INTRODUCTION
Cancer caused 10 million deaths in developed and developing countries in 2020 [1]. World Cancer Research Fund International reported that cervical and colorectal cancers contributed to 342,000 and 935,000 deaths, respectively, in 2020 [2,3]. Therefore, cervical and colorectal cancers have been considered the leading cause of global cancer-related deaths [4,5]. Chemotherapy utilizing an anticancer agent is a widely used treatment to suppress cancer cells’ growth; however, some cancer cell lines have been resistant to commercial anticancer agents [6,7]. Numerous chemical compounds have been elucidated from natural sources and/or synthesized through chemical modifications [8–10]. However, their time-consuming isolation process and/or complicated synthesis procedures limit their real applications for large-scale drug production. Moreover, weak anticancer activity and poor selectivity of the new anticancer drugs attract serious concerns in the war on cancer diseases. [11–13].
Xanthone, an oxygenated heterocyclic compound, has been reported as a potential anticancer agent due to its ease of synthesis and good anticancer activity and selectivity [14]. Kostanecki and Nessler [15] reacted ortho-hydroxybenzoic acid and phenol compounds in the presence of acetic acid anhydride and ZnCl2 as the Lewis acid catalyst. On the other hand, Grover et al. [16] used POCl3 and ZnCl2 as the Lewis acid catalyst to react with ortho-hydroxybenzoic acid and phenolic compounds. Unfortunately, neither method is suitable for all phenolic compounds, especially 1,3-dihydroxybenzene and 1,3,5-trihydroxybenzene. When these phenolic compounds were reacted, the reaction stopped in the formation of benzophenone; thus, the tricyclic xanthone structures were not produced. Thus, researchers are trying to establish a new and efficient synthesis method for xanthone derivatives. Ullman condensation, Friedel–Crafts acylation, Robinson–Nishikawa, Smiles rearrangement, and Tanase synthetic routes have been reported to synthesize xanthones. However, their procedures need several reaction steps; thus, these methods are not categorized as efficient synthesis from the green chemistry point of view [17].
Eaton’s reagent consists of a mixture of 10% wt. P2O5 in methanesulfonic acid as the solvent. Eaton’s reagent has been employed as a Lewis acid catalyst in various cyclization reactions to generate heterocyclic structures of quinolinones, benzimidazoles, and carbazoles [18–20]. Our research group has been using Eaton’s reagent for the one-pot synthesis of 1,6-dihydroxyxanthone, 1,3,6-trihydroxyxanthone, 1,3,8-trihydroxyxanthone, and 1,5,6-trihydroxyxanthone [21]. In continuation of our research, we herein synthesized the other hydroxylated xanthones, i.e., 1-hydroxyxanthone, 1,3-dihydroxyxanthone, and 3,6-dihydroxyxanthone, employing Eaton’s reagent, in a one-pot synthesis procedure.
Xanthone and its derivatives have been investigated as bioactive compounds, including anticancer, anti-inflammatory, antiviral, antioxidant, antidiabetic, antimicrobial, and antimalarial agents [14]. As the anticancer agent, the unmodified xanthone gave poor anticancer activity against the colorectal cancer cell line with a half-maximal inhibitory concentration (IC50) value of 200 mM. The addition of chloro and bromo substituents at the xanthone structure, resulting in 2-chloroxanthone and 2-bromoxanthone, enhanced the anticancer activity with IC50 values of 96.7 and 85.1 mM, respectively. Meanwhile, the addition of a hydroxyl group on the halogenated xanthone exhibited much stronger anticancer activity with IC50 values of 0.15 and 0.15 mM for 2-chloro-1,3-dihydroxyxanthone and 2-bromo-1,3-dihydroxyxanthone, respectively [22]. This result clearly demonstrated that the presence of the hydroxyl group is much more critical than halogen groups for the anticancer activity of xanthone derivatives.
Fatmasari et al. [23] reported the anticancer activity of 1,3,8-trihydroxyxanthone, 1,6-dihydroxyxanthone, and 1,5,6-trihydroxyxanthone against WiDr and HeLa cancer cell lines. These hydroxyxanthones gave the IC50 value of 0.209–0.355 and 0.241–0.322 mM against the WiDr and HeLa cancer cell lines, respectively. It was reported that the anticancer activity of the hydroxyxanthones comes through the Topoisomerase II inhibition, thus leading to anti-proliferative and apoptosis mechanisms [23]. The presence of the hydroxyl group is critical to enhancing anticancer activity through the formation of noncovalent interactions with the key amino acid residues at the Topoisomerase II receptor, which is overexpressed in the cervical and colorectal cancer cells [24]. Unfortunately, a comprehensive discussion on the effect of the number and position of the hydroxyl group on the anticancer activity of xanthone has not been available as of today.
Therefore, in the present work, we synthesized the other hydroxylated xanthones (i.e., 1-hydroxyxanthone, 1,3-dihydroxyxanthone, and 3,6-dihydroxyxanthone) and discussed the effect of the number and location of the hydroxyl group on the anticancer activity and selectivity against HeLa and WiDr cancer cells. The most potent anticancer agent was further examined through molecular docking studies against the Topoisomerase II receptor to reveal its mechanism of action as the anticancer agent.
