INTRODUCTION
The International Agency for Research on Cancer estimates that there were 19.3 million new cases of cancer and 10.0 million deaths worldwide in 2020. By 2040, this number is projected to rise to 28.4 million cases, a 47% increase from 2020. Lung, liver, and stomach cancers were the leading causes of cancer deaths globally, followed by female breast cancer, lung cancer, and prostate cancer [1].
The most popular cancer treatments radiation treatment, surgery, and systemic chemotherapy are also the most likely to have clinical efficacy restrictions. For instance, radiation therapy frequently results in indirect harm to the tissues around the wound, leading to difficulties and slow healing following surgery. It may also result in microscopic and metastatic illness. Chemotherapy frequently results in cancer development and systemic damage. As a result, there is a need to create an improved clinical agent that is more focused, lower risk, and can reduce side effects [2].
The drugs now available to treat cancer are not only expensive but also perilous, damaging both cancer and healthy cells. Finding new, efficient, and safe molecules from natural sources is more essential than ever [3].
Natural products are increasingly being used as medicines to treat human ailments due to their remarkable biological activity and reduced adverse effects when opposed to synthesized compounds [4].
For decades, humans have relied on plants to meet their basic needs, namely food, clothing, and shelter. The ecosystem in which humans live is nourished by plants, which provide food and medical herbs to us and other creatures. These plants’ medicinal usefulness is derived from a class of compounds known as phytochemicals, which have a specific pharmacological effect on the human body. Phytoconstituents were naturally occurring active compounds found in the barks, leaves, fruits, roots, and barks of medicinal plants [5].
The research demonstrates that more than 80% of medicines currently in use come from natural sources (herbal medicines, their bioactive compounds, or microbes) [6]. Throughout tropical and subtropical India, the Malay Peninsula, Africa, Srilanka, China, and America, the understory shrub Triumfetta rhomboidea Jacq (Tiliaceae) is found in large quantities. The perennial herb played a significant part in traditional medicine [7,8].
It contains compounds, such as phytosterol, flavonoids, carbohydrates, steroids, glycosides, tannin, and phenolic compounds, and triterpenoids, which have a variety of therapeutic activities, including antitumor, antioxidant, antibacterial, antimicrobial, diuretic, anti-inflammatory, antidiabetic, antitubercular, antiproliferative, lactogenic effect [9].
Traditional approaches for characterizing bioactive substances include extraction, analysis, chromatographic separation, and spectroscopic identification. Nevertheless, even investing a lot of time and energy into it, the majority of investigation merely results in characterizing a small number of existing compounds so it is challenging to discover appropriate phytoconstituents criteria. Uncovering the complex chemistry of bioactive crude extracts using high throughput and high-resolution techniques is necessary to find pharmaceutically potent bioactives and make it easier to understand their influence on the target [10].
In addition, the time, money, and effort required to screen the biological potential of natural products have been decreased because of advancements in computational biology. To predict how a drug-like molecule would bind to the active site of a receptor and with what affinities, a technique known as molecular docking has become popular. Several different synthetic and natural compounds might hypothetically be tested for activity against a variety of targets, which would save time and effort and allow for quicker identification of the most promising candidates [11].
Since EGFR and VEGFR overexpression is widely observed in a variety of tumor types, such as breast, lung, colon, and ovarian tumors, it is an intriguing therapeutic and imaging target for the study of cancer therapies.
Aggressiveness of the disease and EGFR overexpression in cancer are related. By the stimulation of proliferation, invasion, metastasis, and angiogenesis as well as the inhibition of apoptosis, adhesion, and differentiation, EGFR activation promotes tumor development and progression [2].
Several proangiogenic factors enhance endothelial cell motility (migration, invasion, proliferation, and tubulogenesis) and, eventually, induce neovascularization, destabilize the integrated blood artery, and govern the tumor angiogenic process. VEGF, one of these factors, is essential to the angiogenesis process. The primary angiogenic properties of VEGF are controlled by VEGFR2. Many downstream signaling pathways, especially extracellular signal-regulated kinase and Akt (extracellular signal-regulated kinase), are activated as a result of VEGFR2 phosphorylation, which in turn promotes migration, endothelial cell proliferation, and tube formation. Hence, a key antiangiogenic therapy approach is to block the VEGF/vascular endothelial growth factor (VEGFR)2 signaling system by preventing endogenous VEGF secretion and decreasing VEGF binding to VEGFR2 [12].
Natural products have been demonstrated to inhibit nearly every type of cancer because of their special chemical composition [13]. It has a range of bioactive compounds that interact with the epidermal growth factor receptor (EGFR) and VEGF and block them through several signaling pathways, inhibiting the spread of cancer [14]. In light of this, EGFR and VEGF seem to be attractive new molecular targets for the creation of anti-cancer drugs.
First time attempt was made to use high resolution-liquid chromatography-mass spectrometry analysis (HR-LCMS) to look into different bioactive chemicals from T. rhomboidea. To evaluate the effectiveness of anticancer agents, MCF7 cancer cell lines were used in a wet laboratory experiment, followed by a molecular analysis of interactions between phytoconstituents and EGFR and VEGFR to determine binding efficiency.
MATERIALS AND METHODS
Collection and authentication of T. rhomboidea
Triumfetta rhomboidea specimens were gathered in the Aurangabad region of India. Plant authentication was done by BSI, Pune (Specimen No.—NSKTR-1). After being thoroughly cleaned three to four times, T. rhomboidea leaves are dried for 2 weeks in the shade. To achieve a small particle size, leaves are either manually crushed or run through a mechanical grinder.
