INTRODUCTION
Infections remain the leading cause of death in the developing world, owing to the emergence of new diseases, the re-emergence of controlled diseases, and antimicrobial resistance [1]. Many of these infections have become difficult to treat, and the lack of new antibiotics, particularly for treating gram-negative bacterial infections, necessitates implementing novel antibiotic use strategies [2]. Understanding the relationship of pharmacokinetic/pharmacodynamic (PK/PD) drugs in the fight against bacteria is the key to optimizing antibiotic dosing [3]. The maximum concentration/minimum inhibitory concentration (Cmax/MIC) ratio and the area under the curve over 24 hour/minimum inhibitory concentration (AUC0–24/MIC) ratio are PK/PD predictors of the efficacy of concentration-dependent antibiotics such as aminoglycosides (AMGs) [4,5].
Amikacin (AMK) is one of the AMGs that possesses a narrow therapeutic range with toxic concentration, which is Cmax of >40 μg/ml and trough concentration (Cmin) of >10 μg/ml [6]. AMK is used to treat serious infections caused by Gram-negative bacteria that are resistant to other AMGs, such as gentamicin [7], as evidenced by its high sensitivity level compared to other antibiotics [5,8]. A target Cmax/MIC ratio of ≥8 or an AUC0–24/MIC ratio of ≥80 should be achieved to maximize the effectiveness of AMK therapy [4,9–11]. On the other hand, a prolonged high Cmax or Cmin may be associated with increased AMG toxicity, particularly ototoxic and nephrotoxic [12]. To minimize toxicity, most studies sought a Cmin of <10 or <5 mg/l [13]. AUC has additionally been associated with the development of AMK nephrotoxicity [9].
Therapeutic drug monitoring (TDM) should be performed in AMG due to its limited therapeutic range and high toxicity risk to reduce toxicity and optimize therapy [4,5,14]. However, accurate data (such as the dose, sampling time, and infusion time) must be recorded to interpret serum concentrations and correctly adjust dosages [4]. Using antibiotics at optimal doses and proper routes is critical to improving their efficacy [15], but antibiotic-appropriate routes remain controversial [16]. Due to a lack of data and concerns that an excessively rapid and high Cmax could result in toxicity, such as ototoxicity, intravenous (IV) AMK is not recommended to be administered via bolus IV [17]. As a result, intermittent infusion of AMK for 30–60 minutes is recommended [6,12,18]. According to previous research, most AMK was administered as 30-minute infusions [10,11,19–22], 60-minute infusions [3,23], or both [24–26].
The duration of infusion is one of the factors that may influence the blood drug level, as previously reported in various drugs, such as the extended or continuous versus short-term infusion of carbapenems [27,28] and other beta-lactams [29]. Furthermore, the drug effectiveness and toxicity are also possibly affected by the infusion duration: the toxicity and immunomodulation of IL-2 are dependent on both dose and infusion duration [30], mortality of carbapenem or piperacillin/tazobactam as an extended or continuous infusion (CI) was lower than that for short-term infusion [2], and the clinical outcomes (CO) were different on CI compared to intermittent infusions of antibiotics in infectious disease [31]. Previous research has focused on carbapenem, piperacillin-tazobactam, ceftriaxone, b-lactams, and vancomycin, which are time-dependent antibiotics that require prolonged drug concentrations above the MIC (fT > MIC) to increase their efficacy.
Unlike time-dependent antibiotics, AMK is a concentration-dependent antibiotic with a prolonged post-antibiotic effect (PAE) [4]. It requires a high Cmax to achieve efficacy, but such a high concentration should not be prolonged to reduce toxicity risk. Very few studies have examined the various infusion durations of dose-dependent antibiotics. According to previous research on pediatric cancer patients, administering AMK via IV infusion for 30 minutes resulted in different blood concentrations in which Cmax could be reached faster and higher, while the drug level became significantly lower in the following hours compared to 60-minute infusions [24]. However, the study did not report on treatment outcome achievement. Thus, more research is required to investigate the PK/PD parameters of AMK administered via 30- versus 60-minute infusions in adult patients and their correlation with treatment outcomes and toxicity.
Cmax and Cmin after the administration of IV infusion are clinically important to estimate the plasma drug level at a steady state; thus, whether the level is effective or toxic can be estimated [32]. The weaknesses in measuring the concentrations in biological liquids are usually related to the relatively high monitoring cost, the time appropriateness, and the accuracy of which the samples are taken [33]. Estimating blood drug levels using a PK equation is an alternative strategy for measuring drug levels in the blood. The Bauer and Winter formula can be employed to estimate drug concentration in a steady state [34,35]. Estimating the blood drug levels can be a strategic solution for countries where TDM activity in hospitals has not been practically implemented due to various constraints, such as Indonesia. This study will concentrate on estimating blood AMK levels administered via IV infusions, with the simulation of 30- versus 60-minute infusions, and the relationship of estimated PK/PD ratio with AMK efficacy and toxicity in adult hospitalized patients.
METHODS
This research was conducted through a retrospective study with a cross-sectional design, located in Central General Hospital Dr. Sardjito Yogyakarta Indonesia, one of the referral and teaching hospitals in the Special Region of Yogyakarta. This research obtained ethical approval from the Health Research Ethics Committee of FKKMK UGM Yogyakarta, number KE/FK/0885/EC/2020, continued by KE/FK/1060/EC/2021. The research subjects’ inclusion criteria were the inpatients staying in the hospital from January to December 2020, aged ≥18, receiving AMK therapy administered by IV infusion route, and diagnosed with infectious disease. Meanwhile, the exclusion criteria were patients with pulmonary or extrapulmonary TB or immunocompromised patients.
