated with an increased risk of DN in both types of diabetes.
The study by Viswanathan et al. [24] focused on the South Indian population and assessed the association between ACE gene I/D polymorphism and DN. The results indicated a significant association between the ACE gene I/D polymorphism and an increased risk of DN in the south Indian population.
Araz et al. [25] concentrated on Turkish type-2 diabetic patients and examined the relationship between ACE I/D gene polymorphism and the progression of DN. The researchers found evidence of an association between certain ACE gene polymorphisms and an increased risk of progression in DN among the Turkish patients.
Wyawahare et al. [26] conducted a follow-up study on type 1 diabetic patients to investigate the relationship between ACE gene polymorphism and the progression of nephropathy. The study found that specific ACE gene polymorphisms were associated with an increased risk of progression in DN in these patients.
Another study by El-Barbary et al. [27] investigated the association between the ACE gene I/D polymorphism and DN in Egyptian patients with type 2 diabetes. The researchers reported a significant association between the DD genotype and an increased risk of DN, supporting the notion that ACE gene polymorphism might contribute to disease susceptibility.
A meta-analysis published by Chen et al. [28] pooled data from multiple studies to assess the overall association between the ACE gene I/D polymorphism and DN risk. The meta-analysis included individuals of various ethnicities. The results showed a significant association between the DD genotype and DN, further supporting the potential role of this genetic variant in disease susceptibility.
Conversely, a study by Nair et al. [29] focused on the Indian population with type 2 diabetes and evaluated the association between the ACE gene polymorphism and DN risk. Surprisingly, the researchers did not find any significant association between the ACE gene I/D polymorphism and DN, suggesting that the genetic effect might vary among different ethnic groups. Overall, the findings from these studies suggest that ACE gene polymorphism may play a role in the development and progression of DN. However, it is essential to interpret these results with caution and consider the limitations of each study, as genetic associations can be influenced by various factors and may vary across different populations. Further research and replication studies are necessary to establish a robust and comprehensive understanding of the relationship between ACE gene polymorphism and DN.
In this study, we also aimed to assess the serum ACE levels in DN patients and type-2 diabetic patients. There were higher serum levels of ACE in DN patients than in the control group; however, it was not statistically significant. The analysis of serum ACE levels in different stages of DN and various ACE genotypes did not show significant variations.
A study by Ustundag et al. [30], showed a significant increase in ACE levels in DN patients 47·11 ± 3·70 U l−1 than the type-2 diabetic patients 43·72 ± 2·93 U l−1.
A study by Vaidya et al. [31] examined serum ACE levels in patients with type 1 diabetes and DN. The researchers found that serum ACE levels were significantly higher in patients with DN compared to those without nephropathy. Furthermore, the elevated ACE levels were associated with the severity of kidney disease, suggesting a potential role for ACE in the pathogenesis and progression of DN.
Another study by Elsayed et al. [32] investigated serum ACE levels in patients with type 2 diabetes and various stages of nephropathy. The researchers observed a progressive increase in serum ACE levels with the severity of nephropathy. In addition, they found that higher ACE levels were associated with worse renal function and higher levels of proteinuria, indicating a possible link between elevated ACE and the progression of diabetic kidney disease.
Zhang et al. [33] evaluated serum ACE levels in patients with DN and non-diabetic kidney disease. The researchers found that serum ACE levels were significantly higher in the DN group compared to the non-diabetic kidney disease group. They also observed a positive correlation between serum ACE levels and markers of kidney damage, suggesting that ACE may serve as a biomarker for DN.
Conversely, Kumeda et al. [34] found that serum ACE levels were not significantly different between patients with DN and those without nephropathy. However, the study did report a positive correlation between serum ACE levels and proteinuria, indicating that ACE might still be involved in the progression of kidney damage in diabetes.
When comparing the different stages of albuminuria in different genotypes, no statistically significant difference was observed. However, patients with the wild-type genotype showed a slight protective trend against developing albuminuria, while the carrier patients had a higher proportion of severe albuminuria. The study also explored the relationship between the duration of diabetes and serum ACE levels, albuminuria, and eGFR in DN patients.
The limitation of our study that is having a healthy control arm in the study and having a larger sample size in each group of nephropathy (based on albuminuria) can give more robust results. We conclude from our study that ACE gene polymorphism may be associated with serum levels of ACE that lead to the progression of DN.
