INTRODUCTION
Colon cancer is an abnormal cell growth that usually occurs in glandular epithelial cells of the large intestine and is known as colorectal adenocarcinoma. Colon cancer develops when certain cells in the epithelial tissue undergo a series of genetic or epigenetic mutations, providing opportunities for these cells to grow [1]. These genetic diseases, which can be modified by lifestyle, personal features, and inheritance, can raise the risk of colon cancer [2]. Colon cancer that occurs genetically has hundreds of mutations in different genes, but the number of mutated genes that promote the development of carcinogenesis is still limited.
Colon cancer is a health problem with a high risk of death worldwide [3]. According to the report of the World Health Organization of the Internal Agency for Research on Cancer, colon cancer ranks as the third most diagnosed cancer in 2020 (10.1%) and the second leading cause of death in the world (9.39%) [4]. In 2022, colon cancer accounted for 1.918 million cases in the world and is expected to continue to increase to 3.2 million cases in 2040 [5,6]. Meanwhile, Asia carries the highest colon cancer burden, with more than half of cases leading to death [6]. In Indonesia, colorectal cancer (CRC) ranks second (among males and females), with an addition of 396,914 new cases reported in 2020 based on Global Cancer Statistics (GLOBOCAN) data [7,8]. GLOBOCAN 2020 is an online database that provides incidence and mortality of global cancer statistics for 36 forms of cancer and all cancer sites combined in 185 countries. The data are part of the Global Cancer Observatory of IARCs and are available online at Cancer Today, users may create maps and explore visualizations.
The high number of cases and deaths from colon cancer is due to inadequate initial screening and ineffective treatment [9]. The common treatments for this disease are surgery and chemotherapy. Chemotherapy risks harmful side effects for patients, such as nausea, weakness, hair loss, vomiting, loss of appetite, weight loss, insomnia, skin discoloration, headache, and fever [10]. Currently, the available targeted drugs are limited to growth factors such as the epidermal growth factor receptor signaling pathways, vascular endothelial growth factor, and immune checkpoint inhibitors [11].
Regarding the pathophysiological mechanism, 90% of colon cancer occur due to mutations in the adenomatous polyposis coli (APC) gene, which is about 85% [12,13]. APC gene mutation causes constitutive activation of the Wnt/β-catenin signaling pathway. The wnt/β-catenin signaling pathway is needed for the differentiation and proliferation of the cells during fetal development and for maintaining homeostasis due to the body’s response to the outside environment after birth [14]. Interestingly, the pathway is activated only when needed according to network requirements and will be resting when not required. Meanwhile, under pathological conditions, especially during cancer development, activation of the wnt/β-catenin signaling pathway will continue in the cytoplasm [15,16]. This is thought to be due to mutations in axin-2, which cause failure to convey negative feedback or through other mechanisms that have not yet been explained [17,18].
Clofazimine is a lipophilic riminophenazine used for multidrug-resistant tuberculosis [19,20]. Recent studies have shown to suppress cell growth in breast cancer [21,22]. In vitro studies reported that clofazimine can inhibit the Wnt/β-catenin pathway in triple-negative breast cancer (TNBC) cell panels, hepatocellular, colorectal, and ovarian cancer cell line panels [23]. In vivo, clofazimine efficiently suppresses breast cancer growth by transducing Wnt signals in xenograft mice [24,25]. However, until now, the effects and mechanisms of clofazimine in colon cancer are not clearly understood. In this study, we aimed to determine the effect of clofazimine on the growth of colitis-associated colon cancer (CAC) and its possible mechanism, which is suggested through wnt/β-catenin.
MATERIALS AND METHOD
Reagent and chemicals
Azoxymethane 13.4 M, ≥98% (Cat. No. A5486 ), dextran sulfate sodium (DSS) Mr ~40,000 (Cat. No. 42867-25G), and clofazimine molecular weight: 473.40 (Cat. No. C8895-1G) were purchased from Sigma–Aldrich; Merck. The interleukin-1β (IL-1β) enzyme-linked immunosorbent assay (ELISA) kit 96T (Cat. No. EH0185) and Caspase-3 ELISA kit 96T (Cat. No. EH0546) were purchased from Fine Test. The Rabbit primary antibody β-catenin 1:50–1:200 (Cat. No. A19657) was purchased from ABClonal technology. The rabbit primary antibody axin-2 100 μl (Cat. No.bs-5717R) was purchased from USA Bioss Antibodies. The horseradish peroxidase (HRP)-conjugated secondary antibody 3 mg/ml bovine serum albumin (goat anti-rabbit IgG; Cat. No. FNSA-0001) was purchased from Fine Test.
Animal and treatment
36 BALB/C mice (30 ± 2 g; 7–8 weeks old) were procured from the Bio Farma Laboratory Animal Center in Bandung, Indonesia. Mice were acclimatized for 2 weeks at the Animal House, Department of Pharmacy, University of Indonesia. Six groups of six mice each were formed from the mice: azoxymethane and DSS (AOM-DSS) group, mice treated with curative clofazimine at the following doses 0.2, 0.4, and 0.8 mg/20 g body weight (BW) group, and mice treated with preventive clofazimine dose 0.4 mg/20 g BW group. Health Research Ethics Committee—Faculty of Medicine Universitas Indonesia and Cipto Mangunkusumo Hospital authorized all experimental animal protocols [25].
All groups of mice except the normal control group were treated with 7.5 mg/kg AOM via intraperitoneal injection. After 7 days, mice were given 2% DSS drinking water for 5 days plus regular drinking water for 16 days in one of three cycles of DSS to induce colorectal tumors. Mice in the preventive group were treated with 0.4 mg/20 g BW clofazimine in the second week or when the first 2% DSS cycle started. Meanwhile, mice in the curative groups were treated with 0.2, 0.4, and 0.8 mg/20 g BW clofazimine in the ninth week. The CAC induction process lasted for 12 weeks.