Data collections
The medical record data of inpatients receiving AMK therapy were collected using a total sampling technique. Demographic and clinical characteristics [sex, age, weight, height, diagnosis, comorbidities, length of stay (LOS), and discharge condition], as well as AMK usage pattern data (dosage, frequency, administration route, duration therapy, and other antibiotics), were collected. To assess the efficacy of AMK treatment, vital signs, clinical symptoms, and laboratory data (leukocyte count, procalcitonin, serum creatinine (SCr), culture and sensitivity test results, radiology test results, and others) were collected.
Since the TDM was not performed in our hospital or Indonesia in general due to various constraints, AMK levels in the blood: highest and lowest blood levels at steady state (CSSmax and CSSmin) after the administration of infusion IV (with the simulation of infusion duration for 30 and 60 minutes), calculated as estimated AMK level using the Bauer and Winter PK formula [Equations (1) and (2)]. Meanwhile, the minimum inhibitory concentration (MIC) data of the pathogenic bacteria were obtained from the hospital-integrated laboratory installation and determined based on the examination procedural standard at the related hospital laboratory.
Efficacy and toxicity evaluation
The efficacy of AMK was assessed as the primary outcome (CO) and the secondary outcome (mortality). The CO and toxicity were evaluated at the end of AMK therapy compared to the pre-AMK therapy condition. In contrast, the secondary outcome was measured at the end of hospitalization. When at least one of the general parameters (improving statement from a clinician, a negative result of bacteria culture, or decreasing leukocyte count approaching normal value) and/or two or more specific parameters (improving vital signs, clinical symptoms based on the disease, or other supporting data) had been achieved, the patients’ CO was considered to be improving. The nephrotoxic development was evaluated by the presence of an SCr significant increasing value (>0.5 mg/dl) when the SCr baseline was ≤2 mg/dl or an increase of >30% when the SCr baseline is >2 mg/dl, and/or a urine output reduction (<0.5 ml/kg/hour for >6 hours) [9,36]. Another AMK toxicity was assessed based on clinical signs and symptoms reported by patients, as stated in their medical records, as none of the patients had an audiometric examination.
Data analysis
A univariate analysis was conducted on the patient’s characteristics, AMK usage patterns, and treatment outcome data. The Cmax, Cmin, and PK/PD ratio (Cmax/MIC) values were descriptively analyzed and compared within the infusion duration of 30 versus 60 minutes using the Wilcoxon sign Rank test. The Targets of the parameters are determined based on previous study, which were Cmax ≥64 μg/ml [11,22], Cmin ≤5 μg/ml [13], and Cmax/MIC ≥8× [4,9,10,11]. The correlation of AMK Cmax, Cmin, and Cmax/MIC ratio at various targets with the AMK efficacy (CO and mortality) and signs of toxicity were analyzed using chi-square or Fisher exact test assisted with the Statistical Package for the Social Sciences software version 27.
RESULT AND DISCUSSION
Demographic and clinical characteristics of subjects
Seventy patients met the inclusion criteria and became subjects in this study. Table 1 demonstrates the characteristics of the research subjects. The majority of these subjects were male, in accordance with previous research that the majority of patients were also male with an age range of 45–64 years [37]. Most subjects had normal kidney function, with an initial SCr range of 0.22–5.52 mg/dl. AMK accumulation may occur in patients with renal dysfunction because almost 100% AMGs are cleared through glomerular filtration, necessitating a dosage adjustment or extended dosing interval [38,39]. The t1/2 value of AMK in adults with normal kidney function is ±2 hours (1.4–2.3 hours), whereas it can reach 28–86 hours in patients with anuria and end-stage renal disease [6,40]. Our previous study discovered that renal function was related to the patient’s discharge condition [41]. Because of the narrow therapeutic index of AMGs, the patient’s renal function should be monitored to avoid toxicity, and TDM is frequently required [42].
Most patients receiving AMK therapy in this study had mixed infections, and 64.3% had cardiovascular disease (CVD) or diabetes mellitus (DM) comorbidities. AMK was mostly used to treat sepsis/septic shock patients with pneumonia (28 patients), and the second most common infection was pneumonia [particularly hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia], consistent with previous findings that the majority of sepsis patients receiving AMK had lung infections (community-acquired pneumonia, healthcare-associated pneumonia and HAP) [22]. The majority of sepsis patients also had hypertension, CVD, or DM [37], which can worsen the patient’s condition. High blood pressure and blood glucose levels that are not continuously controlled can damage kidney blood vessels and impair kidney function [43]. AMK PK can be influenced by septic conditions, which can then affect its PD [22]. Sepsis-related pathophysiological changes, such as capillary leak syndrome and/or augmented renal clearance, may result in an increased volume of distribution and clearance. The PK variability is very high in critically ill patients, making optimal dose selection difficult [11].