Preparation of extracts
After selection and authentication of the plant, the whole plant is thoroughly washed with distilled water a few times and shade dried. Then, it is grinded using a mechanical mixer so the dry powder is sieved for a uniform particle size. For extraction by cold maceration process, 500 g of leaves powder was soaked in 2,000 ml of hydroethanolic solution of water and ethanol in equal proportion (1:1) for 24 hours with occasional stirring.
Following muslin material and Whatman’s filter paper no. 1 filtering, the extract was then reduced utilizing a rotary evaporator under reduced pressure. The hydroalcoholic extract aqueous suspension was gradually divided into hexane, chloroform, and ethyl acetate to separate its polar and nonpolar components. The resulting fractions were dried in a rotary evaporator. Until they were required, each fraction was stored at 4°C [15,16].
The following formula was used to determine the yield of each extract:
HR-LCMS analysis of T. rhomboidea
The HR-LCMS was used to investigate the ethyl acetate fractions of the T. rhomboidea since it had the strongest antioxidant activity of the three fractions. The Agilent ([6550 iFunnel Quadrupole Time-of-Flight (Q-TOF)] system, which includes a column component, hip sampler, and electrospray ion generation (ESI) with Agilent Jet Stream [15], was used to analyze the ethyl acetate fraction, using a Q-TOF with a dual ion source and a binary pump to separate chromatograms. A needle wash was used to inject 5 μl of ethyl acetate fraction into an Agilent ultra high performance liquid chromatography system that was fitted with a Hypersil Gold column (C18 100 × 2.1 mm-3 MICRON). The elution solvent was 0.1% formic acid in water (A) and acetonitrile (B) at a flow rate of 0.3 ml/minute. The gradient system changed its composition from 95% A: 5% B to 5% A: 95% B in 50 minutes, returned to the original composition in 10 minutes, and then retained that composition for 5 minutes. The MS analysis was conducted using the positive and negative ionization modes of the ESI. The following MS source parameters were used: capillary voltage 3,500 V, nebulizer pressure of 35 psi, gas temperature 250°C, and drying gas flow 13 l/minute. Acquisition of Q-TOF data and mass spectrometric analysis were done using Agilent Mass Hunter software [15,17,18].
In vitro anticancer activity
In an (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) (MTT) anticancer test of T. rhomboidea against MCF-7 cells, tamoxifen was utilized as a positive control to determine the percentage of inhibition. Tamoxifen, the pioneering selective estrogen-receptor (ER) modulators, blocks estrogen action by binding to the estrogen-receptor in breast cancers [19].
Experimental procedure
To assess the cytotoxic effects of the fractions, the MTT test was used. Cells was maintained in Dulbecco’s modified eagle medium supplemented with 10% fetal bovine serum. For the cell lines of MCF-7 in each well, 1 × 104 cells were planted in 96-well plates and cultured for 24 hours at 37°C. The cells were exposed to various doses of the fraction (0–80 μg/ml) for 72 hours. The plates were incubated for 24 hours, and cell proliferation was measured by adding 10 µl of MTT (thiazolyl blue tetrazolium bromide) dye (5 mg/ml in phosphate-buffered saline) per well. The plates were incubated for a further 4 hours at 37°C in a humidified chamber containing 5% CO2. Formazan crystals formed due to the reduction of dye by viable cells in each well were dissolved in 200 µl DMSO. All of the tests were performed three times and the data were expressed as mean ± standard deviation (SD).
The wells were examined under a microscope to check for the formation of formazan crystals. The quantity of live cells directly relates to the amount of formazan that is produced. A microplate reader set at 570 nm was used to measure the absorbance. In this study, tamoxifen served as the positive control [19,20].
Molecular docking
It is possible to understand and explain how small molecules behave at the binding site of protein targets by using a molecular docking approach to observe the interaction between a ligand or small molecule and a protein at the atomic level. Identification of the binding site, searching for the ligand that fits the receptor or protein the best, and obtaining the scoring function to make a comparison of the various binding conformation energies produced by each ligand-protein interaction are the three interconnected steps of the molecular docking procedure [21].
Retrieval and protein preparation
From (www.rcsb.org), structural models of the kinase domains of the EGFR in complex with AFN941 (PDB: 2ITW) and the VEGFR in complex with Axitinib (PDB: 4AG8) were retrieved from the Protein Data Bank [22]. All docking tests were conducted on a single monomer because both structural models are monomeric and functionally active. The “Make macromolecule” command from PyRx’s AutoDock menu was used to prepare the receptor molecule in the conventional pdbqt format after charging the receptor coordinate file.
Ligands preparation
The open source Pubchem database [https://pubchem.ncbi.nlm.nih.gov/], which contains the 3-D structures of the phytochemicals in SDF format, was used to retrieve these structures. Molecular docking calculations were performed using AutoDock4.2, which was implemented in Python Prescription 0.8 (PyRx). These molecules were reduced for more than 200 steps using UFF forcefield and the conjugate gradient optimization algorithm [22] before being converted to AutoDock readable pdbqt format [21].