Isolate colon tissue process
The isolation was initiated by dissecting the abdomen to identify the cecum. Subsequently, the proximal colon was isolated by making a distal cut. The distal colon, including the anus, was removed by cutting through the pelvis. The colon was flushed with phosphate-buffered saline (PBS) using a 10 ml syringe and placed lengthwise on the Whatman paper. Along the proximal-distal axis, the colon was longitudinally cut for 10 cm. For IHC analysis, the tissue was preserved in 10% formal saline, subjected to tissue processing, and embedded in paraffin blocks. The colon tissue was stored at −80°C for ELISA examination until it was used.
Conduct an ELISA analysis
The manufacturer’s instructions carried out the analysis. Total protein was extracted by centrifuging 1 g of homogenate tissue with 9 ml PBS at 5,000 × g for 5 minutes at 4°C. 300 μl sample solution was mixed with 300 μl dilution buffer and vortexed for 5 seconds. Enter 100 μl of the mixed solution into the well according to the sample group. Each sample group carried out three repetitions (triple). Then incubated for 90 minutes while closed at 37°C. The plate was washed by wash buffet twice, then 100 μl biotin antibodies were added to each well, and the well was incubated for 60 minutes in a closed condition at 37°C. The plates were washed with wash buffer for 1–2 minutes three times, 100 μl HRP-streptavidin conjugated was added into each well, and then the well was incubated for 30 minutes at 37°C in a closed state. The plate was washed with wash buffer five times, then 90 μl of 3,3’,5,5’-tetramethylbenzidine substrate was added to each well, and then the well was incubated for 10–20 minutes at 37°C. Finally, the incubation can be stopped when the blue discoloration is maximized, then 50 μl stop solution is added, and the color will change to yellow. Then, the results were read with an ELISA Reader (Biochrom EZ) at a wavelength of 450 nm.
The system analyzes and scores hematoxylin and eosin
The colon tissue was processed and stained hematoxylin-eosin as previously described. The colon that had been fixed with 10% formalin was embedded in paraffin using an Excelsior/Citadel 200 automatic machine (DP Elcelsior, DP Citadel 200) and tissue embedding (DP Embedding Center) with histoplast and then frozen on a cold plate (DP cold plate). The paraffin block was cut to a thickness of 4–5 μm and placed on the kada slide. The sections to be analyzed were stained with hematoxylin and eosin [26].
The criteria for scoring tissue damage were observed in five visual fields at 40× magnification. Scoring criteria based on the features of inflammatory cell infiltration, degree/extent of inflammation, and crypt damage with scores refer to Cui et al. [27] summarized in Table 1.
Immunohistochemical
The protein expression of β-catenin and axin-2 was assessed by immunohistochemistry. The paraffin-embedded sample was cut 3 μm then deparaffinized with Xylol for 5 minutes. Samples were rehydrated with alcohol for 4 minutes. Samples were washed and blocked with peroxide for 10 minutes. Incubate samples with primary antibody (β-catenin or axin-2) for 60 minutes and wash with PBS pH 7.4 for 4 minutes. UltraTek Anti-Polyvalent incubates the sample for 10 minutes, and PBS pH 7 washes it for 4 minutes. The samples were incubated with UltraTek HRP for 10 minutes, and then the slides were washed with PBS pH 7.4 for 4 minutes. The sample was incubated with 3,3’-diaminobenzidine substrate secondary antibody for 1–5 minutes, and then the slides were washed with running water. The slides were stained with hematoxylin for 1–2 minutes, the sample was washed with running water, and the bluing reagent was added. Finally, the slides were dehydrated with graded alcohol for 4 minutes, cleared with xylol, and then covered with a cover glass.
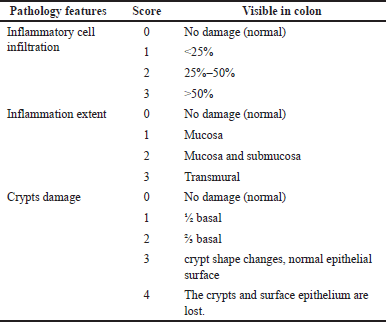 | Table 1. Scoring criteria for the extent of colon tissue damage. [Click here to view] |
Evaluate statistically of data
ELISA data were presented as mean ± SEM and tissue damage level data as median values. A one-way analysis of variance (ANOVA) test and a nonparametric test carried out the process of statistical analysis. The ANOVA test is considered to have a significant difference if the p-value < 0.05. After the ANOVA test, it is continued with the Dunnett post-hoc test to compare the sample means with the normal and AOM-DSS groups.
RESULTS
The effect of clofazimine on BW in mice-induced CAC
Weight loss occurred in the AOM/DSS induced group compared to the normal group, where until the last week of treatment, the animal weight in the AOM/DSS group still decreased by around 0.03%. While in the group that was given clofazimine curatively (dose of 0.2, 0.4, and 0.8 mg/20 g BW) and preventively showed that the percentage of BW began to increase from the 9th to 12th weeks. Figure 1b shows a fluctuating pattern, meaning that the mice in each group show weight loss and gain. At the time of acclimatization, the mice’s BW was 25–30 g. The AOM/DSS group showed greater relative weight loss than the other groups, namely at weeks 2, 6, 8, 10, and 12. Overall, weight loss occurred when the DSS cycle was given, namely at weeks 2, 5, and 8. ANOVA statistical analysis showed a p-value > 0.05, meaning that there was no significant difference between the relative weight of mice in each treatment group.
The effect of clofazimine on survival probability in mice-induced CAC
In addition to weight loss, AOM/DSS can cause death in animal models. This mortality rate will continue to increase as the cycle of induction with DSS. The data of this study also showed that the number of animals that died in the group that was given AOM/DSS alone was more prominent than the group that AOM/DSS + clofazimine induced.