MATERIALS AND METHODS
Chemistry
The 2-hydroxybenzoic acid, 2,4-dihydroxybenzoic acid, 1,3-dihydroxybenzene, 1,3,5-trihydroxybenzene, and Eaton’s reagent were purchased from Merck in proanalytical grade. The synthesis and anticancer activity of 1,6-dihydroxyxanthone, 1,3,6-trihydroxyxanthone, 1,3,8-trihydroxyxanthone, and 1,5,6-trihydroxyxanthone have been reported in the previous work [23]. The infrared spectra of the synthesized compounds were measured in the form of a KBr pellet using a Fourier-transform infrared spectrophotometer (FTIR, Shimadzu Prestige21). The proton and carbon nuclear magnetic resonance (NMR) spectra of the products were analyzed in deuterated methanol with tetramethylsilane as the internal standard using a JNM-ECZ500R/S1 spectrometer. The mass spectra (MS) were recorded on a Shimadzu QP2010S spectrometer. Meanwhile, the 3-D structure of Topoisomerase II was retrieved from a protein data bank (https://www.rcsb.org) with 4G0V as the ID number. The software utilized for molecular docking studies was Gaussian09W, Chimera 1.13.1, AutoDock Tools 1.5.6, and Discovery Studio Visualizer 2019. These software programs were supported by Austrian–Indonesian Center for Computational Chemistry, Universitas Gadjah Mada.
METHODS
One-pot synthesis of hydroxylated xanthones
An equivalent mixture of hydroxybenzoic acid (15 mmol) and hydroxybenzene (15 mmol) was mixed in the presence of Eaton’s reagent (8 ml) as the Lewis acid catalyst and solvent. The mixture was then reacted at 80°C. The reaction was monitored by thin-layer chromatography analysis using a mixture of n-hexane:ethyl acetate in a 7:3 volume ratio as the mobile phase. After 3 hours, the mixture was cooled to reach room temperature and then poured into cold distilled water (30 ml) to precipitate the product. The crude product was filtered, dried, and purified by preparative thin-layer chromatography using a mixture of n-hexane:ethyl acetate in a 7:3 volume ratio as the mobile phase. The yellow spot was taken, rinsed with methanol (10 ml), filtered, and evaporated under a vacuum to obtain the target compound. The product was characterized using FTIR, NMR, and MS analyses. The 1-hydroxyxanthone was obtained from the reaction of 2-hydroxybenzoic acid and 1,3-dihydroxybenzene. The 1,3-dihydroxyxanthone was obtained from the reaction of 2-hydroxybenzoic acid and 1,3,5-trihydroxybenzene. The 3,6-dihydroxyxanthone was obtained from the reaction of 2,4-dihydroxybenzoic acid and 1,3-dihydroxybenzene. Meanwhile, the 1,3,6-trihydroxyxanthone has been prepared from 2,4-dihydroxybenzoic acid and 1,3,5-trihydroxybenzene, according to the previous work [24].
1-Hydroxyxanthone was obtained as a yellow solid in 6.60% yield. FTIR (cm−1): 3,124 (O-H stretching), 1,613 (C-O), 1,450 (C=C stretching), and 1,119 (C=O stretching) (Fig. S1). 1H-NMR (500 MHz) δ (ppm): 6.79 (1H, doublet, J = 7.5 Hz, H2), 7.01 (1H, doublet, J = 7.5 Hz, H4), 7.45 (1H, triplet, J = 8.5 Hz, H7), 7.57 (1H, doublet, J = 8.5 Hz, H5), 7.66 (1H, triplet, J = 7.5 Hz, H3), 7.84 (1H, triplet, J = 8.5 Hz, H6), and 8.26 (1H, doublet, J = 8.5 Hz, H8) (Fig. S2). 13C-NMR (125 MHz) δ (ppm): 108.31, 109.99, 111.42, 119.20, 121.79, 125.54, 126.87, 137.31, 138.41, 157.77, 157.90, 163.17 (12 aromatic carbons), and 183.75 (C=O) (Fig. S3). MS: m/z = 213 ([M+1]+, 15%), 212 ([M]+ and base peak, 100%), 184 (25%), 155 (5%), 128 (30%), and 77 (10%) (Fig. S4).
1,3-Dihydroxyxanthone was obtained as a yellow solid in 46.50% yield. FTIR (cm−1): 3,387 and 3,140 (O-H stretching), 1,612 (C-O), 1,458 (C=C stretching), and 1,172 (C=O stretching) (Fig. S5). 1H-NMR (500 MHz) δ (ppm): 6.19 (1H, doublet, J = 2.0 Hz, H2), 6.35 (1H, doublet, J = 2.0 Hz, H4), 7.39 (1H, triplet, J = 8.0 Hz, H7), 7.48 (1H, doublet, J = 8.0 Hz, H5), 7.76 (1H, triplet, J = 8.0 Hz, H6), and 8.18 (1H, doublet, J = 8.0 Hz, H8) (Fig. S6). 13C-NMR (125 MHz) δ (ppm): 95.36, 99.44, 103.89, 118.81, 121.78, 125.26, 126.62, 136.43, 157.50, 159.56, 164.94, 168.14 (12 aromatic carbons), and 181.82 (C=O) (Fig. S7). MS: m/z = 229 ([M+1]+, 15%), 228 ([M]+ and base peak, 100%), 200 (20%), 171 (35%), and 77 (5%) (Fig. S8).