Testing validity of AutoDock 4.2 and virtual screening
By evaluating a docking program’s capacity to replicate an experimental ligand’s binding mode, a docking system’s validity can be verified. Following the docking experiment, the root mean square deviation (RMSD) value of the expected poses to the experimentally verified positions is determined. If the RMSD value is frequently found to be less than 2.00 Å, the prediction of binding mode is regarded to have been effective because it represents the degree of spatial similarity between two structures. The Auto-grid application was used to get a grid file. The affinity grid of 60 × 60 × 60 points was set with a spacing of 0.375 to completely cover the active site. The Lamarckian genetic algorithm was used to conduct a conformational search to find the ideal binding pose. There were 10 runs assigned to each Lamarckian job [18]. The final structures were clustered by utilizing the native autodock scoring algorithm. The top-ranking conformations of each ligand were chosen. The docking experiment with crystallographic [15] ligands Axitinib and AFN941 back in inhibitors binding sites of VEGFR and EGFR, respectively, yielded RMSD values of 1.06 and [22] 1.92. All of the necessary functional groups and significant structural moieties are predicted to be oriented in the right positions, as shown by these values and structural analysis of superimposition, indicating that the docking mechanism is valid. Furthermore, there is a striking similarity between the predicted binding mode and the conformer from X-ray crystallography [22].
Post virtual screening analysis
The resultant docked complexes were visualized and the images were rendered using PyMOL 0.99 (http://www.pymol.org). A JAVA-based GUI of the LigPlot program called LigPlot+ was used to infer the precise interactions of phytochemicals with EGFR and VEGFR and PoseView was used to discover possible interactions in pi stacking [23].
 | Table 1. Percentage yield of fraction obtained from T. rhomboidea. [Click here to view] |
RESULT AND DISCUSSION
Percentage yield of different fractions
The weights of hexane, chloroform, and ethyl acetate fractions are presented in Table 1.
HR-LCMS analysis of T. rhomboidea
Triumfetta rhomboidea ethyl acetate fraction was analyzed using HR-LCMS, which identified 100 compounds as being present. 15 major compounds were identified in the T. rhomboidea ethyl acetate fraction based on retention time, mass, and molecular formula, as shown in Table 2. On a chromatogram, Figure 1 depicts the approximations of the various component concentrations that are present in T. rhomboidea and that are eluted following the retention time. The height of the peak was used to calculate the relative concentration of the bioactive substances found in the plants. The compounds which were eluted at different times are examined by the mass spectrometer to ascertain the make-up and structure of the compounds. Positive and negative ion modalities were used to identify phytochemicals with proton-donor and proton-acceptor properties. The foundation of a fragment at a particular mass ion was predicted to be phytocompounds, and a database search supported this prediction.
The principal substances identified related to several secondary metabolite groups, such as aralkyl amines, terpene, sesquiterpenoids, cyclic polyol, flavone, flavonoids, fatty acid, polyphenols, furanoid lignans, and anthraquinone based on the study of HR-LCMS and comprehensive literature search. The distinctive mass spectra of the T. rhomboidea bioactives that have been isolated are shown in Figure 2.
Anticancer activity of T. rhomboidea in MCF-7 using MTT assay
In this study, the MTT test was used to investigate the cytotoxic effect of T. rhomboidea hexane and ethyl acetate fraction on the MCF 7 cell line at concentrations ranging from 0–80 μg/ml.
Figure 3 shows the % inhibition of T. rhomboidea in MCF7 by different fractions. The ethyl acetate fraction shows % inhibition from 45.37% ± 5.11% to 60.87% ± 2.22% and n-hexane fractions show 40.8% ± 0.88% to 49.6% ± 2.58% inhibitions. Ethyl acetate and hexane fraction have IC50 values of 17.15 and 26.69 in MCF 7 whereas that of standard drug Tamoxifen is 4.54.
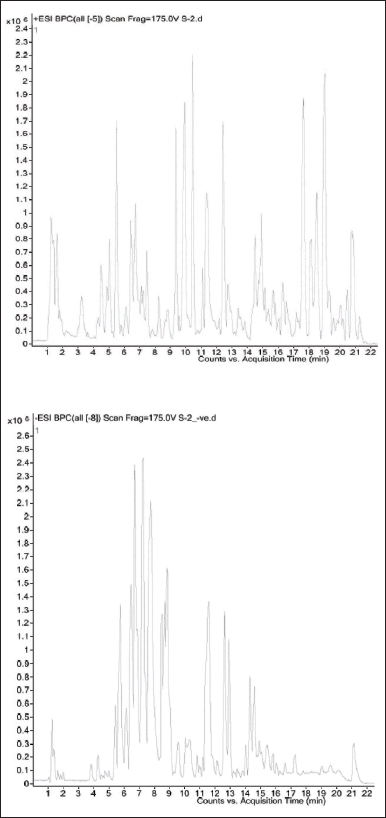 | Figure 1. Triumfetta rhomboidea HR-LCMS chromatogram for the ethyl acetate fraction. [Click here to view] |
At a concentration of 17.15 and 26.69 μg/ml the ethyl acetate fraction and hexane fraction of T. rhomboidea showed 50% inhibition in the MCF7 cell line. IC50 value indicates that the fractions are more cytotoxic to cells.
Both fractions of T. rhomboidea exhibit anticancer properties, according to in vitro MTT studies on breast cancer cells. When compared to n-hexane fraction, the ethyl acetate fraction exhibits strong anticancer activity in MCF 7 cell lines.