The LOS in this study ranged from 4 to 63 days, and 43.6% of subjects were hospitalized for 15–30 days. At the end of AMK therapy (EoT), 37.1% of patients improved in clinical conditions, demonstrating the therapy’s efficacy. At the end of hospitalization, 41 of 70 patients had no improvement in their conditions and died (Table 1). According to a previous study, the LOS of most sepsis patients was 1–5 days, with a 62.5% mortality rate [37]. A study of critically ill patients reported the length of intensive care unit (ICU) stay was 20.8 days, 30-day mortality was 37.6%, the clinical response at EoT was 57.6%, and a Cmax/MIC ratio was associated with treatment response [9]. AMK is a concentration-dependent antibiotic, meaning its antimicrobial activity and clinical efficacy are best linked to its PK/PD index (Cmax/MIC ratio) [42]. A review found that clinical cure rates were generally high, and AMK was at least as effective as other AMGs, depending on organism sensitivity [13]. This study’s results showed that the efficacy of AMK at the end of therapy is still quite low, with a relatively high mortality rate, indicating that further research into the accuracy of the AMK regimen and PK/PD studies is required.
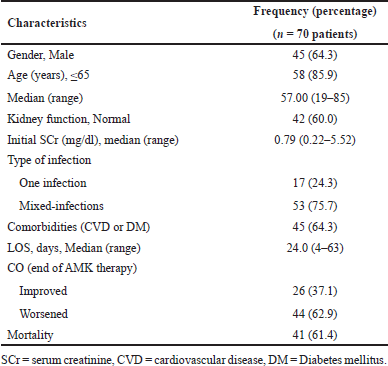 | Table 1. Demographic and clinical characteristics of research subjects. [Click here to view] |
Pathogenic bacteria
The microbial culture test of the patients’ specimens revealed 97 pathogenic bacteria in this study. Almost all subjects had microbial cultures performed before or during AMK therapy. The results were good and should be improved by the hospital so patients can get definitive antibiotic therapy based on the pathogenic bacteria found. There were 10 patients who had negative test results and only 2 patients who did not have the culture tests listed in their medical records. Table 2 indicates the pathogenic bacteria’s characteristics. In most subjects, two or more pathogenic bacteria causing infection were found. Of 97 bacterial isolates, 87.6% were Gram-negative; the most common pathogenic bacteria were Acinetobacter baumannii, Klebsiella pneumoniae, and Escherichia coli. Similar results unveiled that K. pneumoniae, A. baumanii, Pseudomonas aeruginosa, and E. coli were bacteria mostly found in ICU patients [22] and K. pneumoniae was the main pathogenic bacteria in critically ill patients [9]. Gram-negative bacteria (Klebsiella sp.) were also reported as the most pathogenic bacteria, although microbial culture tests were only performed on 16 of 40 patients [37].
The bacteria MIC values in this research had an average of 6 μg/ml (Table 2). Most bacteria causing infection were sensitive to AMK, with MIC values of less than 2 μg/ml. Only one bacterium (A. baumannii) had a MIC of 64 μg/ml and was resistant to AMK based on the culture results before AMK therapy. The bacteria were discovered in the wound bed culture of a patient with a mixed infection (MODS sepsis, HAP, and wound dehiscence). The patient with impaired renal function had a pleural effusion, and the sputum cultures revealed the presence of A. baumannii still sensitive to AMK, so the clinician prescribed AMK therapy at a dose of 450 mg/24 hours. AMK is an antibiotic that kills Gram-negative bacteria, including those with severe infection [44,45].
Amikacin therapy
Table 3 shows that most AMK antibiotics were used as definitive therapy, and only 24.4% of regimens were considered single AMK therapy. Meropenem was the antibiotic most commonly combined with AMK in this study. Kato et al. [25] also reported that most patients received combination therapy (97.1%). An empirical combination therapy containing at least one antimicrobial agent to which the pathogen is susceptible reduces mortality and improves outcomes in patients with serious infections caused by Gram-negative pathogens [46]. In patients with a high risk of mortality or when there is concern that the causative pathogen may be resistant to more commonly used agents, AMGs are frequently combined with b-lactams for the empirical treatment of severe sepsis and certain nosocomial infections [47,48]. Following recent guidelines and expert opinion on treating sepsis, beta-lactam/AMK combination therapy has been recommended, particularly in patients with septic shock or suspected P. aeruginosa infection [48–50].
 | Table 2. Characteristics of pathogenic bacteria result from culture testb. [Click here to view] |
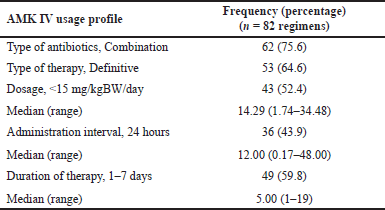 | Table 3. AMK IV usage profile at Dr. Sardjito Hospital during 2020. [Click here to view] |
AMK was obtained at a median dose of 14.29 mg/kgBW/day in this study, with doses ranging from 1.74 to 34.48 mg/kgBW/day. There were 18 of 82 AMK regimens administered at a dose of less than 10 mg/kgBW/day, which might make achieving the Cmax/MIC target difficult and possibly affect therapeutic outcomes in these patients. Previous studies reported that AMK was given at an average dosage of 10 [25], 18.5 [22], and 23.4 mg/kgBW/day. Larger doses may be required in critically ill patients due to an insufficient Cmax target attainment rate, but it raises concerns about the risk of nephrotoxicity associated with high trough concentrations [11].