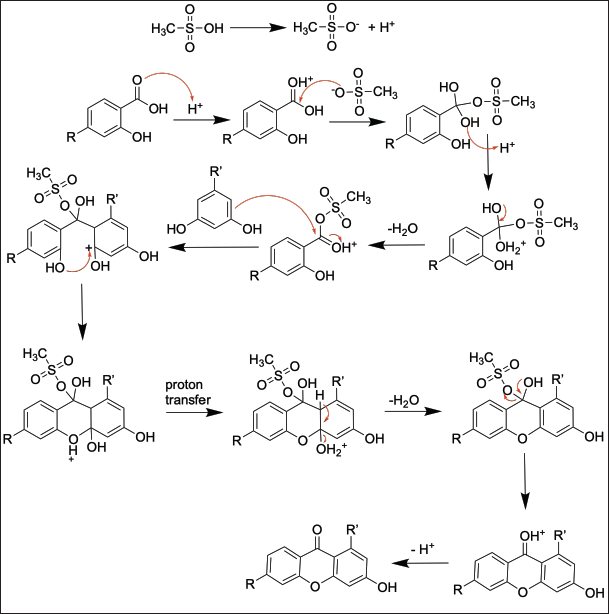 | Figure 1. The reaction mechanism in the production of the hydroxylated xanthones. [Click here to view] |
3,6-Dihydroxyxanthone was obtained as a yellow solid in 21.93% yield. FTIR (cm−1): 3,325 (O-H stretching), 1,621 (C=O), 1,458 (C=C stretching), and 1,165 (C-O stretching) (Fig. S9). 1H-NMR (500 MHz) δ (ppm): 6.81 (2H, doublet, J = 2.5 Hz, H2), 6.85 (2H, doublet of doublet, J = 2.5 and 6.0 Hz, H4), and 8.06 (2H, doublet, J = 6.0 Hz, H8) (Fig. S10). 13C-NMR (125 MHz) δ (ppm): 103.31, 115.92, 115.59, 129.12, 159.88, 165.52 (6 aromatic carbons), and 177.69 (C=O) (Fig. S11). MS: m/z = 229 ([M+1]+, 20%), 228 ([M]+ and base peak, 100%), 200 (70%), 171 (40%), 155 (5%), and 77 (5%) (Fig. S12).
Anticancer activity assay of hydroxylated xanthones
The anticancer activity of hydroxylated xanthones was investigated through in vitro assay following the reported procedure employing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent [23]. Meanwhile, molecular docking was conducted the same as reported before [23]. Briefly, the 3-D structure of the Topoisomerase II receptor was prepared using Chimera 1.13.1, while the 3-D structure of hydroxylated xanthone was optimized using Gaussian09W with DFT-B3LYP-6,31G as the basis set. The molecular docking of hydroxylated xanthone was conducted in a 50 × 50 × 50 Å grid box with a spacing of 0.375 Å. The minimal and maximal coordinates in the grid box were fixed at (x, y, z) = (26.124, 79.227, 41.381) and (44.874, 97.977, 60.131), respectively, for 100 calculations of Lamarckian genetic algorithms. The final coordinate of hydroxylated xanthone, Gibbs free energy, ligand efficiency, binding constant, and root-mean-square-deviation (RMSD) parameters were retrieved from the calculations using AutoDock Tools 1.5.6. On the other hand, the formed noncovalent interactions between the hydroxylated xanthone and the active site of the Topoisomerase II protein receptor were visualized using Discovery Studio Visualizer 2019 software.
RESULTS AND DISCUSSION
One-pot synthesis of hydroxylated xanthones
In this work, hydroxylated xanthones were synthesized by reacting hydroxybenzoic acid with hydroxybenzene derivatives under acidic conditions. Eaton’s reagent facilitates the formation of the cyclic structure of xanthone. At first, methanesulfonic acid donates a proton to the carbonyl group of benzoic acid. Consequently, the electrophilicity of benzoic acid is higher and ready to be attacked by hydroxybenzene. The acylation reaction of hydroxybenzene with activated benzoic acid derivative leads to the formation of a benzophenone structure as the intermediate. Afterward, both hydroxyl groups at the ortho position were eliminated as water molecule to form the tricyclic structure of hydroxylated xanthones, as shown in Figure 1 [25]. The synthesis scheme of hydroxylated xanthones in this work is shown in Figure 2. In this work, the hydroxylated xanthones have been successfully synthesized as yellow solids in 6.60%–46.50% yield. The lowest yield of 1-hydroxyxanthone (6.60%) was caused by the competition reaction with the formation of 3-hydroxyxanthone. Meanwhile, the other hydroxylated xanthones were obtained in low-to-medium yield (21.93%–46.50%) due to a short reaction time (3 hours), indicating that the reaction might not be optimized yet.
The chemical structures of hydroxylated xanthones have been confirmed by using FTIR, NMR, and also MS. The FTIR data confirmed the presence of O-H hydroxyl, C=O carbonyl, C=C aromatic ring, and C-O ether functional groups on the structure of hydroxylated xanthones. The C=O functional group was observed in 1,612–1,621 cm−1 due to the conjugation with two aromatic rings that agreed with the other reported works [23]. The 1-hydroxyxanthone showed seven aromatic protons and twelve aromatic carbons on its NMR spectra. The 1,3-dihydroxyxanthone showed six aromatic protons and twelve aromatic carbons on its NMR spectra, while the 3,6-dihydroxyxanthone showed three types of aromatic proton and six types of aromatic carbon on its NMR spectra due to its symmetrical structure. The presence of C=O carbonyl group of 1-hydroxyxanthone, 1,3-dihydroxyxanthone, and 3,6-dihydroxyxanthone on their FTIR spectra was confirmed by the signal at 183.75, 181.82, and 177.69 ppm, respectively, on the 13C-NMR spectrum. The molecular mass of the hydroxylated xanthones was confirmed by the appearance of molecular ion (M+) at m/z = 212 and 228. These molecular ions became the base peak of MS because of the highly stable hydroxylated xanthones’ structure.