Survival studies revealed a substantial difference in survival time between treatment groups. Figure 1d shows that the AOM/DSS + clofazimine group preventively survived 100% with a survival value of 1. Meanwhile, the AOM/DSS-only group showed a curve that decreased during the experiment days. The group that was given clofazimine as a curative showed a lower survival value than the preventive group. Thus, the preventive administration of clofazimine showed a greater effect on increasing mice survival rate than the curative group.
Effect of clofazimine on colon weight in mice-induced CAC
Compared to the normal control group, macroscopic observation of the colon tissue showed a difference in the colon’s wall. The mice induced by AOM/DSS alone showed wall thickening and higher colon weight (Fig. 2a). In mice that were not treated (control), the large intestine walls tended to be thinner and break more easily if pulled; their weight was also lower. In contrast to the group treated with clofazimine, the wall of the colon was not too thick and even tended to be similar to the normal colon (especially the curative dose group of 0.8 mg/20 g BW and preventive), but the colon weight was lower than the untreated group.
Figure 2a shows the difference in the weight of the colon for each group. The colon weight of AOM/DSS-induced mice has increased almost twice compared to the normal group. The colon weight in the group receiving clofazimine was lower than in the AOM/DSS induction group alone. Of the three curative dose groups and one preventive group, it was shown that the doses of 0.4 and 0.8 mg/20 g BW and the preventive dose showed that colon weight was the closest to normal, although there were no significant differences between the groups dose three and others doses.
Clofazimine decreases Il-1β expression in mice-induced CAC
IL-1β is an activating cytokine from the IL-1β family [28]. The results of this study showed that the AOM/DSS-induced group had higher IL-1β concentration compared to the normal control group and the AOM/DSS + clofazimine group (Fig. 2b). The results of this study also indicated that the greater the dose of clofazimine, the lower the concentration of IL-1β. In this study, the results showed that the AOM/DSS induction group had higher concentrations of IL-1β (mean + SEM) when compared to the normal control group (mean + SEM), p = <0.001. Clofazimine administration significantly decreased IL-1β expression (mean ± SEM), p = <0.001. The decrease in IL-1β expression levels varied in each dose group and administration method (curative and preventive). In the curative dose group, administration of clofazimine at a dose of 0.8 mg/20 g BW had lower concentrations of IL-1β than other curative doses and a very significant difference in concentration compared to the group induced only by AOM/DSS (mean ± SEM), p = <0.001. Meanwhile, in the preventive dose group, the lowest concentration of IL-1β was obtained among all treatment groups, and this concentration had a very significant difference compared to the AOM/DSS group (mean ± SEM), p = <0.001. The results of this study indicated that administration of clofazimine at a dose of 0.8 mg/20 g BW for AOM/DSS induction had the lowest IL-1β concentration compared to doses of 0.4 and 0.2 mg/20 g BW.
Effect of clofazimine on caspase-3 expression in mice-induced CAC
In this study, the concentration of caspase-3 was analyzed using the ELISA method to determine its expression level in the colon tissue of experimental animals. This study showed that the concentration of caspase-3 protein in the colon tissue of the AOM/DSS-induced group alone was lower than that of the normal control group. Meanwhile, the concentration of caspase-3 in the group induced by AOM/DSS accompanied by clofazimine at a dose of 0.2 mg/20 g BW showed lower caspase-3 concentration than doses of 0.4, 0.8 mg/20 g BW, and preventive doses. However, greater than the AOM/DSS-induced group only (Fig. 2c). In this study, statistical analysis using ANOVA revealed that the concentration caspase-3 in each group differed significantly. The concentration of caspase-3, which was almost close to the normal group, was obtained in the curative dose group of 0.8 mg/20 g BW and the preventive group of 0.4 mg/20 g BW, this shows that there is a dose-dependent trend, which shows that an increase will follow the greater dose of clofazimine in caspase-3 concentration close to the normal group. In this study, the administration of clofazimine at a higher curative dose of 0.8 mg/20 BW showed a higher concentration of caspase-3 in experimental animals induced by AOM/DSS.
 | Figure 1. Physical and colon changes of mice after induction. (a) Treatment process; (b) BW change; (c) colon shape difference; and (d) survival rate. Normal = no treatment; AOM/DSS = induction group without clofazimine; CD1 = curative dose of 0.2 mg/kg BW; CD2 = curative dose of 0.4 mg/kg BW; CD3 = curative dose 0.8 mg/kg BW; and PD = preventive dose of 0.4 mg/kg BW. [Click here to view] |
 | Figure 2. Histological analysis and ELISA. (a) Colon weight; (b) IL-1β concentration; (c) caspase-3 concentration; and (d) histopathological scoring to quantify the affected tissues. Normal = no treatment; AOM/DSS = induction group without clofazimine; CD1 = curative dose of 0.2 mg/kg BW; CD2 = curative dose of 0.4 mg/kg BW; CD3 = curative dose 0.8 mg/kg BW; and PD = preventive dose of 0.4 mg/kg BW. [Click here to view] |
Effects of clofazimine on CAC-induced tissue damage
Based on histological analysis, this study’s AOM/DSS model has not yet caused the cancer appearance. However, the inflammatory process followed by the changes in mucosa and sub-mucosa layers was prominently seen. The histological changes were quantified, and the scoring system was used. The level of tissue damage to the colon tissue in the group that was given AOM/DSS alone showed the highest degree of damage score (score 6). The inflammatory process mainly characterizes this damage as depicted by the inflammatory cell infiltration, inflammation extending to the submucosa layer, and minor crypt changes with goblet cells that appear full of mucin (Fig. 3).