AMK was mostly administered once a day, and most subjects in this study received AMK therapy for ≤7 days. There were nine patients who died after receiving AMK for only 1 day, the shortest duration of AMK therapy. All of these patients were diagnosed with mixed infection with sepsis or shock-sepsis, and six were more than 60 years old. Their condition had worsened at the time of initial AMK therapy. Thus, the timing of starting AMK therapy may also influence its success. The longest duration of AMK therapy was 19 days as a definitive therapy given to a 20-year-old male patient with HAP, septicemia, and TSAH following a traffic accident. The patient had normal renal function and was discharged from the hospital in an improved condition. A once-daily dose of AMGs is as effective and likely less nephrotoxic than multiple-daily dosing regimens [4]. Peak concentrations in multiple-daily dose regimens should be calculated to ensure therapeutic concentrations are achieved. AMGs serum concentrations also must be monitored for courses lasting more than 2–3 days to ensure efficacy and avoid excessive Cmin to avoid toxicity [42]. Unfortunately, TDM has not been performed, particularly in our hospital and in general in Indonesia; thus, special attention from stakeholders and policymakers is required to obtain optimal antibiotic therapy.
Impact of duration of infusion on the estimated AMK level
In this study, we investigated how the duration of AMK infusion (simulated infusion for 30 versus 60 minutes) affected AMK blood levels and their PK/PD ratio. According to Spencer et al. [17], there are no AMGs indicated for the administration of injection bolus IV. AMK was demonstrated to be more effective when administered via CI rather than bolus IV on rabbits [51]. AMK 7.5 mg/kg administered for 2 minutes resulted in a serum Cmax with a toxic potential of 68–122 μg/ml [52]. The bolus IV resulted in an abrupt increase in serum drug concentration, which can result in toxicity and side effects. This can be avoided by using a slow bolus IV or controlling the IV infusion [53]. Due to the higher risk factors, multiple administration of bolus IV was rarely performed, so the administration through infusion IV constantly in a short duration between 30 and 60 minutes, repeated with certain time intervals. The combination of repeated bolus IV and constant infusion IV predicts drug levels in plasma [32]. The AMK blood levels in this study were estimated using PK equation formulas. This concept of PK interpretation can be employed for calculating dosages and estimating drug concentrations in blood. If blood drug levels cannot be measured directly, this PK estimation concept approach can be used, which can also reduce the cost of sample collection [33,54]. Previous studies used this PK estimation to estimate drug levels in the blood, evaluate dosage appropriateness, and assess clinical response [34,35,55,56].
The results of this study, illustrated in Figure 1 and Table 4, clearly demonstrated that AMK given as 30-minute infusions resulted in a higher Cmax (Fig. 1A) and Cmax/MIC ratio (Fig. 1C) when compared to AMK given as 60-minute infusions. The higher the MIC value of AMK against the pathogen, the lower the Cmax/MIC ratio. According to PK estimation, the average AMK Cmax was 38.64 and 35.95 μg/ml for 30 and 60 minutes of infusion, respectively, while the average Cmax/MIC ratio was 14.14 and 13.13, respectively (Table 4). The Cmax will be reached at the end of the infusion, resulting in faster achievement of Cmax with AMK infusion for 30 minutes. Meanwhile, the average AMK Cmin values between 30 and 60 minutes of infusion were 2.63 and 2.71 μg/ml, respectively (Table 4), with a trend showing that 30 minutes AMK infusion Cmin values were relatively similar or lower than 60 minutes of infusion (Fig. 1B). These results are consistent with previous reports that administering AMK infusion for 30 versus 60 minutes in pediatric patients resulted in faster and higher Cmax and significantly lower in the following 2, 4, and 6 hours. The peak and trough serum concentrations measured in the first 3 days of therapy did not differ from those measured after 3 days in the same patients [24].
The statistical analysis results using the Wilcoxon sign Rank test revealed that the estimation of AMK level in the blood was significantly different when administered via infusion for 30 versus 60 minutes. The maximum level (Cmax), trough level (Cmin), and PK/PD ratio (Cmax/MIC) all had p-values of <0.001 (Table 4). It demonstrates that the duration of infusion affects the estimation of drug levels in the patient’s blood and the PK/PD ratio, as previously stated in several studies. A study that examined the PK/PD properties of carbapenems found that extended or CIs kept blood concentrations above the MIC better than short-term infusions [27,28]. Longer exposure with more frequent dosing, extended infusions, or CIs has improved the PD profile of beta-lactams. The duration of infusion of beta-lactams has been demonstrated to impact their fT > MIC. To achieve therapeutic effectiveness in time-dependent antibiotics, levels above the MIC must be maintained for an extended period of time with a target of 50%–100% fT > MIC to ensure efficacy [29], so extended or CIs are preferable. However, infusion durations that provide Cmax/MIC ratio values exceeding the target will be more appropriate for concentration-dependent antibiotics with long PAE, such as AMK.
The proportion of patients who reached the target of Cmax > 64 μg/ml and Cmax/MIC > 8 times as the PK/PD target was a little bit higher in 30 minutes infusion, regardless of whether the proportion of patients based on the Cmin target in both durations of AMK infusion is similar. The number of patients who reached the target Cmax of > 64 μg/ml was only 12.2% and 9.8% in the 30 and 60 minutes infusions, respectively. Interestingly, more patients achieved the Cmax/MIC target of >8× (61.2% and 59.7% patients, respectively), as well as the Cmin < 5 μg/ml target (both of 85.4% patients) (Table 4). This shows the importance of knowing the MIC value of pathogens in AMK therapy to obtain the appropriate target and determine the right individualized dose. As a concentration-dependent antibiotic, higher AMK concentrations in the blood may result in better antibacterial activity, so higher AMK Cmax and Cmax/MIC ratios are expected to result in better therapy efficacy. However, the low Cmin should be maintained to reduce the risk of toxicity. This study discovered that AMK delivered a higher Cmax with a comparable Cmin when administered via IV infusion for 30 versus 60-minute infusions. Based on the results of the estimation of blood level in this study, AMK administered as a 30-minute infusion may offer a faster onset and better antibacterial activity with a relatively similar toxicity risk. Furthermore, it is necessary to test the correlation between these estimated levels and therapeutic outcomes or toxicity.