Anticancer activity investigation of hydroxylated xanthones
The anticancer activity of hydroxylated xanthones is shown in Table 1, while their chemical structures are displayed in Figure 2. It was found that 1-hydroxyxanthone, 1,3-dihydroxyxanthone, 1,6-dihydroxyxanthone, 3,6-dihydroxyxanthone, 1,3,6-trihydroxyxanthone, 1,3,8-trihydroxyxanthone, and 1,5,6-trihydroxyxanthone gave the IC50 values of 1.198, 0.086, 0.322, 0.162, 0.203, 0.277, and 0.241 mM, respectively, against HeLa cancer cells. A lower IC50 value represents a higher anticancer activity [26].
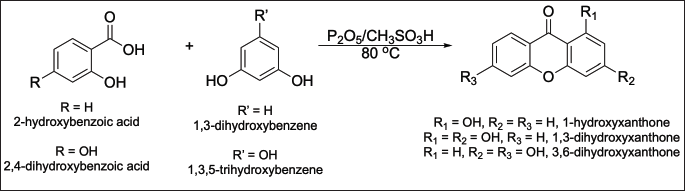 | Figure 2. The synthesis scheme of hydroxylated xanthones. [Click here to view] |
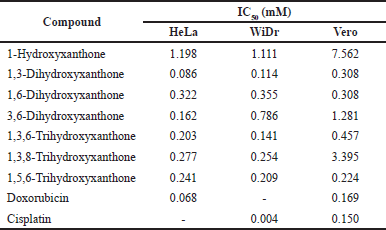 | Table 1. Anticancer activity and cytotoxicity of hydroxylated xanthones against HeLa and WiDr cancer cells. [Click here to view] |
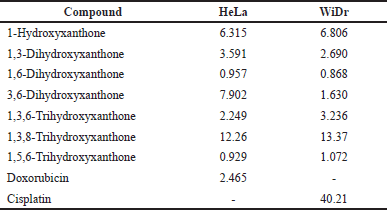 | Table 2. Selectivity index of hydroxylated xanthones as the anticancer agents. [Click here to view] |
The 1-hydroxyxanthone showed the weakest anticancer activity against HeLa cancer cells (IC50 = 1.198 mM) due to the presence of a single hydroxyl group. Additional hydroxyl groups in 1-hydroxyxanthone structure, i.e., 1,3-dihydroxyxanthone, 1,6-dihydroxyxanthone, 3,6-dihydroxyxanthone, 1,3,6-trihydroxyxanthone, 1,3,8-trihydroxyxanthone, and 1,5,6-trihydroxyxanthone, enhanced its anticancer activity (IC50 = 0.086–0.322 mM). The addition of a hydroxyl group on the 3-position of 1-hydroxyxanthone, named 1,3-dihydroxyxanthone, exhibited better anticancer activity (IC50 = 0.086 mM) compared to the addition of hydroxyl group on the 6-position (IC50 = 0.322 mM). It suggested that the 3-hydroxyl group gave a higher anticancer activity enhancement of 1-hydroxyxanthone against HeLa cancer cells compared to the 6-hydroxyl group. A combination of 3-hydroxyl and 6-hydroxyl groups at 3,6-dihydroxyxanthone gave the IC50 value of 0.162 mM, in which the IC50 value was in between the anticancer activity of 1,3-dihydroxyxanthone (IC50 = 0.086 mM) and 1,6-dihydroxyxanthone (IC50 = 0.322 mM).
Further addition of hydroxyl group on the 1,6-dihydroxyxanthone (IC50 = 0.322 mM), i.e., 1,3,6-trihydroxyxanthone and 1,5,6-trihydroxyxanthone, slightly enhanced its anticancer activity (IC50 = 0.203–0.241 mM). In contrast, the additional hydroxyl group on the 1,3-dihydroxyxanthone (IC50 = 0.086 mM), i.e., 1,3,6-trihydroxyxanthone and 1,3,8-trihydroxyxanthone, demarcated its anticancer activity (IC50 = 0.203–0.277 mM). As reported before, this result could be explained by stronger intramolecular and/or intermolecular hydrogen bonds between the trihydroxylated xanthones during the anticancer examination [13]. From these data, the anticancer activity order against HeLa cancer cells was as follows: 1-hydroxyxanthone < 1,6-dihydroxyxanthone < 1,3,8-trihydroxyxanthone < 1,5,6-trihydroxyxanthone < 1,3,6-trihydroxyxanthone < 3,6-dihydroxyxanthone < 1,3-dihydroxyxanthone. It meant that 1,3-dihydroxyxanthone exhibited the strongest anticancer activity against HeLa cancer cells with an IC50 value of 0.086 mM.
Since the hydroxylated xanthones showed high anticancer activity against HeLa cancer cells, the cytotoxicity evaluation was also performed against non-cancer (normal) cells, i.e., the Vero cell line. The IC50 values of hydroxylated xanthones against normal cells are listed in Table 1. The IC50 value of hydroxylated xanthones against normal cells is expected to be higher than the HeLa cancer cells. If so, the hydroxylated xanthones prefer to attack HeLa cancer cells rather than normal cells. From Table 1, only 1,6-dihydroxyxanthone and 1,5,6-trihydroxyxanthone gave lower IC50 values against Vero cells (0.308 and 0.224 mM) than HeLa cancer cells (0.322 and 0.241 mM). These results indicated that both hydroxylated xanthones were more toxic to normal cells than HeLa cancer cells, which is unfavorable.