CONCLUSION
Overall, the study provides insights into the serum ACE levels and ACE gene polymorphism in DN patients. The lack of significant differences in serum ACE levels between DN stages and different genotypes suggests that other factors might be more influential in the development and progression of DN. In addition, the study highlights the need for larger and more comprehensive investigations to fully understand the relationship between ACE gene polymorphism, serum ACE levels, and DN. It is also important to consider other factors that could influence the progression of DN, such as diabetes duration, glycaemic control, and comorbidities. Further research in these areas may provide a more comprehensive understanding of the genetic and molecular mechanisms underlying DN and potentially aid in the development of personalized treatment strategies.
ACKNOWLEDGMENT
The authors would like to acknowledge NITTE (Deemed to be University) and RSSDI for the funding.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study was approved by the Central Ethics Committee’s (Ref. No. NU/CEC/2020/65), Date: 24/03/2021 and written informed consent was taken from the study participants.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Sreenivasamurthy L. Evolution in diagnosis and classification of diabetes. J Diabetes Mellitus. 2021;11(5):200–7. CrossRef
2. Pandey AR, Aryal KK, Shrestha N, Sharma D, Maskey J, Dhimal M. Burden of diabetes mellitus in Nepal: an analysis of global burden of disease study 2019. J Diabetes Res. 2022;2022:1–15. CrossRef
3. O’Rahilly S, Barroso I, Wareham NJ. Genetic factors in type 2 diabetes: the end of the beginning? Science. 2005;307(5708):370–3. CrossRef
4. Papatheodorou K, Banach M, Bekiari E, Rizzo M, Edmonds M. Complications of diabetes 2017. J Diabetes Res. 2018;2018:3086167. CrossRef
5. Tusa BS, Weldesenbet AB, Tessema ZT, Ayele TA. Incidence of diabetic nephropathy and its predictors among type 2 diabetes mellitus patients at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. J Nutr Metab. 2021;2021:2090–97. CrossRef
6. Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, et al. Nephropathy in diabetes. Diabetes Care. 2004;27(1):S79–83. CrossRef
7. Ma H, Yu C, Wang R. Association of ACE polymorphism and diabetic nephropathy susceptibility. Int J Clin Exp Med. 2015;8(2):2962–5.
8. Ha SK, Park HC, Park HS, Kang BS, Lee TH, Hwang HJ, et al. ACE gene polymorphism and progression of diabetic nephropathy in Korean type 2 diabetic patients: effect of ACE gene DD on the progression of diabetic nephropathy. Am J Kidney Dis. 2003;41(5):943–9. CrossRef
9. Jiang ZS, Jia HX, Xing WJ, Han CD, Wang J, Zhang ZJ, et al. Investigation of several biomarkers associated with diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2015;123:1–6. doi: https:// doi:10.1055/s-0034-1385875
10. Chawla T, Sharma D, Singh A. Role of the renin-angiotensin system in diabetic nephropathy. World J Diabetes. 2010;1(5):141–5.
11. El-Baz R, Settin A, Ismaeel A, Khaleel AA, Abbas T, Tolba W, et al. MTHFR C677A1298C and ACE I/D polymorphisms as risk factors for diabetic nephropathy among type 2 diabetic patients. J Renin Angiotensin Aldosterone Syst. 2012;13:472–7. CrossRef
12. Zeng WL, Yang SK, Song N, Chu FF. The impact of angiotensin converting enzyme insertion/deletion gene polymorphism on diabetic kidney disease: a debatable issue [published online ahead of print]. Nefrologia. 2021;42(4):415–31. CrossRef
13. Shen W, Jiang XX, Li YW, He Q. I/D polymorphism of ACE and risk of diabetes-related end-stage renal disease: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2019;23:1652–60. CrossRef
14. Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Investig. 1990;86:1343–6.