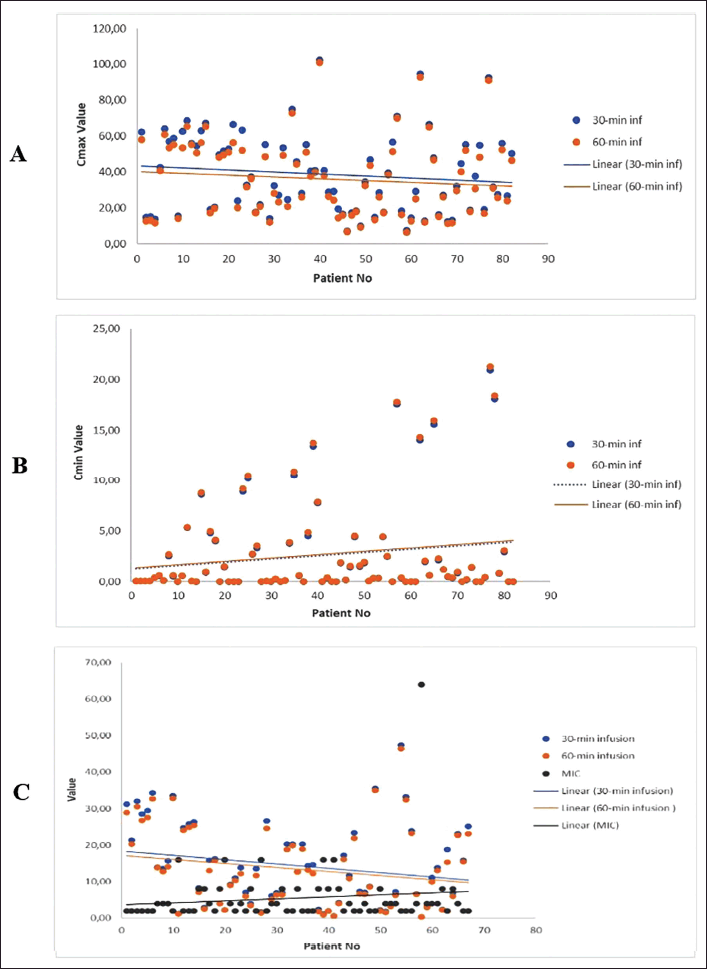 | Figure 1. Scattered plots of estimated plasma levels of AMK administered by 30- versus 60-minute infusions in adult patients at steady state: (A) Cmax values; (B) Cmin values; and (C) Cmax/MIC ratios. [Click here to view] |
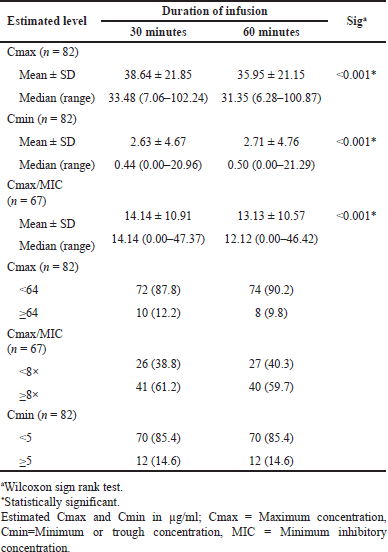 | Table 4. Impact of infusion duration on estimated AMK levels. [Click here to view] |
Several studies have discovered that the duration of infusion may influence therapeutic outcomes. Thompson et al. [30] reported that the toxicity and immunomodulation by IL-2 are dose and infusion duration dependent, with CI being maximal compared with two hours or divided into 15-minute infusions every 8 hours. A review of the impact of IV administration duration on CO in patients treated with carbapenem or piper/tazobactam revealed that extended or CI resulted in lower mortality than short-term infusion [2]. A study conducted by Chen et al. [31] compared CI to traditional intermittent infusions of IV antibiotics for patients with infection, and the subgroup analysis revealed a shorter ICU stay, higher mortality, and a longer antibiotic duration.
Correlation of estimated AMK levels and PK/PD with treatment outcome and toxicity
Considering both PK and PD factors is necessary to evaluate a drug’s responses. PK principles are used in clinical settings to produce effective and safe drug therapy [32]. Table 5 indicates the cross-tabulation results of the estimated AMK levels (Cmax, Cmin, and Cmax/MIC ratio) with the CO, final discharge condition, and presence of AMK signs of toxicity. In this analysis, we tested the estimated AMK blood levels when administrated as a 30-minute infusion by simulating various target levels. The result showed that the proportion of patients who did not reach the Cmax target was greater than the patients who achieved the target. On the other hand, more patients achieved both Cmax/MIC and Cmin at all targets, which is consistent with previous findings from Table 4. The AMK levels (Cmax and Cmin) at all targets revealed a trend of more patients having a worsening CO at the end of AMK therapy, dying after hospitalization, and no signs of toxicity. The most common sign of toxicity found was nephrotoxicity. Furthermore, Cmax/MIC data revealed similar trends but showed relatively similar numbers in both COs and mortality.