The selectivity index is a well-known parameter to determine the toxicity of anticancer agents. An anticancer agent is labeled a toxic drug when the selectivity index value is lower than 2.00 [27]. The selectivity index is calculated by the division of the IC50 value against normal cells over the IC50 value of cancer cells. The selectivity index of hydroxylated xanthones from the IC50 values data against HeLa cancer cells is shown in Table 2. The selectivity indexes of 1-hydroxyxanthone, 1,3-dihydroxyxanthone, 3,6-dihydroxyxanthone, 1,3,6-trihydroxyxanthone, and 1,3,8-trihydroxyxanthone were 6.315, 3.591, 7.902, 2.249, and 12.26, respectively, which were higher than 2.00. Thus, these hydroxylated xanthones are not considered toxic to normal cells.
From these data, the order of toxicity was 1,3,8-trihydroxyxanthone < 3,6-dihydroxyxanthone < 1-hydroxyxanthone < 1,3-dihydroxyxanthone < 1,3,6-trihydroxyxanthone < 1,6-dihydroxyxanthone < 1,5,6-trihydroxyxanthone. This order might suggest that 1,3,8-trihydroxyxanthone was the best anticancer agent with the lowest toxicity to normal cells. However, both sensitivity (low IC50 value) and selectivity (high selectivity index) shall be carefully considered in the design and development of anticancer agents. The selectivity index of 1,3,8-trihydroxyxanthone (12.26) was higher than 1,3-dihydroxyxanthone (3.591); however, its anticancer activity (IC50 = 0.277 mM) was lower than 1,3-dihydroxyxanthone (IC50 = 0.086 mM). Furthermore, the IC50 value of 1,3-dihydroxyxanthone (IC50 = 0.086 mM) was close to doxorubicin (IC50 = 0.068 mM) as the standard cancer drug. In contrast, it shall be noted that the 1,3-dihydroxyxanthone was 1.5 times less toxic than doxorubicin, which was remarkable.
On the other hand, 1-hydroxyxanthone, 1,3-dihydroxyxanthone, 1,6-dihydroxyxanthone, 3,6-dihydroxyxanthone, 1,3,6-trihydroxyxanthone, 1,3,8-trihydroxyxanthone, and 1,5,6-trihydroxyxanthone exhibited an IC50 value of 1.111, 0.114, 0.355, 0.786, 0.141, 0.254, and 0.209 mM, respectively, against WiDr cancer cells. The trend for the anticancer activity of hydroxylated xanthones against the WiDr cancer cells was similar to the HeLa cancer cells. The 1-hydroxyxanthone showed the weakest anticancer activity (IC50 = 1.111 mM) against WiDr cancer cells due to the presence of a single hydroxyl group. Additional hydroxyl groups in 1-hydroxyxanthone structure, i.e., 1,3-dihydroxyxanthone, 1,6-dihydroxyxanthone, 3,6-dihydroxyxanthone, 1,3,6-trihydroxyxanthone, 1,3,8-trihydroxyxanthone, and 1,5,6-trihydroxyxanthone, increased the anticancer activity (IC50 = 0.114–0.786 mM).
The addition of a hydroxyl group on the 3-position of 1-hydroxyxanthone, named 1,3-dihydroxyxanthone, also exhibited better anticancer activity (IC50 = 0.114 mM) against WiDr cancer cells compared to the addition of hydroxyl group on the 6-position (IC50 = 0.355 mM). It showed that the presence of the 3-hydroxyl group yielded a higher anticancer activity enhancement of 1-hydroxyxanthone compared to the 6-hydroxyl group. Further addition of hydroxyl group on the 1,6-dihydroxyxanthone (IC50 = 0.355 mM), i.e., 1,3,6-trihydroxyxanthone and 1,5,6-trihydroxyxanthone, slightly enhanced its anticancer activity (IC50 = 0.141–0.209 mM) against WiDr cancer cells.
In contrast, the additional hydroxyl group on the 1,3-dihydroxyxanthone (IC50 = 0.114 mM), i.e., 1,3,6-trihydroxyxanthone and 1,3,8-trihydroxyxanthone, suppressed its anticancer activity (IC50 = 0.141–0.254 mM). These data could be explained by stronger intramolecular and/or intermolecular hydrogen bonds between the hydroxylated xanthones during the anticancer examination which agreed with the reported literature [23]. From these data, the order of anticancer activity against WiDr cancer cells was 1-hydroxyxanthone < 3,6-dihydroxyxanthone < 1,6-dihydroxyxanthone < 1,3,8-trihydroxyxanthone < 1,5,6-trihydroxyxanthone < 1,3,6-trihydroxyxanthone < 1,3-dihydroxyxanthone. It meant that 1,3-dihydroxyxanthone exhibited the strongest anticancer activity against WiDr cancer cells with an IC50 value of 0.114 mM.
From Table 1, only 1,6-dihydroxyxanthone gave a lower IC50 value against the normal cell line (0.308 mM) than the WiDr cancer cell line (0.355 mM). Table 2 reveals that three hydroxylated xanthones, i.e., 1,6-dihydroxyxanthone, 3,6-dihydroxyxanthone and 1,5,6-trihydroxyxanthone were not qualified as safe anticancer agents because their selectivity index values were less than 2.00. In contrast, 1-hydroxyxanthone, 1,3-dihydroxyxanthone, 1,3,6-trihydroxyxanthone, and 1,3,8-trihydroxyxanthone yielded a selectivity index of 6.806, 2.690, 3.236, and 13.37, respectively. Therefore, they are considered safe anticancer agents that are nontoxic to normal cells.