However, the AOM/DSS induction did not induce aberrant crypt foci, dysplasia, or carcinoma in the colon tissue (Fig. 3). Thus, it is more likely as colitis induced by AOM/DSS. The results showed that the colon tissue in the normal control group had the lowest damage score, and the AOM/DSS induction group had the highest score (Fig. 2d). Administration of clofazimine to the AOM/DSS induction showed a lower damage effect (score) than the AOM/DSS induction group alone. Curative administration of clofazimine at a dose of 0.8 mg/20 g BW showed a minimal damage score close to the normal score. However, tissue damage in the group given clofazimine preventively at a dose of 0.4 mg/20 g BW had a better score than the curative dose of 0.8 mg/20 g BW.
 | Figure 3. Histological features of the colon (H&E). Normal = no treatment; AOM/DSS = induction group without clofazimine; CD1 = curative dose of 0.2 mg/kg BW; CD2 = curative dose of 0.4 mg/kg BW; CD3 = curative dose 0.8 mg/kg BW; and PD = preventive dose of 0.4 mg/kg BW. [Click here to view] |
Effects of clofazimine on β-catenin expression in mice-induced-CAC
The expression of β-catenin in colon tissue is shown in Figure 4a. This study examined the control for positive expression of β-catenin protein in colon cancer tissue. The brown color in the expressed β-catenin antibody indicates positive expression results. Meanwhile, on negative control slides that were not given primary antibodies, no brown color was found, which means no β-catenin expression was found (Fig. 4a). Based on descriptive observations, it appears that the expression of β-catenin was found to be thicker/darker in intensity in colon tissue induced by AOM/DSS without clofazimine. β-catenin expression appears localized in the cell membrane, cytoplasm, and nucleus. Administration of clofazimine to experimental animals induced by AOM/DSS showed a reduction in the intensity of the brown color with increasing dose and duration of treatment.
Meanwhile, the IHC results were quantified using ImageJ software by reading the percentage of brown color on each slide. Based on ANOVA analysis showed that there was a significant difference in the percentage expression of β-catenin protein between the AOM/DSS group, normal, and those treated with clofazimine with p <0.001 (Fig. 5). Based on the quantification method, the decrease in β-catenin expression showed the best expression in the curative dose group of 0.8 mg/kg BW.
Effects of clofazimine on axin-2 expression in mice-induced CAC
The positive control for axin-2 protein expression was examined in the brain (cerebellum) tissue. Positive expression results are indicated by the brown color of the axin-2 antibody expressed on neuron cells. On negative control slides that were not given primary antibodies, no brown color or axin-2 expression was found. Figure 4b represents the results of examining the axin-2 protein detected in the cytoplasm of the epithelial cells of the colon mucosa. Axin-2 expression, marked in brown, can be found in all study groups. The results of descriptive observations showed that the intensity of the brown color in the normal group was relatively lower in intensity compared to the AOM/DSS group. Meanwhile, the group curatively treated with clofazimine showed lower color intensity as the dose increased. The group given clofazimine showed preventive action and had almost the same color intensity as the normal group.
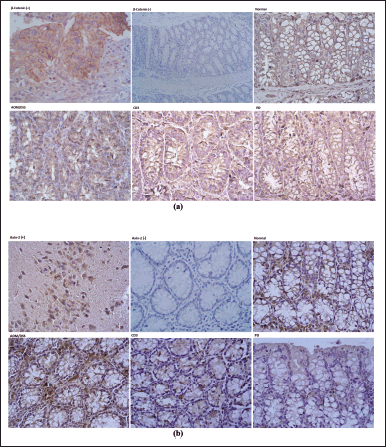 | Figure 4. The expression of β-catenin and axin-2 in each colon tissue using 40×. (a) β-catenin immunohistochemistry staining and (b) axin-2 immunohistochemistry staining. Normal = no treatment; AOM/DSS = induction group without clofazimine; CD1 = curative dose of 0.2 mg/kg BW; CD2 = curative dose of 0.4 mg/kg BW; CD3 = curative dose 0.8 mg/kg BW; and PD = preventive dose of 0.4 mg/kg BW. [Click here to view] |
IHC results were quantified using the ImageJ device and analyzed for the percentage of axin-2 protein expression marked brown on each slide. Based on the ANOVA test, the results revealed that there were substantial differences in the proportion of axin-2 protein expression between the normal, AOM/DSS, and clofazimine groups (Fig. 5). The result showed that the best administration of clofazimine to decrease in axin-2 expression was in the curative dose group of 0.8 mg/kg BW and the preventive dose of 0.4 mg/kg BW.
DISCUSSION
This study aimed to analyze the possible mechanism of clofazimine in attenuating colon cancer associated with colitis observed in the wnt/β-catenin signaling pathway using the AOM/DSS induction model in mice. The colon cancer model with the AOM/DSS combination is known as a model that can trigger cancer development in about 10 weeks [21]. This can be very helpful in observing the pathophysiology of colon cancer due to the relatively short induction time compared to human cases. But at the same time, it is a challenge for researchers to keep the animals alive until the end of the modeling and treatment process. In this study, all animals were observed for their BW and movement activity development during model making. BW is measured daily during the induction process and once every 2–3 days after the induction is stopped. Based on the observations in this study, the animals induced by AOM/DSS experienced more frequent weight loss than the normal and preventive groups given clofazimine. AOM/DSS-induced weight loss in animals follows the model previous researchers made. The research by Parang et al. [29] explained that during the induction process, experimental animals should be weighed every day because there will be a 10%–20% weight loss. The test animals’ BW will return 2–3 days after the induction cycle is stopped [21,24,25]. Statements in previous studies follow the results of observations of animal BW in this study, which showed graphical fluctuations of BW loss when the DSS cycle begins and increases again when the DSS cycle is stopped.
 | Figure 5. IHC analysis of β-catenin and axin-2 expression level. Normal = no treatment; AOM/DSS = induction group without clofazimine; CD1 = curative dose of 0.2 mg/kg BW; CD2 = curative dose of 0.4 mg/kg BW; CD3 = curative dose 0.8 mg/kg BW; and PD = preventive dose of 0.4 mg/kg BW. [Click here to view] |
In addition to weight loss, AOM/DSS can cause death in animal models. This is evidenced by the results of observations during research where animals induced by AOM could be in a critical period and even die within 12–72 hours [21]. This mortality rate will continue to increase as the cycle of induction with DSS. The data of this study also showed that the number of animals that died in the group that was given AOM/DSS alone was more than the normal animal group and the group that AOM/DSS + clofazimine induced. Survival studies revealed a substantial difference in survival time between the therapy groups. Our result showed that the colon cancer model of male balb/c mice given clofazimine showed preventive effect and survived 100% with a survival value of 1.