According to the statistical analysis results, almost all of the parameters did not influence the patients’ COs at the end of AMK therapy. Interestingly, Cmin in a target of <2.5 μg/ml was significantly different COs (p-value = 0.039). It can be seen that the percentage of improved and worsened CO is almost similar in patients who have Cmin < 2.5 μg/ml but was worse in Cmin ≥ 2.5 μg/ml. Parameters that showed correlation with the secondary outcome (mortality) were Cmax with a target of ≥64 μg/ml and Cmin at all targets (2.5, 5, and 10 μg/ml). These parameters also showed significant differences (p-value < 0.05) in the signs of toxicity, except for Cmin with a target of <10 μg/ml. Patients who reached the estimated Cmax of ≥64 μg/ml, Cmin > 2.5, >5, and >10 μg/ml actually experienced more deaths. It means that the risk of patient death is greater with higher estimated Cmax and Cmin. Conversely, the lower the estimated Cmax and Cmin values, the lower the occurrence of signs of toxicity. AMK is associated with nephrotoxicity and ototoxicity, particularly auditory toxicity [13]. Meanwhile, the Cmax/MIC ratios at all targets did not differ significantly in COs, discharge conditions, or signs of toxicity (p-value > 0.05). This result is in contrast to previous studies that the achievement of Cmax/MIC targets is related to the efficacy of AMK therapy [4,9,11]. In general, continuous drug administration and drug levels in the blood reflect drug levels in receptors, which results in pharmacological effects. Drugs with a narrow therapy index require special attention because small dosage changes can result in subtherapeutic or toxic effects due to the narrow limits between effect and safety. As a result, precise dosage regimens are required so that the drug level falls within the therapeutic range [32].
Overall, the results of this study illustrate that AMK administered via IV infusion for 30 minutes achieves faster and higher peak levels (Cmax), with trough levels (Cmin) that are comparable to administration via infusion for 60 minutes. But in the other hand, the estimated Cmax and Cmin showed a significant association with mortality rate and development signs of toxicity, although they did not influence the primary COs. Thus, based on the estimated AMK blood levels, administering AMK by 60 minutes infusions may be more appropriate for use in hospitalized patients, as it is expected to decrease mortality while posing the same toxicity risk. This was consistent with a previous report that suggested the optimal AMK was administered over 60 minutes [24].
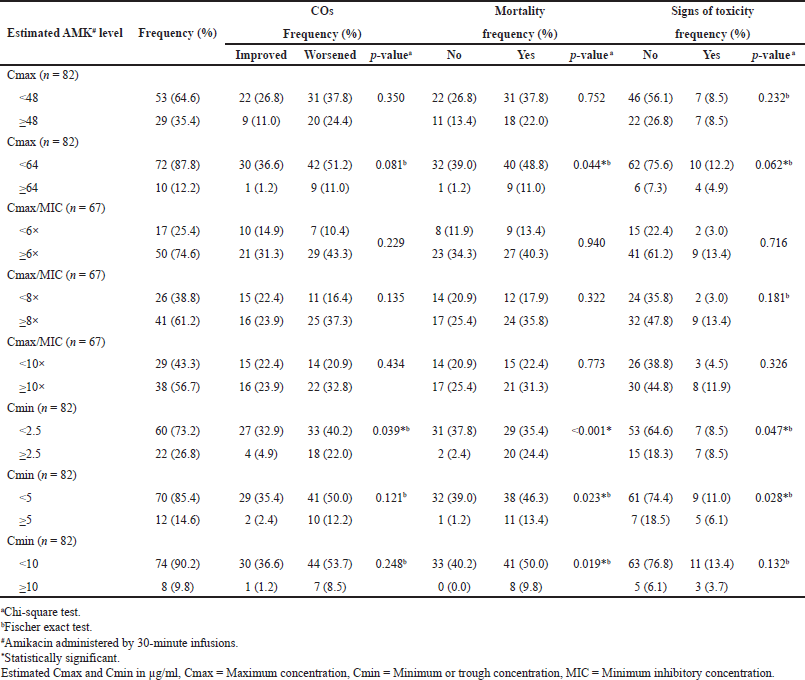 | Table 5. Relationship between estimated AMK level with CO and toxicity. [Click here to view] |
The limitations of this study include the fact that the TDM was not implemented in our hospital, so the estimation of AMK blood levels in this study was only performed using PK formulas based on available patient data (body weight, height, AMK dose, SCr). So it may not accurately represent the real or actual blood levels of AMK in patients. The actual AMK blood levels may be affected by the patient’s pathophysiological and clinical condition, which cannot be predicted by this PK formula. As a result, more research is urgently needed to determine the real blood AMK levels and the relationship between patient COs and adverse drug reactions (ADRs) or toxicity.
Based on the findings of this study, the practical implications include the use of TDM activities in hospitals or the determination of blood levels of AMK (and other drugs with a narrow therapeutic index, PK variability, and serious toxicity) to optimize AMK therapy regimens to achieve optimal therapeutic outcomes while minimizing the toxicity. It is also hoped that the appropriate AMK therapy regimen will prevent the development of AMK resistance. If TDM cannot be done, the estimation of drug levels in the blood using PK estimation, as performed in this study, can be used as an alternative for determining AMK individualized dose regimens based on the target of blood AMK levels to be achieved for each patient. The findings of this study can assist policymakers in improving drug management cycle standards, particularly in controlling AMK administration to patients in the health center, as well as implementing TDM, in order to create optimal, effective, and safe AMK therapy.