The order of toxicity was 1,3,8-trihydroxyxanthone < 1-hydroxyxanthone < 1,3,6-trihydroxyxanthone < 1,3-dihydroxyxanthone < 3,6-dihydroxyxanthone < 1,5,6-trihydroxyxanthone < 1,6-dihydroxyxanthone. Even though the selectivity index of 1,3,8-trihydroxyxanthone (13.37) was higher than 1,3-dihydroxyxanthone (2.690), however, its anticancer activity (IC50 = 0.254 mM) was lower than 1,3-dihydroxyxanthone (IC50 = 0.114 mM). Furthermore, Tables 1 and 2 show that 1,3-dihydroxyxanthone gave a comparable IC50 value and higher selectivity index than doxorubicin as the positive control. Therefore, as the 1,3-dihydroxyxanthone had fulfilled the requirement for low toxicity against normal cells, 1,3-dihydroxyxanthone was selected as the best candidate for an anticancer agent in this study.
Molecular docking studies of hydroxylated xanthones
To reveal the mechanism of action of 1,3-dihydroxyxanthone as an anticancer agent, molecular docking studies were conducted against the Topoisomerase II receptor, a protein that plays a pivotal role in the multiplication of cancer cells [28]. The Topoisomerase II receptor was selected as this receptor was overexpressed in 86 cervical and colorectal cancer patients [29]. The first step in the molecular docking of 1,3-dihydroxyxanthone was locating the 1,3-dihydroxyxanthone in the active site of the Topoisomerase receptor in the same position as mitoxantrone as the native ligand. The 1,3-dihydroxyxanthone had a similar structure to mitoxantrone, thus 1,3-dihydroxyxanthone is expected to be able to bind in the active of the Topoisomerase II receptor (Fig. 3).
The whole complex structure of 1,3-dihydroxyxanthone in the active site of the Topoisomerase II receptor is shown in Figure 4a. To simplify, the active site of Topoisomerase II is focused on in Figure 4b. Figure 4b shows that the hydroxyl and carbonyl groups of 1,3-dihydroxyxanthone interacted with Guanine13, Cytosine14, Lysine505, and Alanine521 through the hydrogen bonds. This result confirmed the importance of 1-hydroxyl and 3-hydroxyl groups as described in the in vitro assay. The 1,3-dihydroxyxanthone also interacted with Arginine503 and Glutamic acid522 through noncovalent interactions, as shown in Figure 4b.
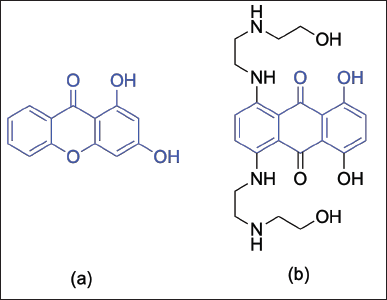 | Figure 3. The chemical structure of (a) 1,3-dihydroxyxanthone and (b) mitoxantrone. [Click here to view] |
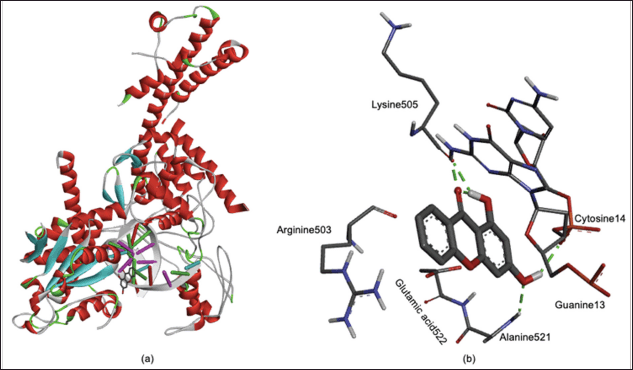 | Figure 4. (a) Whole and (b) focused on three dimensional-structure of 1,3-dihydroxyxanthone in the active site of Topoisomerase II. [Click here to view] |
The more detailed noncovalent interactions of 1,3-dihydroxyxanthone in the active site of Topoisomerase II are displayed in a 2-D contour (Fig. 5 and Table 3). The 1,3-dihydroxyxanthone interacted with Guanine13 through its carbonyl group, with Lysine505 through its 1-hydroxyl group, and with Cytosine14 and Alanine521 through its 3-hydroxyl group with the hydrogen bond distance in a range of 3.87–7.01 Å. The six-membered rings of 1,3-dihydroxyxanthone interacted with Glutamic acid522 through pi-anion interaction with a distance of 5.62 and 7.05 Å, respectively. Instead of a hydrogen bond with Guanine13 through its carbonyl group, the 1,3-dihydroxyxanthone also interacted with Guanine13 through pi-pi T-shape interaction with a distance of 4.26 and 4.95 Å, respectively. Furthermore, the aromatic ring of 1,3-dihydroxyxanthone interacted with Arginine503 and Alanine521 amino acid residues through pi-alkyl interactions with a distance of 4.97 and 6.72 Å, respectively. The van der Waals interactions with Adenine12, Glycine504, Isoleucine506, Leucine507, and Asparagine520 are also observed in Figure 5.