Meanwhile, mice given AOM/DSS without being treated with clofazimine showed a curve that continued to decrease. The group given clofazimine showed a curative effect and had a lower survival value than the preventive group. Thus, the preventive administration of clofazimine showed a greater effect on increasing survival than the curative group. This is consistent with the study of Zaccagnino et al. [30], who reported that clofazimine could reduce the viability of cancer cells in the PDAC pathway containing p53 mutations.
Macroscopic observation of the colon tissue of mice induced by AOM/DSS showed no significant tumor lesions compared to the normal control group. The difference lies in the colon wall, whereas in the group of mice induced by AOM/DSS alone, wall thickening and higher colon weight. In mice that were not treated (control), the large intestine walls tended to be thinner and break more easily if pulled; their weight was also lower. In contrast to the group treated with clofazimine, the colon wall was not too thick and tended to be similar to the normal colon (especially the curative dose of 0.8 mg/20 g BW and preventive), but the colon weight was lower than the untreated group. The colon weight of mice induced by CAC using AOM/DSS increased almost twice that of the normal mouse colon. The colon weight in the group receiving clofazimine was lower than in the AOM/DSS induction group alone. Of the three curative dose groups and one preventive group, it was shown that the doses of 0.4 and 0.8 mg/20 g BW, as well as the preventive dose for heavy colon were the closest to normal, although there were no significant differences between the three. These findings are comparable to those of a recent study by Schepelmann et al. [31], who observed the same parameters and found that the colon weight of Balb/c mice produced by AOM/DSS was larger than that of untreated controls.
One of the signs of the formation of colitis that leads to colon cancer is an increase in the level of IL-1β and caspase-3. IL-1β is an activating cytokine from the IL-1β family, which is known as the pro-inflammatory cytokine [22]. IL-1β induces downstream signaling cascades and transcription of many genes, including inflammatory pathways and the immune system [23]. This study showed that the AOM/DSS-induced group had higher IL-1β concentration than the normal control group and the AOM/DSS + clofazimine group. The results of this study also indicated that the greater the dose of clofazimine, the lower the expression level of IL-1β. Therefore, the mechanism of clofazimine may be anticancer through its suppressive effect on IL-1β expression. As a pro-inflammatory protein, IL-1β is known to promote cancer development. So, if its expression is suppressed or inhibited, it can reduce the development of existing cancer. Research by Zhang and Veeramachaneni [32] reported that inhibition of IL-1β by Canakinumab administration may reduce the occurrence of lung cancer in cancer patients.
Chronic inflammatory triggers cause high IL-1β expression in colon tissue through the administration of DSS. Activated IL-1β can increase the expression of miR-181a, thereby inhibiting the expression of phosphatase and tensin homolog and triggering the growth of colon cancer [28]. Other research has found that IL-1 can promote colon tumor growth and invasion by activating the self-renewal of cancer stem cells, epithelial-mesenchymal transition, and zinc finger protein E-box binding homeobox-1 (Zeb1) [29]. In addition, several studies have also shown that IL-1β can increase the proliferative process in colon cancer cells, trigger tumorigenesis, and mature the tumor microenvironment [31,33]. In line with that, Li et al. [34] explained that IL-1β was expressed more fully in phase 2 colon cancer patients. Other studies found large amounts of IL-1β detected APC colon cancer models. The results of this study indicated that administration of clofazimine at a dose of 0.8 mg/20 g BW for AOM/DSS induction had the lowest IL-1β expression level compared to doses of 0.4 and 0.2 mg/20 g BW. If converted to a human dose, the 0.8 mg/20 g dose is 300 mg, which is still a safe therapeutic dose. This result suggests that clofazimine can limit cancer formation by reducing IL-1 expression. The 0.8 mg/20 g dose is 300 mg when translated to a human dose, which is still a safe therapeutic dose. This result suggests that clofazimine can limit cancer formation by reducing IL-1 expression.
Caspase-3 belongs to the cysteine-aspartic acid protease (Caspase) family and is a key execution protein in proteolytic breakdown during apoptosis. Procaspase-3 is dormant until it is transformed into the active form of caspase-3 through the proteolytic process of aspartate residues. Caspase-8, caspase-9, or caspase-10 activate the conversion process of procaspase-3 to caspase-3. These proteolytic events are responsible for apoptotic events, including nuclear condensation, DNA fragmentation, and plasma membrane blebbing [35]. Apoptosis is essential for maintaining cellular homeostasis and eliminating damaged cells [32]. Thus, faulty regulation of caspases and apoptosis underlies the pathogenesis of many human diseases, including cancer [36,37]. This study showed that the expression level of caspase-3 protein in the colon tissue of the AOM/DSS-induced group alone was lower than that of the normal control group.
Meanwhile, the expression level of caspase-3 in the group induced by AOM/DSS accompanied by clofazimine at a dose of 0.2 mg/20 g BW showed lower caspase-3 expression levels than doses of 0.4, 0.8 mg/20 g BW, and preventive doses, but greater than the AOM/DSS induced group only. Caspase-3 activation in normal colon tissue physiologically occurs to trigger intrinsic apoptosis, also known as mitochondrial apoptosis [36]. This pathway is activated due to cellular stresses, including growth factor deficiency, cytoskeletal disorders, DNA damage, accumulation of unfolded proteins, hypoxia, and other factors [38,39]. Meanwhile, in colon cancer tissue, caspase-3 activation is inhibited so that apoptotic cell death does not occur and causes cancer tissue to develop. Gastric cancer patients with Caspase-3 expression exhibited a reduced recurrence rate than those without Caspase-3 expression in a prior study by Huang et al. [40]. In hepatocellular carcinoma, it was shown that patients with low Caspase-3 expression had a higher recurrence rate after curative surgery. The same thing was also reported by Asadi et al. [41], that the expression level of caspase-3 in colon tissue from cancer patients was less than in normal colon tissue.