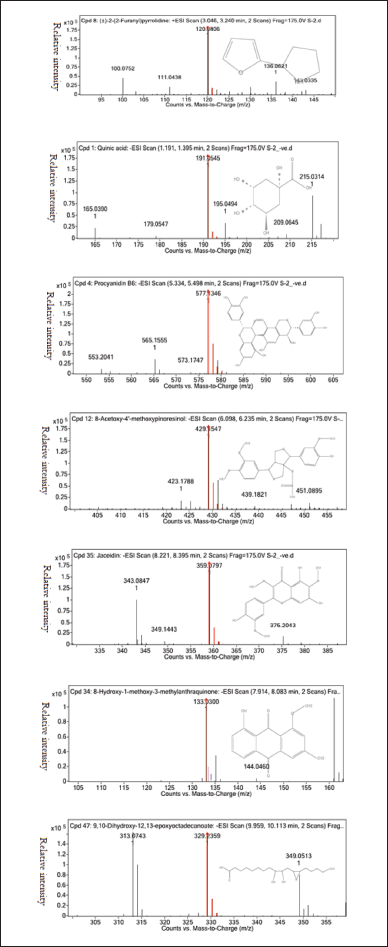 | Figure 2. Mass fragmentation of isolated bioactives (a) 2-(2-furanyl) pyrrolidine, (b) quinic acid, (c) procyanidin B6, (d) 8-acetoxy-4’-methoxypinoresinol, (e) jaceidin, (f) 8-hydroxy-1-methoxy-3-methylanthraquinone, and (g) 9,10-dihydroxy-12,13-epoxyoctadecanoate. [Click here to view] |
Molecular docking analysis
Theoretical predictions of ligand-target interactions have been effectively applied in silico investigations for a better understanding of the molecular underpinnings of the biological action of natural products. Also, it offers more information on the potential method of action and manner of binding of substances that are active against enzymes. 15 compounds from the HR-LCMS profile of the ethyl acetate fraction were docked against the EGFR and VEGFR enzymes in an effort to grasp more deeply the examined compounds capacity to inhibit the enzyme and to correlate the results of experimental enzyme inhibition. Six of the 15 molecules from the EGFR and 7 from the VEGFR exhibit strong binding interactions. Matching the phytochemicals to the corresponding pharmaceutical inhibitors of the proteins, a molecular docking study showed that the phytochemicals exhibited strong interactions with the proteins. The interaction of phytochemicals with EGFR and VEGFR and their binding energy is shown in Tables 3 and 4.
IN SILICO STUDY
EGFR and T. rhomboidea phytochemicals interactions
Annofoline interacts with C797 by a nonpolar interaction while procyanidin B6 interacts polarly. One of the most significant residues that is vital to the irreversible form of EGFR inhibition is C797. CL-387785, HKI-272, and EKB-569 are examples of irreversible EGFR kinase inhibitors. They work by exposing an electrophilic moiety (such as a crotonamide group) to a C797 nucleophilic attack, which results in the creation of a covalent bond with EGFR. Despite the phytochemicals’ apparent interaction with C797, test flavonoids appear to have very little possibility of creating a covalent bond because no effective electrophilic substitute can cause C797 to successfully attack nucleophiles [22,24]. So, we presume that phytochemicals’ interaction with C797 may result in the stability of the phytochemicals-EGFR complex rather than in an irreversible covalent change.
G719 interacts nonpolarly with substances including annofoline, procyanidin B6, and 8-hydroxy-1-methoxy-3-methylanthraquinone. It is thought that G719, a conserved residue in the N-terminal lobe of the kinase that is close to the phosphate-binding “P loop,” is one of the key elements affecting catalysis and EGFR suppression. According to Yun et al. [25] results, G719S mutations cause interactions that maintain the inactive conformation to break down, which activates the kinase. It is well known that polar interactions involving K745 alter how certain inhibitors inhibit EGFR. K745’s drug-binding mechanisms and affinities are known to be affected by drug interaction [22].
Several phytochemicals, including annofoline, luteolin, robinetinidol, and 8-hydroxy-1-methoxy-3-methylanthraquinone, have been seen to form hydrogen bonds with M793, as shown in Table 3. Due to its critical location at the junction of the EGFRK C and N lobes, M793 may control the inhibitory effects of a variety of medications, including gefitinib and AFN941. It is interesting to note that structural examination of the binding mechanisms of experimentally proven inhibitors such as gefitinib, WZ4002, TAK-285, and AFN941 demonstrates that these inhibitors interact with EGFR M793 through backbone atoms [22]. This fact perfectly aligns with our docking predictions that all of the sample phytochemicals similarly interact with M793 through backbone atoms [25].
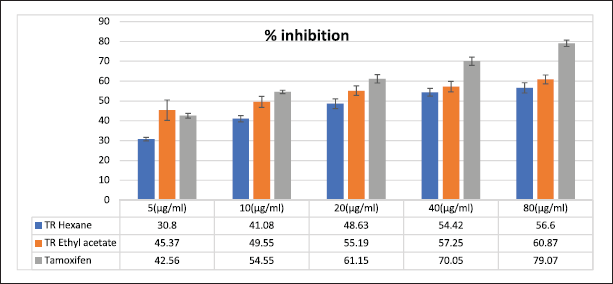 | Figure 3. Anticancer activity of T. rhomboidea in MCF 7 cell line. [Click here to view] |
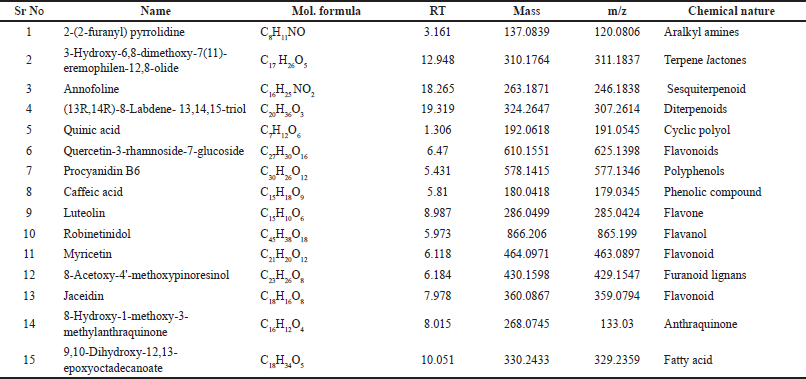 | Table 2. Different phytochemicals isolated by HR-LCMS from ethyl acetate fraction of T. rhomboidea. [Click here to view] |
T790, a gatekeeper residue in the EGFR, interacts polarly with jaceidin while annofoline, luteolin, robinetinidol, and 8-hydroxy-1-methoxy-3-methylanthraquinone interact nonpolarly. Its prominent position at the opening to a hydrophobic region at the rear of the ATP binding cleft [22] is the reason for its given name. This demographic information makes it a key factor in defining the selectivity of inhibitors for protein kinases. It is crucial for maintaining both the active and dormant states. The effectiveness of EGFR inhibitors may be restricted by mutation at this location, which is thought to cause resistance to kinase inhibitor binding. It appears that interaction with these conserved residues may be what causes the anticancer impact that phytochemicals have proven [22,26].