The 1,3-dihydroxyxanthone was located in a final coordinate of (x, y, z) = (34.415–36.444, 84.007–93.235, 48.450–53.379). With the aforementioned noncovalent interactions above, the 1,3-dihydroxyxanthone generated four types of energy. The first energy consisted of hydrogen bonds, van der Waals, de-solvation, and electrostatic energy with a total of −7.47 kcal/mol. The other energies were final total internal energy, torsional free energy, and unbonded system with a value of −0.67, +0.60, and −0.67 kcal/mol, respectively. The Gibbs free energy (−6.85 kcal/mol) was calculated by and summarizing all energies. Meanwhile, the ligand efficiency, binding constant, and RMSD values were 0.40, 9.56 × 10−6 mol/l, and 0.15 Å, respectively. These results demonstrated that 1,3-dihydroxyxanthone interacted well with key amino acid residues in the active site of the Topoisomerase II receptor, thus, disturbing the protein’s function in the multiplication of cancer cells. It was reported that disturbance of Topoisomerase II receptors’ function led to apoptotic cancer cell death [23]. Furthermore, Negri et al. [30] and Zhao et al. [31] reported that Topoisomerase II inhibition decreased the cancer cells’ viability, as well as the rate of deoxyribonucleic acid synthesis in the cancer cells. This result could be the reason for the strong anticancer activity of 1,3-dihydroxyxanthone with IC50 values of 0.086 and 0.114 mM against HeLa and WiDr cancer cell lines.
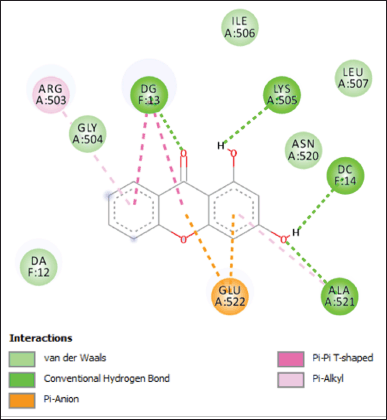 | Figure 5. Non-covalent interactions of 1,3-dihydroxyxanthone in the active site of Topoisomerase II. [Click here to view] |
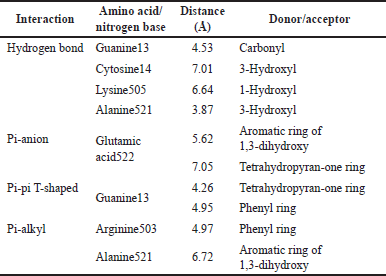 | Table 3. Observed interactions of 1,3-dihydroxyxanthone in the active site of Topoisomerase II receptor. [Click here to view] |
CONCLUSION
The hydroxylated xanthones have been successfully synthesized through a one-pot reaction in 6.60%–46.50% yield. Seven hydroxylated xanthones have been evaluated as anticancer agents against HeLa cervical and WiDr colorectal cancer cells through in vitro MTT and in silico molecular docking studies. The addition of the 3-hydroxyl group gave a stronger anticancer activity than the addition of the 1-hydroxyl group against both cancer cells. Among the investigated hydroxylated xanthones, 1,3-dihydroxyxanthone exhibited the strongest anticancer activity with IC50 and selectivity index values of 0.086–0.114 mM and 2.690–3.591, respectively. Moreover, 1,3-dihydroxyxanthone exhibited comparable anticancer activity to doxorubicin with less toxicity to normal cells. From the molecular docking studies, 1,3-dihydroxyxanthone interacted with amino acid and nitrogen base residues through non-covalent interactions. These findings demonstrated that 1,3-dihydroxyxanthone could disturb the function of Topoisomerase II on the multiplication of cancer cells and lead to the death of cervical and colorectal cancer cells.
LIST OF ABBREVIATIONS
FTIR: Fourier-transforms infrared spectrophotometer; IC50: Half-maximal inhibitory concentration; MS: Mass spectra; MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NMR: Nuclear magnetic resonance; RMSD: Root-mean-square-deviation.
ACKNOWLEDGMENT
The authors thank Final Project Recognition Grant (RTA) Universitas Gadjah Mada Number 5075/UN1.P.II/Dit-Lit/PT.01.01/2023. The first author acknowledges the Indonesia Endowment Fund for Education (LPDP), Ministry of Finance, The Republic of Indonesia for the provided scholarship to pursue doctoral study at Universitas Gadjah Mada (2022-2026). The authors also thank the Austrian-Indonesian Center for Computational Chemistry (AIC), Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Gadjah Mada for providing Gaussian09 licenses in this work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics 2022. CA Cancer J Clin. 2021;72:7–33. CrossRef
2. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival and risk factors. Gastroenterol Rev. 2019;14:89–103. CrossRef
3. Burmeister CA, Khan SF, Schafer G, Mbatani N, Adams T, Moodley J, et al. Cervical cancer therapies: current challenges and future perspectives. Tumour Virus Res. 2022;13:200238. CrossRef
4. Guimaraes YM, Godoy LR, Longatto-Filho A, dos Reis R. Management of early-stage cervical cancer: a literature review. Cancers. 2022;14:575. CrossRef
5. Hossain MS, Karuniawati H, Jairoun AA, Urbi Z, Ooi DJ, John A, et al. Colorectal cancer: a review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers. 2022;14(7):1732. CrossRef
6. Bachelard CM, Coquan E, du Rusquec P, Paoletti X, Tourneau CL. Risks and benefits of anticancer drugs in advanced cancer patients: a systematic review and meta-analysis. eClinicalMedicine. 2021;40:101130. CrossRef
7. Ramirez-Malule H, Cardona GW. Bibliometric analysis of recent research on 5-fluorouracil (2015-2020). J Appl Pharm Sci. 2022;12(01):70–7. CrossRef
8. Mohamed NR, Khaireldin NY, Fahmy AF, El-Sayed AA. Facile synthesis of fused nitrogen containing heterocycles as anticancer agents. Der Pharma Chem. 2010;2(1):400–17.