In this study, colon tissue damage was observed microscopically through histological preparations with H&E staining. Colon tissue damage was observed based on the presence of inflammation (infiltration of inflammatory cells), the extent of inflammation in the lining of the colon wall, and the severity levels of damage to the crypts. Previous research stated that the induction of CAC with DSSis characterized by inflammatory cell infiltration and changes in the shape of the crypts, which will damage colon tissue [42]. Based on histological analysis, the level of damage to the colon tissue in the group that was given AOM/DSS alone showed the highest degree of damage score (score 6). This damage is characterized by inflammatory ce
ll infiltration, inflammation that extends to the submucosa layer, and visible changes in the shape/damage of the crypts with goblet cells that appear full of mucin. However, there has not been any dysplasia and disorganization of the mucosal structures of the colon as a whole, which are cancer markers. This contrasts a study by Parang et al. [29], which showed lymphoid aggregation, inflammatory cell infiltration, large polyps, severe crypt damage, and nuclear hyperchromatic, which are early signs of tumor appearance and malignancy in the large intestine.
This study’s colitis induction model associated with colon cancer follows the model in previous studies based on the induction duration of 10 weeks and 2% DSS concentration. Tanimura et al. [43] revealed that modeling CAC using AOM/DSS for 10–20 weeks showed signs of early adenomas, such as changes in the shape of the cell nucleus to become larger, round, or ovoid in shape and nuclear polarity. Cells are almost gone [44]. Meanwhile, tumor infiltration into the submucosa at week 10 was only 24%, and there was no vessel invasion [45]. In line with this, Schepelmann et al. [31] reported that DSS concentration affected tumor severity, where mice receiving 3% DSS showed the development of high-grade dysplasia to carcinoma in male Balb/c mice, while 1% DSS concentration only showed crypt abnormalities in some mice [25]. Thus, our AOM/DSS model will likely have CAC rather than prominent cancer.
Activity in the wnt/β-catenin pathway was determined based on the expression of β-catenin and axin-2 proteins. β-Catenin is an important protein to confirm activity in the Wnt signaling pathway. β-Catenin accumulation is regulated by the APC-axin-GSK3β complex (as its degradation complex). Mutations of the APC gene and other degradation components in the Wnt signaling pathway can lead to excessive accumulation of β-Catenin. This causes the activation of various transcription factors in the Wnt pathway to increase, including proliferation and tumorigenesis [46,47]. The results of this study confirmed that the colon tissue induced by AOM/DSS has a higher expression of β-Catenin than the normal group. In the AOM/DSS-induced group, β-Catenin appeared to be detected stronger (darker brown color) in the cell membrane and cytoplasm compared to the normal group. The results obtained in this study are in line with the research of Yoshida et al. [48] that in normal cells, β-Catenin is localized in the cytoplasm at a low expression level, while in tumor cells, β-Catenin is detected in the membrane, nucleus, and cytoplasm. The presence of β-catenin in the nucleus is a marker of excessive activation of the Wnt signaling pathway, leading to the formation of colon cancer [43,46,48,49]. This finding was confirmed by quantitative data analyzed using ImageJ software, where the percentage of brown areas was more visible in the AOM/DSS-induced group than in the other groups. Administration of clofazimine at a curative dose of 0.8 mg/20 g BW and a preventive dose of 0.4 mg/20 g BW showed a significant reduction in the percentage of brown areas. This indicates that the higher the dose of clofazimine, the less faded the brown color will be. Thus, clofazimine shows its potential as a drug that can inhibit signaling in the Wnt pathway by inhibiting β-catenin expression.
Axis inhibitor protein-2 (axin-2) is a destruction complex that provides negative feedback and controls signaling of the wnt/β-catenin pathway. Expression of axin-2 in colon tissue is one of the markers of tumor development. This is associated with the finding that in colon cancer cells, mutations occur in the axin-2 protein, and the function of axin-2 as a destruction complex becomes weak. As a result, the expression axin-2 increases in colon cancer cells as a response to activating the β-catenin/T-cell factor complex in the Wnt signaling pathway, but this axin-2 has no function whatsoever [14,50,51]. The IHC staining results show that normal colon tissue is less brown, indicating a small amount of axin-2 expression in the cytoplasm. Meanwhile, in the colon tissue induced by AOM/DSS, the brown color is more intense and predominant, which indicates more axin-2 expression and is localized in the cytoplasm.
Meanwhile, quantitative analysis showed similar results, namely, the percentage of brown areas in colon tissue induced by AOM/DSS was higher than the others. The group was curatively given clofazimine at 0.2 mg/20 g BW showed a lower percentage of axin-2 expression than the AOM/DSS-only group. The same thing was also shown in the group that was given clofazimine curatively at a dose of 0.4 mg/20 g BW. The lowest percentage of axin-2 expression was shown in the group given clofazimine curatively at a dose of 0.8 mg/20 g BW and preventive clofazimine at 0.2 mg/20 g BW. Localization of axin-2 expression has also been confirmed in previous studies. Schaal et al. [52] reported that regulation of axin-2 in CRC tissue showed high expression in the cytoplasm in both tumor center cells and invasive tumors, and axin-2 was expressed in small amounts in normal colon cells [52,53,54]. Rennoll et al. [17] confirmed that axin-2 was also expressed in the nucleus in normal cells, tumor tissue, and CRC cell lines. In normal cells, axin-2 expression in the nucleus acts as a rheostat to control myelocytomatosis oncogene (MYC) expression in response to Wnt signaling [13]. Meanwhile, in tumor cells and CRC cell lines, axin-2, expressed in the nucleus, cannot suppress MYC expression, thus triggering uncontrolled cell growth [13,40,55].