Figures 4–5 depicts the interactions between T. rhomboidea phytochemicals and EGFR.
VEGFR and T. rhomboidea phytochemicals interactions
As shown in Table 4, several residues, including D1046, N923, E917, and K868, appear to interact with phytochemicals. Numerous phytochemicals were seen to form polar interaction with VEGFR residue D1046. It was discovered that D1046 from VEGFR was interacting polarly with caffeic acid, luteolin, robinetinidol, myricetin, and jaceidin. Again, both the backbone and the side-chain atoms play a role in the formation of these connections. The DFG loop of VEGFR, which controls the enzyme’s active and inactive states, contains D1046 as its initial residue [27,28]. According to crystallographic studies, the carbonyl group of well-known medications like benzimidazole interacts with the D1046 residue [29]. As part of another experiment to investigate the amino-benzoxazole molecules, it was noted that a strong hydrogen bond was created between the side chain of D1046 and the endocyclic nitrogen from the active functional group [30]. As a result, it is reasonable to believe that the observed anti-cancer activity of the plant sample may be mediated by phytochemicals interaction with VEGFR D1046 in light of circumstantial literature and the findings of this investigation [15].
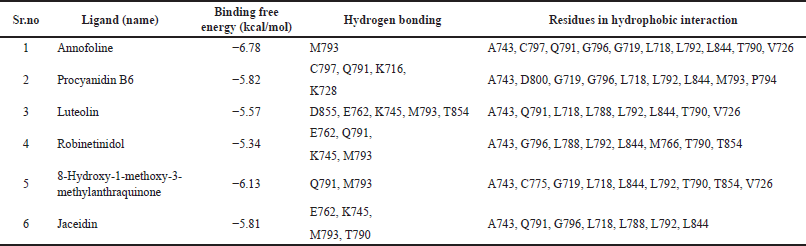 | Table 3. Details obtained from T. rhomboidea structural analysis and docking with EGFR kinase domain. [Click here to view] |
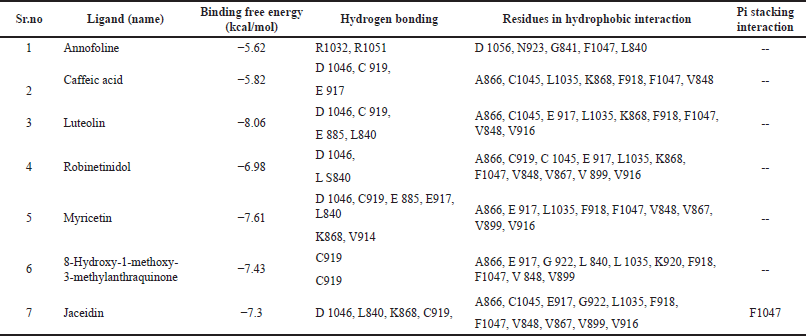 | Table 4. Details obtained from T. rhomboidea structural analysis and docking with VEGFR kinase domain. [Click here to view] |
With N923, Annofoline exhibits a hydrophobic contact. Intriguingly, experimental evidence suggests that N923 plays a crucial role in the inhibition of VEGFR. Harris et al. [31], for instance, examined a variety of oxazole compounds for the inhibition of VEGFR and found that both polar and nonpolar interaction from VEGFR orient the sulfone functional group of the oxazole substances for effective inhibition [15,18].
Caffeic acid, luteolin, myricetin, 8-hydroxy-1-methoxy-3-methylanthraquinone, and jaceidin all establish highly conserved hydrogen bonds with C919 via their hydroxyl substituents, according to structural studies of docked complexes. This connection is essential for VEGFR inhibitory function by Hasegawa et al. [29]. According to biochemical evidence, the inhibitors’ inability to form hydrogen bonds with C919 carbonyl results in a significant decline in enzyme activity. For determining the inhibitor specificity for VEGFR2, these hydrogen bonds are considered to be of utmost importance. Consequently, based on structural findings from this study and circumstantial evidence from supported crystallographic and biochemical data, it is possible that the polar contact of different hydroxyl groups from phytochemical scaffolds with C919 [22] may be crucial in the suppression of VEGFR [15].