9. El-Sayed AA, El-Saidi MMT, Khattab R. Unexpected reactions of azido-p-benzoquinone derivatives towards lawesson’s reagent & molecular docking study as a promising anticancer agent. Egyptian J Chem. 2019;62:315–26. CrossRef
10. El-Saidi MMT, El-Sayed, AA, Pedersen EB, Tantawy MA, Mohamed NR, Gad WA. Synthesis, characterization and docking study of novel pyrimidine derivatives as anticancer agents. Indones J Chem. 2020;20(5):1163–77. CrossRef
11. Chen EY, Ragunathan V, Prasad V. An overview of cancer drugs approved by the US Food and Drug Administration based on the surrogate end point of response rate. JAMA Int Med. 2019;179(9):915–21. CrossRef
12. Ibrahim A, Siswandono S, Wardojo BPE. Potential anticancer activities of chloroform subfraction from peronema leaf on colon cancer HT-29 cells in vitro. J Appl Pharm Sci. 2021;11(12):82–9. CrossRef
13. Ang AMG, Tabugo SRM, Uy MM. Antiproliferative, proapoptotic, and antimigration activities of marine sponges against human colon cancer cell line (HCT116). J Appl Pharm Sci. 2023;13(7):186–92. CrossRef
14. Shagufta S, Ahmad I. Recent insight into the biological activities of synthetic xanthone derivatives. Eur J Med Chem. 2016;116:267–80. CrossRef
15. Kostanecki SV, Nessler B. Synthesen von oxyxanthonen. Ber Dtsch Chem Ges. 1891;24:1894–7.
16. Grover PK, Shah GD, Shah RC. Xanthones. Part IV. A new synthesis of hydroxyxanthones and hydroxybenzophenones. J Chem Soc. 1955;1:3982–5. CrossRef
17. Resende DISP, Duraes F, Maia M, Sousa E, Pinto MMM. Recent advances in the synthesis of xanthones and azaxanthones. Org Chem Front. 2020;7:3027–66. CrossRef
18. Yuan J, Jin L, Chen R, Tang X, Xie X, Tang Y, et al. Eaton’s reagent assisted aromatic C-C coupling of carbazoles for optoelectronic applications. New J Chem. 2018;42:14704–8. CrossRef
19. Olvera-Mancilla J, Palacios-Alquisira J, Alexandrova L. Eaton’s reagent in polybenzimidazole synthesis: the influence of temperature and microwave irradiation. High Perform Polym. 2018;30(6):699–709. CrossRef
20. Ulysse LG, Yang Q, McLaws MD, Keefe DK, Guzzo PR, Haney BP. Process development and pilot-scale synthesis of new cyclization conditions of substituted phenylacetamides to tetrahydroisoquinoline-2-ones using Eaton’s reagent. Org Process Res Dev. 2010;14(1):225–8. CrossRef
21. Yuanita E, Ulfa M, Sudirman S, Sumarlan I, Sudarma IM, Dharmayani NKT, et al. Synthesis, cytotoxic evaluation and molecular docking of bromo-substituted 1,3,6-trihydroxyxanthone as protein tyrosine kinase inhibitor. Malaysian J Chem. 2021;23:24–32.
22. Liu J, Zhang J, Wang H, Liu Z, Zhang C, Jiang Z, et al. Synthesis of xanthone derivatives and studies on the inhibition against cancer cells growth and synergistic combinations of them. Eur J Chem. 2017;133:50–61. CrossRef
23. Fatmasari N, Kurniawan YS, Jumina J, Anwar C, Priastomo Y, Pranowo HD, et al. Synthesis and in vitro assay of hydroxyxanthones as antioxidant and anticancer agents. Sci Rep. 2022;12:1535. CrossRef
24. Yuanita E, Pranowo HD, Siswanta D, Swasono RT, Mustofa M, Zulkarnain AK, et al. One-pot synthesis, antioxidant activity and toxicity evaluation of some hydroxyxanthones. Chem Chem Technol. 2018;12:290–5.
25. Kurniawan YS, Priyangga KTA, Jumina J, Pranowo HD, Sholikhah EN, Zulkarnain AK, et al. An update on the anticancer activity of xanthone derivatives: a review. Pharmaceuticals. 2021;14:1144. CrossRef
26. Kumar P, Nagarajan A, Uchil PD. Analysis of cell viability by the MTT assay. Cold Spring Harb Protoc. 2018;2018(6):119–26. CrossRef
27. Badisa RB, Darling-Reed SF, Joseph P, Cooperwood JS, Latinwo LM, Goodman CB. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009;29:2993–6.
28. Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9:338–50. CrossRef
29. McLeod HL, Douglas F, Oates M, Symonds RP, Prakash D, van der Zee AG, et al. Topoisomerase I and II activity in human breast, cervix, lung and colon cancer. Int J Cancer. 1994;59(5):607–11. CrossRef
30. Negri C, Bernardi R, Donzelli M, Scovassi AI. Induction of apoptotic cell death by DNA topoisomerase II inhibitors. Biochimie. 1995;77:893–9. CrossRef
31. Zhao Q, Li H, Zhu L, Hu S, Xi X, Liu Y, et al. Bioinformatics analysis shows that Top2A functions as a key candidate gene in the progression of cervical cancer. Biomed Rep. 2020;13(4):21. CrossRef