The limitation of this study is that the AOM-DSS model was very lethal. Therefore, our research uses many mice to experiment to have good significant data. The suggestions that can be improved for the continuity of further research are as follows:
1. The induction process can be carried out longer by increasing the per cycle DSS induction period and extending the cycle so that tumors and malignancies can form.
2. It is advisable to examine the expression of β-catenin and axin-2 quantitatively to confirm that there has been a decrease in the expression of the two proteins in each treatment group.
CONCLUSION
This study observed several parameters to evaluate clofazimine in colitis-induced colon cancer model mice. As conclusions were obtained, clofazimine at a dose of 0.8 mg/20 g BW as a curative agent showed a similar effect with a dose of 0.4 mg/20 g BW as a preventive agent in AOM/DSS-induced colitis mice. Both methods showed a higher expression of caspase-3 in colon tissue and lower IL-1β expression, a lower score of colon tissue damage, and a lower difference in the expression of β-catenin and axin-2 compared to the negative group.
AUTHOR CONTRIBUTIONS
SR: Design, collecting data, provide some antibody, and writing manuscript.
AB: Design, analyzing data and review manuscript, provide fund for research.
DW: Design, analyzing data and review manuscript, provide some antibody.
SS: Review manuscript and providing fund for research.
FINANCIAL SUPPORT
This research is funded by the Directorate of Research and Development, Universitas Indonesia, under Hibah PUTI 2023 (Grant No. NKB-586/UN2.RST/HKP.05.00/2023).
CONFLICTS OF INTEREST
All authors declared that there are no conflicts of interest.
ETHICAL APPROVALS
Health Research Ethics Committee—Faculty of Medicine Universitas Indonesia and Cipto Mangunkusumo Hospital authorized all experimental animal Protocol no. 22-08-0781.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. CrossRef
2. Church J. Molecular genetics of colorectal cancer. Semin Colon Rectal Surg. 2016;27(4):172–5. CrossRef
3. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89–103. CrossRef
4. GLOBOCAN. Colorectal cancer incidence in the world. Global Cancer Observatory. 2020;419:1–2.
5. Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14(10):101174. CrossRef
6. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. CrossRef
7. Pardamean CI, Sudigyo D, Budiarto A, Mahesworo B, Hidayat AA, Baurley JW, et al. Changing colorectal cancer trends in Asians: epidemiology and risk factors. Oncol Rev. 2023;17(May):1–10. CrossRef
8. GLOBOCAN. Cancer Incident in Indonesia. Int Agency Res Cancer. 2020;858:1–2.
9. Doubeni CA, Fedewa SA, Levin TR, Jensen CD, Saia C, Zebrowski AM, et al. Modifiable failures in the colorectal cancer screening process and their association with risk of death. Gastroenterology. 2019;156(1):63–74. CrossRef
10. Indra RL, Saputra B. Perception of cancer patients on chemotherapy side effects. J Ris Kesehat. 2021;10(1):71–6. CrossRef
11. Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5(1):22. CrossRef
12. Grady WM. Epigenetic events in the colorectum and in colon cancer. Biochem Soc Trans. 2005;33(4):684–8. CrossRef
13. Grady WM, Markowitz SD. Genetic and epigenetic alterations in colon cancer. Annu Rev Genomics Hum Genet. 2002;3(37):101–28. CrossRef
14. Richmond CA, Shah MS, Carlone DL, Breault DT. Factors regulating quiescent stem cells: insights from the intestine and other self-renewing tissues. J Physiol. 2016;594(17):4805–13. CrossRef
15. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–205. CrossRef
16. Stastna M, Janeckova L, Hrckulak D, Kriz V, Korinek V. Human colorectal cancer from the perspective of mouse models. Genes (Basel). 2019;10(10):1–33. CrossRef
17. Rennoll SA, Konsavage WM, Yochum GS. Nuclear AXIN2 represses MYC gene expression. Biochem Biophys Res Commun. 2014;443(1):217–22. CrossRef
18. Zhao G, Kim KY, Zheng Z, Oh Y, Yoo DS, Lee ME, et al. AXIN2 and SNAIL expression predict the risk of recurrence in cutaneous squamous cell carcinoma after Mohs micrographic surgery. Oncol Lett. 2020;19(3):2133–40. CrossRef
19. Lange C, Dheda K, Chesov D, Mandalakas AM, Udwadia Z, Horsburgh CR. Management of drug-resistant tuberculosis. Lancet. 2019;394(10202):953–66. CrossRef
20. Cholo MC, Steel HC, Fourie PB, Germishuizen WA, Anderson R. Clofazimine: current status and future prospects. J Antimicrob Chemother. 2012;67(2):290–8. CrossRef
21. Ahmed K, Koval A, Xu J, Bodmer A, Katanaev VL. Towards the first targeted therapy for triple-negative breast cancer: repositioning of clofazimine as a chemotherapy-compatible selective Wnt pathway inhibitor. Cancer Lett. 2019;449:45–55. CrossRef
22. Koval AV, Vlasov P, Shichkova P, Khunderyakova S, Markov Y, Panchenko J, et al. Anti-leprosy drug clofazimine inhibits growth of triple-negative breast cancer cells via inhibition of canonical Wnt signaling. Biochem Pharmacol. 2014;87(4):571–8. CrossRef
23. Xu J, Koval A, Katanaev VL. Beyond TNBC: repositioning of clofazimine against a broad range of Wnt-dependent cancers. Front Oncol. 2020;10(December):602817. CrossRef
24. Koval A, Bassanini I, Xu J, Tonelli M, Boido V, Sparatore F, et al. Optimization of the clofazimine structure leads to a highly water-soluble C3-aminopyridinyl riminophenazine endowed with improved anti-Wnt and anti-cancer activity in vitro and in vivo. Eur J Med Chem. 2021;222(June):113562. CrossRef