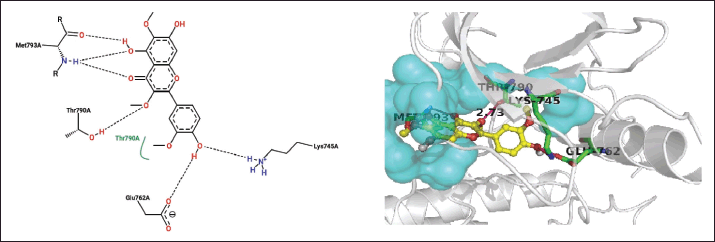 | Figure 4. Closest contacts between T. rhomboidea jaceidin and EGFR active site residues in 2-D (right) and 3-D (left) spaces. [Click here to view] |
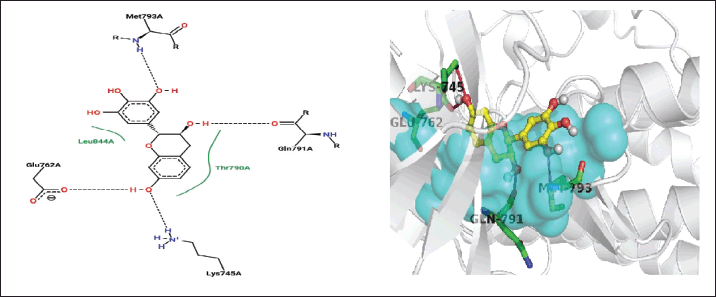 | Figure 5. Closest contacts between T. rhomboidea robinetinidol and EGFR active site residues in 2-D (right) and 3-D (left) spaces. [Click here to view] |
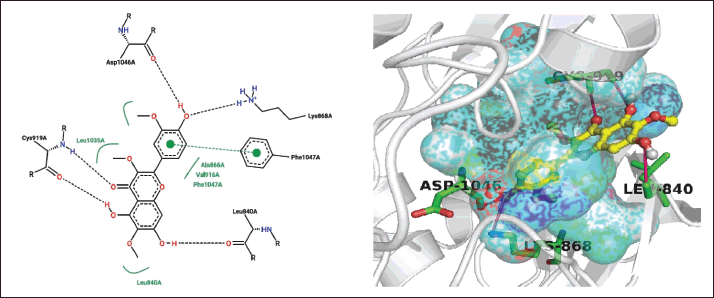 | Figure 6. Polar and π-π T-shaped interactions in 2-D (right) and 3-D (left) space between the Jaceidin from T. rhomboidea and the VEGFR active site residues. [Click here to view] |
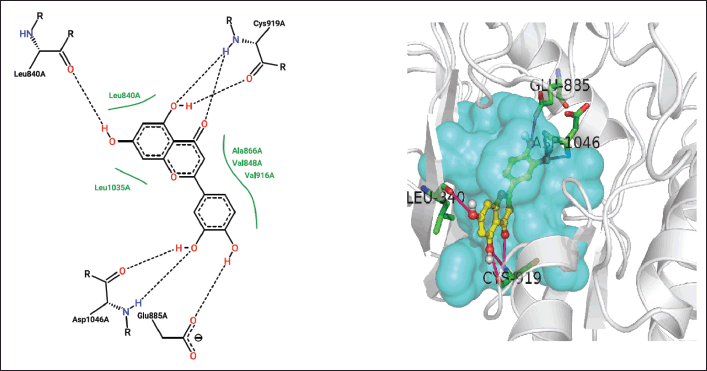 | Figure 7. Closed interactions between T. rhomboidea luteoline and VEGFR active site residues in 2-D (right) and 3-D (left) space. [Click here to view] |
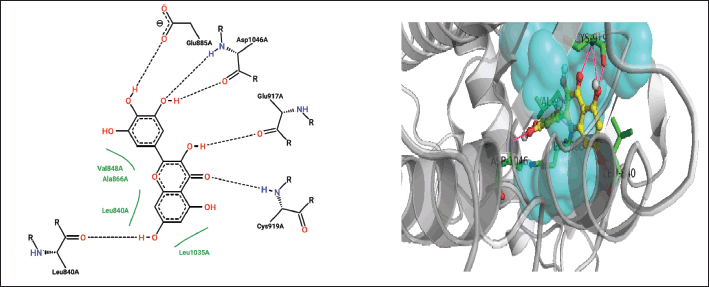 | Figure 8. Closed interactions between T. rhomboidea myricetin and VEGFR active site residues in 2-D (right) and 3-D (left) space. [Click here to view] |
Since it is the first residue in the ATP binding cleft, E917 plays a crucial role in both the catalytic and inhibitory functions of VEGFR [22]. Luteolin, robinetinidol, 8-hydroxy-1-methoxy-3-methylanthraquinone, and Jaceidin interact with E917 nonpolarly while caffeic acid and myricetin interact polarly.
Triumfetta rhomboidea myricetin and jaceidin are actively involved in a hydrogen bond with K868. Several substances, including caffeic acid, luteolin, and robinetinidol from T. rhomboidea, interact through nonpolar contact. K868 has been demonstrated to play a crucial role in the efficacy of the anilino-aryloxazoles line of medications to inhibit VEGFR [18,31]. During catalysis, this residue serves as a container for the ribose sugar component of the nucleotide ATP. In a study using artificial mutagenesis, this lysine was changed to methionine, leading to mutants with a total loss of kinase function [32]. K868 is situated in a crucial region where ligand binding is modulated by nucleotide binding and offers both polar and nonpolar interactions [31]. Using diverse phytoconstituents, we detected both polar and nonpolar contribution of this residue in the most recent docking data, which is in accord with the experimental [15] observations. Myricetin and jaceidin may thereby effectively inhibit VEGFR by reacting with K868 residue.
In addition, many hydrophobic interactions were regarded with F918 and F 1047 by annofoline, caffeic acid, luteolin, robinetinidol, myricetin, 8-hydroxy-1-methoxy-3-methylanthraquinone, and jaceidin.