15. Rahimi Z. ACE insertion/deletion (I/D) polymorphism and diabetic nephropathy. J Nephropathol. 2012 Oct;1(3):143–51.
16. Huang K, Liang Y, Wang K, Ma Y, Wu J, Luo H. Elevated ACE levels indicate diabetic nephropathy progression or companied retina impaired. Front Clin Diabetes Healthc. 2022;3:1–7. CrossRef
17. Anderson S, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension. J Clin Invest. 1986;77:1993–2000. CrossRef
18. Merker L. Nephropathy in diabetes. MMW Fortschr Med. 2021;163:48–51.
19. Umanath K, Lewis JB. Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis. 2018;71:884–95. CrossRef
20. Selby NM, Taal MW. An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab. 2020;22:3–15. CrossRef
21. Wolf G, Sharma K. Chapter 30 pathogenesis, clinical manifestations, and natural history of diabetic nephropathy. Comprehensive clinical nephrology. 5th ed. Amsterdam, The Netherlands: Elsevier. 354–71 pp. CrossRef
22. Taha MM, Mahdy-Abdallah H, Shahy EM, Helmy MA, Elliathy LS. Diagnostic efficacy of cystatin-c in association with different ACE genes predicting renal insufficiency in T2DM. Sci Rep. 2023;13:1–9. CrossRef
23. Ruggenenti P, Bettinaglio P, Pinares F, Remuzzi G. Angiotensin converting enzyme insertion/deletion polymorphism and renoprotection in diabetic and nondiabetic nephropathies. Clin J Am Soc Nephrol. 2008 Sep;3(5):1511–25. CrossRef
24. Viswanathan V, Zhu Y, Bala K, Dunn S, Snehalatha C, Ramachandran A, et al. Association between ACE gene polymorphism and diabetic nephropathy in South Indian patients. JOP. 2001;2(2):83–7.
25. Araz M, Yilmaz N, Gungor K, Okan V, Kepekci Y, Aynacioglu AS. Angiotensin-converting enzyme gene polymorphism and microvascular complications in Turkish type 2 diabetic patients. 2001;54(2):95–104. CrossRef
26. Wyawahare M, Neelamegam R, Vilvanathan S, Soundravally R, Das AK, Adithan C. Association of angiotensin-converting enzyme gene polymorphisms and nephropathy in diabetic patients at a Tertiary Care Centre in South India. Clin Med Insights Endocrinol Diabetes. 2017 Aug 29;10:1179551417726779. CrossRef
27. El-Barbary AM, Hussein MH, Rageh EM, Hamouda MH, Elwaseef AM. Association of angiotensin-converting enzyme gene polymorphism with diabetic nephropathy in type 2 diabetic Egyptian patients. Ren Fail. 2018;40(1):22–7.
28. Chen L, Liu X, Zhang X, Liu Y, Liu H, Sun L. Association of angiotensin-converting enzyme gene I/D polymorphism with diabetic nephropathy risk: a meta-analysis including 11,042 subjects. Nephrol Dial Transplant. 2017;32(1):78–85.
29. Nair AK, Nair KS, Bhat DN, Ibrahim M, Joshi S. No evidence of association of angiotensin-converting enzyme (ACE) gene polymorphism with diabetic nephropathy in Asian Indians with type 2 diabetes. Acta Diabetol. 2015;52(1):177–83.
30. Ustundag B, Canatan H, Cinkilinc N, Halifeoglu I, Bahcecioglu H. Angiotensin converting enzyme (ACE) activity levels in insulin-independent diabetes mellitus and effect of ACE levels on diabetic patients with nephropathy. Cell Biochem Function. 2000;18:23–8. CrossRef
31. Vaidya VS, Niewczas MA, Ficociello LH, Johnson AC, Collings FB, Warram JH, et al. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-β-D-glucosaminidase. Kidney Int. 2010;79(4):464–70. CrossRef
32. Elsayed M. E, Sharif MU, Stack AG, Salem MM. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in diabetic kidney disease. Adv Chronic Kidney Dis. 2015;22(3):200–7. CrossRef
33. Zhang, J, Liu, J, Su K, Wang P, Tan B, Tian Y. Serum angiotensin-converting enzyme is an independent risk factor for the presence and severity of nephropathy and retinopathy in Chinese patients with type 2 diabetes. Clin Biochem. 2013;46(7):643–7. CrossRef
34. Kumeda Y, Inaba M, Shoji S, Imanishi Y, Yamauchi, M, Nishizawa Y, et al. Significant correlation between the ratio of urinary angiotensin-converting enzyme to proteinuria in random urine and the renal severity and progression in patients with type 2 diabetic nephropathy. J Diabetes Investig. 2012;3(5):449–55. CrossRef