25. Sitorus RS. Health Research Ethics Commitee—Faculty of Medicine Universitas Indonesia [Internet]. 2022. p 1. Available from: https://komite-etik.fk.ui.ac.id/p/index.php/p/about
26. Bialkowska AB, Ghaleb AM, Nandan MO, Yang VW. Improved swiss-rolling technique for intestinal tissue preparation for immunohistochemical and immunofluorescent analyses. J Vis Exp. 2016;2016(113):1–8. CrossRef
27. Cui H, Cai Y, Wang L, Jia B, Li J, Zhao S, et al. Berberine regulates Treg/Th17 balance to treat ulcerative colitis through modulating the gut microbiota in the colon. Front Pharmacol. 2018;9(MAY):1–17. CrossRef
28. Garlanda C, Dinarello ChA, Mantovani A. The interleukin-1 family: back to the future. NIH Public Access. 2014;39(6):1003–18. CrossRef
29. Parang B, Barrett CW, Williams CS. AOM/DSS model of colitis-associated cancer. Methods Mol Biol. 2016;1422:297–307. CrossRef
30. Zaccagnino A, Managò A, Leanza L, Gontarewitz A, Linder B, Azzolini M, et al. Tumor-reducing effect of the clinically used drug clofazimine in a SCID mouse model of pancreatic ductal adenocarcinoma. Oncotarget. 2017;8(24):38276–93. CrossRef
31. Schepelmann M, Kupper N, Gushchina V, Mesteri I, Manhardt T, Moritsch S, et al. AOM/DSS induced colitis-associated colorectal cancer in 14-month-old female Balb/C and C57/Bl6 Mice—a pilot study. Int J Mol Sci. 2022;23(5278):1–8. CrossRef
32. Zhang J, Veeramachaneni N. Targeting interleukin-1β and inflammation in lung cancer. Biomark Res. 2022;10(1):1–9. CrossRef
33. Beaugerie L, Svrcek M, Seksik P, Bouvier AM, Simon T, Allez M, et al. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology. 2013;145(1):166–75.e8. CrossRef
34. Dmitrieva-Posocco O, Dzutsev A, Posocco DF, Hou V, Yuan W, Thovarai V, et al. Cell-type-specific responses to interleukin-1 control microbial invasion and tumor-elicited inflammation in colorectal cancer. immunity. 2019;50(1):166–80.e7. CrossRef
35. Weber A, Wasiliew P, Kracht M. Interleukin-1β (IL-1β) processing pathway. Sci Signal. 2010;3(105):2–4. CrossRef
36. Hai Ping P, Feng Bo T, Li L, Nan Hui Y, Hong Z. IL-1β/NF-kb signaling promotes colorectal cancer cell growth through miR-181a/PTEN axis. Arch Biochem Biophys. 2016;604:20–6. CrossRef
37. Muzny DM, Bainbridge MN, Chang K, Dinh HH, Drummond JA, Fowler G, et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–7. CrossRef
38. Bergman M, Levin GS, Bessler H, Djaldetti M, Salman H. Resveratrol affects the cross talk between immune and colon cancer cells. Biomed Pharmacother. 2013;67(1):43–7. CrossRef
39. Pastille E, Wasmer MH, Adamczyk A, Vu VP, Mager LF, Phuong NNT, et al. The IL-33/ST2 pathway shapes the regulatory T cell phenotype to promote intestinal cancer. Mucosal Immunol. 2019;12(4):990–1003. CrossRef
40. Huang Q, Li F, Liu X, Li W, Shi W, Liu F-F, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med [Internet]. 2011 Jul 3;17(7):860–6. Available from: https://pubmed.ncbi.nlm.nih.gov/21725296 CrossRef
41. Asadi M, Shanehbandi D, Kermani TA, Sanaat Z, Zafari V, Hashemzadeh S. Expression level of caspase genes in colorectal cancer. Asian Pacific J Cancer Prev. 2018;19(5):1277–80.
42. Fadeel B, Orrenius S. Apoptosis: A basic biological phenomenon with wide-ranging implications in human disease. J Intern Med. 2005;258(6):479–517. CrossRef
43. Tanimura Y, Fukui T, Horitani S, Matsumoto Y, Miyamoto S, Suzuki R, et al. Long-term model of colitis-associated colorectal cancer suggests tumor spread mechanism and nature of cancer stem cells. Oncol Lett. 2021;21(1):1–10. CrossRef
44. Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol. 2015;16(9):907–17. CrossRef
45. Bhattacharya I, Barman N, Maiti M, Sarkar R. Assessment of beta-catenin expression by immunohistochemistry in colorectal neoplasms and its role as an additional prognostic marker in colorectal adenocarcinoma. Med Pharm Reports. 2019;92(3):246–52. CrossRef
46. McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2015;7(4):a008656. CrossRef
47. Thompson C. Apoptosis in the pathogenesis and treatment of disease. Rom J Morphol Embryol. 1996;267(5203):1456–62. CrossRef
48. Yoshida N, Kinugasa T, Ohshima K, Yuge K, Ohchi T, Fujino S, et al. Analysis of Wnt and β-catenin expression in advanced colorectal cancer. Anticancer Res. 2015;35(8):4403–10.
49. Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet. 2018;392(10159):1736–88. CrossRef
50. Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6(2):99–104. CrossRef
51. Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275(5307):1787–90. CrossRef
52. Schaal U, Grenz S, Merkel S, Rau TT, Hadjihannas MV, Kremmer E, et al. Expression and localization of axin 2 in colorectal carcinoma and its clinical implication. Int J Colorectal Dis. 2013;28(11):1469–78. CrossRef
53. Murphy DJ, Junttila MR, Pouyet L, Karnezis A, Shchors K, Bui DA, et al. Distinct thresholds Govern Myc’s biological output in vivo. Cancer Cell. 2008;14(6):447–57. CrossRef
54. Huang SMA, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–20. CrossRef
55. Zhang X, Wei L, Wang J, Qin Z, Wang J, Lu Y, et al. Suppression colitis and colitis-associated colon cancer by anti-S100a9 antibody in mice. Front Immunol. 2017;8(DEC):1774. CrossRef