Furthermore, interactions via π stacking and π-π T-shaped interactions between the phytochemical jaceidin have been discovered (Fig. 6). As an illustration, it was found that the aromatic ring from the aforementioned chemicals was stacked to the F1047 residue from the VEGFR. The second component of the DFG motif is F1047 [22]. Such π stacking involves a medicinal substance like motesanib diphosphate. It is known that this interaction controls where the F1047 residue is located, which controls the transition of the VEGFR kinase domain from active to inactive. Triumfetta rhomboidea anticancer activity may therefore be defined by the nonpolar π stacking and π-π T-shaped interaction provided by F1047 from VEGFR [15,18].
The results of the molecular docking analysis showed that the tested substance might interact with the important amino acids in the ATP active site of VEGFR-2. The observed experimental in vitro anticancer and VEGFR-2 kinase inhibitory activities for these drugs are consistent with the binding interactions and energy binding scores. Figures 6–8 depicts all of T. rhomboidea polar and π-π T-shaped interactions with VEGFR.
CONCLUSION
Triumfetta rhomboidea underwent a complete phytochemical analysis for the first time, employing HR-LCMS, a state-of-the-art methodology, that found a large number of active phytochemicals with potential therapeutic effects. A successful method for discovering and creating novel drugs is the utilization of secondary metabolites generated from natural sources.
The results of the phytochemical investigation suggest that T. rhomboidea could serve as a supply of advantageous chemicals.
The results of this study indicate that T. rhomboidea is a significant source of biogenic compounds with high structural and biological potential. The discovery of numerous bioactive compounds throughout this analysis confirms the historical use of T. rhomboidea for a range of ailments.
Both fractions of T. rhomboidea exhibit anticancer properties, according to in vitro MTT studies on breast cancer cells. When compared to n-hexane fraction, the ethyl acetate fraction exhibits strong anticancer activity in MCF 7 cell lines.
The structural and functional elements of interaction between phytochemicals and retained EGFR and VEGFR residues have been developed in-silico overall, which may provide a logical basis for the reported phytochemical-mediated anticancer activity. To sum up, T. rhomboidea phytochemical displayed higher EGFR and VEGFR enzyme binding abilities. The observed experimental in vitro anticancer and EGFR and VEGFR-2 kinase inhibitory activities for T. rhomboidea are consistent with the binding interactions and energy binding scores.
As a result, as anticipated by docking scores, these phytochemicals can be employed to treat cancer efficiently. The knowledge gathered from this research can be used to build anti-cancer medications with new targets and mechanisms of action in experimental investigations. To corroborate the physiological consequences of these findings, additional in vitro and in vivo research might be conducted. Future efforts for drug development and biological, pharmaceutical, and pharmacological research may benefit from the knowledge gathered.
In order to describe our current understanding of T. rhomboidea and to identify and encourage prospective areas for further research, this study intends to present information about this organism.
ACKNOWLEDGMENTS
The Indian Institute of Technology, Bombay (IIT Bombay), Sophisticated Analytical Instrument Facility (SAIF), performed an HR-LCMS analysis.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors did not report any conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Yuan M, Zhang G, Bai W, Han X, Li C, Bian S. The role of bioactive compounds in natural products extracted from plants in cancer treatment and their mechanisms related to anticancer effects. Oxid Med Cell Longev. 2022;2022:1429869. CrossRef
2. Nerdy N, Lestari P, Fahdi F, Putra EDL, Amir SAB, Yusuf F, et al. In silico studies of sesquiterpene lactones from Vernonia amygdalina Delile on the expression of EGFR and VEGFR as a new anticancer potential. Pharmacogn J. 2022;14:91–7. CrossRef
3. Karthick V, Akhila C, Ganesh Kumar V, Subashini D, Dhas TS, Govindaraju K, et al. In vitro anticancer activity of Sargassum sp. polysaccharides against MCF-7 cell lines. Indian J Mar Sci. 2019;48(8):1267–73.
4. Marudhupandi T, Ajith Kumar TT, Lakshmanasenthil S, Suja G, Vinothkumar T. In vitro anticancer activity of fucoidan from Turbinaria conoides against A549 cell lines. Int J Biol Macromol. 2015;72:919–23. CrossRef
5. Ajoko IT, Martin B, Amos-Tautua W, Songca SP. Ethnomedicinal and economical profile of Triumfetta cordifolia: a mini-review. J Med Plants Stud. 2020;8:208–12.
6. Gahamanyi N, Munyaneza E, Dukuzimana E, Tuyiringire N, Pan CH, Komba EVG. Ethnobotany, ethnopharmacology, and phytochemistry of medicinal plants used for treating human diarrheal cases in rwanda: a review. Antibiotics. 2021;10:1231. CrossRef
7. Joshi RK. GC-MS analysis of volatile organic constituents of traditionally used medicinal plants from the Western Ghats of India: Blumea lanceolaria (Roxb.) Druce., Heliotropium indicum L. and Triumfetta rhomboidea Jacq. J Mex Chem Soc. 2020;64:1–9. CrossRef
8. Devmurari VP, Ghodasara TJ, Jivani NP. Antibacterial activity and phytochemical study of ethanolic extract of Triumfetta rhomboidea Jacq. Int J Pharm Sci Drug Res. 2010;2:40–2.