CONCLUSION
The duration of AMK infusion showed an impact on the estimated AMK levels (Cmax, Cmin, Cmax/MIC). Estimated AMK Cmax and Cmin did show a significant correlation with mortality rate and signs of toxicity. Only Cmin showed a significant difference in primary COs. AMK administered via 30-minute infusion resulted in faster and higher Cmax, with relatively similar Cmin value, but AMK might be more appropriate to be administered via 60 minutes infusion in order to decrease mortality. Further research is urgently needed to evaluate the actual AMK plasma levels when administered via 30 versus 60 minutes infusions.
ACKNOWLEDGMENT
The authors would like to thank Gadjah Mada University for funding this research through the 2022 Final Project Recognition (RTA) Grant and Dr. Intan Pristian Yuliyani, Randu Fajar Alyansyah, and Siti Nurul Mahmudah for the assistance in data collection processes in the hospital.
AUTHOR CONTRIBUTIONS
All authors have substantial contributions to the conception and design of the work, acquisition or analysis and interpretation of research data; took part in drafting or revising the manuscript; agreed to submit to this journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements.
CONFLICTS OF INTEREST
All of the authors report no financial or any other conflicts of interest in this research.
ETHICAL APPROVALS
This research obtained ethical approval from the Health Research Ethics Committee of FKKMK UGM Yogyakarta, approval number KE/FK/0885/EC/2020, continued by KE/FK/1060/EC/2021.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Kapoor G, Saigal S, Elongavan A. Action and resistance mechanisms of antibiotics: a guide for clinicians. J Anaesthesiol Clin Pharmacol. 2017;33(3):300–5.
2. Falagas ME, Tansarli GS, Ikawa K, Vardakas KZ. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: a systematic review and meta-analysis. Clin Infect Dis. 2013;56(2):272–82.
3. Alhadab AA, Ahmed MA, Brundage RC. Amikacin pharmacokinetic-pharmacodynamic analysis in pediatric cancer patients. Antimicrob Agents Chemother. 2018;62(4):e01781–17.
4. Avent ML, Rogers BA, Cheng AC, Paterson DL. Current use of aminoglycosides: indications, pharmacokinetics and monitoring for toxicity: aminoglycosides: review and monitoring. Intern Med J. 2011;41(6):441–9.
5. Krause KM, Serio AW, Kane TR, Connolly LE. Aminoglycosides: an overview. Cold Spring Harb Perspect Med. 2016;6(6):a027029.
6. Dana WJ, Fuller MA, Goldman MP, Golembiewsky JA, Gonzales JP, Lowe JF, et al. Drug information handbook. 22nd ed. Macedonia, OH: Lexicomp; 2013.
7. Kemenkes RI. Pedoman Pelayanan Kefarmasian Untuk Terapi Antibiotik. Jakarta, Indonesia: Kementerian Kesehatan Republik Indonesia; 2011.
8. Huang Q, Zhou Q, Ju T, Xu H, Wang W. Meropenem and amikacin for management of post-neurosurgical infections from Acinetobacter baumannii. Surg Infect. 2019;20(4):292–7.
9. Ruiz J, Ramirez P, Company MJ, Gordon M, Villarreal E, Concha P, et al. Impact of amikacin pharmacokinetic/pharmacodynamic index on treatment response in critically ill patients. J Glob Antimicrob Resist. 2018;12:90–5.
10. Alqahtani S, Abouelkheir M, Alsulta A, Elsharawy Y, Alkoraishi A, Osman R, Mansy W. Optimizing amikacin dosage in pediatrics based on population pharmacokinetic/pharmacodynamic modeling. Pediatr Drugs. 2018;20:265–72.
11. Boidin C, Bourguignon L, Cohen S, Roger C, Lefrant J-Y, Roberts JA, et al. Amikacin initial dose in critically ill patients: a nonparametric approach to optimize a priori pharmacokinetic/pharmacodynamic target attainments in individual patients. Antimicrob Agents Chemother. 2019;63(11):e00993–19.
12. American Society of Health-System Pharmacists. American hospital formulary service drug information. 44th ed. Bethesda, MD: American Society of Health-System Pharmacists; 2002.
13. Jenkins A, Thomson AH, Brown NM, Semple Y, Sluman C, MacGowan A, et al. Amikacin use and therapeutic drug monitoring in adults: do dose regimens and drug exposures affect either outcome or adverse events? J Antimicrob Chemother. 2016;71(10):2754–9.
14. Hazarika I. Therapeutic drug monitoring (TDM): an aspect of clinical pharmacology and pharmacy practice. Res Rev: J Pharmacol (RRJoP). 2015;5(3):27–34.
15. Chastre J, Luyt CE. Continuous beta-lactam infusion to optimize antibiotic use for severe sepsis. A knife cutting water? Am J Respir Crit Care Med. 2015;192(11):1266–8.
16. Shiu J, Wang E, Tejani AM, Wasdell M. Continuous versus intermittent infusions of antibiotics for the treatment of severe acute infections. Cochrane Database Syst Rev. 2013; 2013:CD008481.