9. Kendre N, Wakte P. Triumfetta rhomboidea: a review on its phytochemical and pharmacological profile. Int J Pharm Sci Res. 2022;13:3458. CrossRef
10. Marulasiddaswamy KM, Nuthan BR, Sunilkumar CR, Bajpe SN, Kumara KKS, Sekhar S, et al. HR-LC-MS based profiling of phytochemicals from methanol extracts of leaves and bark of Myristica dactyloides Gaertn. From Western Ghats of Karnataka, India. J Appl Biol Biotechnol. 2021;9:124–35. CrossRef
11. Amin E, Abdel-Bakky MS, Mohammed HA, Hassan MHA. Chemical profiling and molecular docking study of Agathophora alopecuroides. Life. 2022;12:1852. CrossRef
12. Lei X, Chen M, Nie Q. In vitro and in vivo antiangiogenic activity of desacetylvinblastine monohydrazide through inhibition of VEGFR2 and AXL pathways. Am J Cancer Res. 2016;6:843–58.
13. Sundaram MK. In silico discovery of seaweed molecules against matrixmetalloproteinase-26. J Adv Bioinform Appl Res. 2015;6:52–61.
14. Iheagwam FN, Ogunlana OO, Ogunlana OE, Isewon I, Oyelade J. Potential anti-cancer flavonoids isolated from Caesalpinia bonduc young twigs and leaves: molecular docking and in silico studies. Bioinform Biol Insights. 2019;13:1–16. CrossRef
15. Salunke M, Wakure B, Wakte P. HR-LCMS assisted phytochemical screening and an assessment of anticancer activity of Sargassum squarrossum and Dictyota dichotoma using in vitro and molecular docking approaches. J Mol Struct. 2022;1270:133833. CrossRef
16. Mohini S, Balaji W, Pravin W. Phytochemical analysis of Acanthophora najadiformis using high-resolution liquid chromatography mass spectrometry (HR-LCMS) and FTIR. J Pharm Negative Results. 2022;13:2215–8. CrossRef
17. Salunke M, Wakure B, Wakte P. High-resolution liquid chromatography mass spectrometry (HR-LCMS) and 1H NMR analysis of methanol extracts from marine seaweed Gracilaria edulis. Nat Prod Res. 2022;13:2215–8. CrossRef
18. Salunke MA, Wakure BS, Wakte PS. High-resolution liquid chromatography and mass spectrometry (HR-LCMS) assisted phytochemical profiling and an assessment of anticancer activities of Gracilaria foliifera and Turbinaria conoides using in vitro and molecular docking analysis. J Biomol Struct Dyn. 2022;11(17):1–4. CrossRef
19. Martinkovich S, Shah D, Planey SL, Arnott JA. Selective estrogen receptor modulators: tissue specificity and clinical utility. Clin Interv Aging. 2014;9:1437–52. CrossRef
20. Esmaeili S, Hamzeloo-Moghadam M, Ghaffari S, Mosaddegh M. Cytotoxic activity screening of some medicinal plants from south of Iran. Res J Pharmacogn. 2014;1:19–25.
21. Qawoogha SS, Shahiwala A. Identification of potential anticancer phytochemicals against colorectal cancer by structure-based docking studies. J Recept Signal Transduct Res. 2020;40:67–76. CrossRef
22. Gacche RN, Meshram RJ, Shegokar HD, Gond DS, Kamble SS, Dhabadge VN, et al. Flavonoids as a scaffold for development of novel anti-angiogenic agents: an experimental and computational enquiry. Arch Biochem Biophys. 2015;577–8:35–48. CrossRef
23. Stierand K, Maaß PC, Rarey M. Molecular complexes at a glance: automated generation of two-dimensional complex diagrams. Bioinformatics. 2006;22:1710–6. CrossRef
24. Gajiwala KS, Feng J, Ferre R, Ryan K, Brodsky O, Weinrich S, et al. Insights into the aberrant activity of mutant EGFR kinase domain and drug recognition. Structure. 2013;21:209–19. CrossRef
25. Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, Meyerson M, et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11:217–27. CrossRef
26. Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92.
27. Mctigue MA, Wickersham JA, Pinko C, Showalter RE, Parast CV, Tempczyk-Russell A, et al. Crystal structure of the kinase domain of human vascular endothelial growth factor receptor 2: a key enzyme in angiogenesis. Structure. 1999;7:319–30.
28. Regan J, Capolino A, Cirillo PF, Gilmore T, Graham AG, Hickey E, et al. Structure-activity relationships of the p38α MAP kinase inhibitor 1-(5-tert-butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-[4-(2-morpholin-4-yl-ethoxy) naphthalen-1-yl] urea (BIRB 796). J Med Chem. 2003;46:4676–86. CrossRef
29. Hasegawa M, Nishigaki N, Washio Y, Kano K, Harris PA, Sato H, et al. Discovery of novel benzimidazoles as potent inhibitors of TIE-2 and VEGFR-2 tyrosine kinase receptors. J Med Chem. 2007;50:4453–70. CrossRef
30. Potashman MH, Bready J, Coxon A, Demelfi TM, DiPietro L, Doerr N, et al. Design, synthesis, and evaluation of orally active benzimidazoles and benzoxazoles as vascular endothelial growth factor-2 receptor tyrosine kinase inhibitors. J Med Chem. 2007;50:4351–73. CrossRef
31. Harris PA, Cheung M, Hunter RN, Brown ML, Veal JM, Nolte RT, et al. Discovery and evaluation of 2-anilino-5-aryloxazoles as a novel class of VEGFR2 kinase inhibitors. J Med Chem. 2005;48:1610–9. CrossRef
32. Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 isessential for VEGF-A-dependent activation of PLC-gand DNA synthesis in vascular endothelial cells. Eur Mol Biol Organ. 2001;20:2768–78.