17. Spencer S, Ipema H, Hartke P, Krueger C, Rodriguez R, Gross AE, et al. Intravenous push administration of antibiotics: literature and considerations. Hosp Pharm. 2018;53(3):157–69.
18. Medscape.com [Internet]. Amikacin (Rx) administration. [cited 2020 Feb 9]. Available from: https://reference.medscape.com/drug/amikacin-342516#11
19. Burdet C, Pajot O, Couffignal C, Armand-Lefèvre L, Foucrier A, Laouénan C, et al. Population pharmacokinetics of single-dose amikacin in critically ill patients with suspected ventilator-associated pneumonia. Eur J Clin Pharmacol. 2015;71:75–83.
20. Pajot O, Burdet C, Couffignal C, Massias L, Armand-Lefevre L, Foucrier A, et al. Impact of imipenem and amikacin pharmacokinetic/pharmacodynamic parameters on microbiological outcome of Gram-negative bacilli ventilator-associated pneumonia. J Antimicrob Chemother. 2015;70(5):1487–94.
21. Roger C, Wallis SC, Muller L, Saissi G, Lipman J, Lefrant J-Y, et al. Influence of renal replacement modalities on amikacin population pharmacokinetics in critically ill patients on continuous renal replacement therapy. Antimicrob Agents Chemother. 2016;60(8):4901–9.
22. Aliska G, Setiabudy R, Purwantyastuti, Karuniawati A, Sedono R, Dewi TU, et al. Optimal amikacin levels for patients with sepsis in intensive care unit of Cipto Mangunkusumo Hospital, Jakarta, Indonesia. Acta Med Indones Indones J Intern Med. 2017;49(3):227–35.
23. Petitcollin A, Dequin P-F, Darrouzain F, Vecellio L, Boulain T, Garot D, et al. Pharmacokinetics of high-dose nebulized amikacin in ventilated critically ill patients. J Antimicrob Chemother. 2016;71(2):3482–6.
24. Cleary TG, Pickering LK, Kramer WG, Culbert S, Frankel LS, Kohl S. Amikacin pharmacokinetics in pediatric patients with malignancy. Antimicrob Agents Chemother. 1979;16(6):829–32.
25. Kato H, Hagihara M, Hirai J, Sakanashi D, Suematsu H, Nishiyama N, et al. Evaluation of amikacin pharmacokinetics and pharmacodynamics for optimal initial dosing regimen. Drugs R D. 2017;17:177–87.
26. Illamola SM, Huynh HQ, Liu X, Bhakta ZN, Sherwin CM, Liou TG, et al. Population pharmacokinetics of amikacin in adult patients with cystic fibrosis. Antimicrob Agents Chemother. 2018;62(10):e00877–18.
27. Thalhammer F, Traunmuller F, El Menyawi I, Frass M, Hollenstein UM, Locker GJ, et al. Continuous infusion versus intermittent administration of meropenem in critically ill patients. J Antimicrob Chemother. 1999;43(4):523–7.
28. Jaruratanasirikul S, Sriwiriyajan S, Punyo J. Comparison of the pharmacodynamics of meropenem in patients with ventilator-associated pneumonia following administration by 3-hour infusion or bolus injection. Antimicrob Agents Chemother. 2005;49(4):1337–9.
29. Veiga RP, Paiva J-A. Pharmacokinetics-pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit Care. 2018;22:1–34.
30. Thompson JA, Lee DJ, Lindgren CG, Benz LA, Collins C, Levitt D, et al. Influence of dose and duration of infusion of interleukin-2 on toxicity and immunomodulation. J Clin Oncol. 1988;6(4):669–78.
31. Chen C-H, Chen Y-M, Chang Y-J, Wang S-H, Chang C-Y, Yen H-C. Continuous versus intermittent infusions of antibiotics for the treatment of infectious diseases. Medicine. 2019; 98(10):e14632.
32. Wahyono D. Farmakokinetika Klinik: Konsep Dasar Dan Terapan Dalam Farmasi Klinik. Yogyakarta, Indonesia: Gadjah Mada University Press; 2013.
33. Rugelj N, Trobec K?, Pišlar M, Brguljan PM, Košnik M, dan Mrhar A. Evaluation of theophylline therapeutic drug monitoring service. Slov Med J. 2015;84(3):191–202.
34. Pratiwi TA. Evaluasi Pendosisan Aminoglikosida terhadap Efektivitas dan Fungsi Ginjal pada Pasien dengan Gangguan Ginjal Kronis [Thesis]. Yogyakarta, Indonesia: Fakultas Farmasi Universitas Gadjah Mada; 2018.
35. Endriastuti NE, Wahyono D, Sukarno R. Evaluasi Pendosisan Gentamisin pada Pasien Anak Pneumonia Berat. J Manaj Pelayanan Farm. 2015;5(1):27–32.
36. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):1–8.
37. Hidayati, Arifin H, Raveinal. Kajian Penggunaan Antibiotik pada Pasien Sepsis dengan Gangguan Ginjal, J Sains Farm Klin. 2016;2(2):129–37.
38. Doogue MP, Polasek TM. Drug dosing in renal disease. Clin Biochem Rev. 2011;32(2):69–73.
39. Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient—concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev. 2014 Nov;77:3–11.
40. Bauer LA. Applied clinical pharmacokinetics. 2nd ed. New York, NY: McGraw-Hill Medical; 2008.
41. Utami ED, Puspitasari I, Asdie RH, Lukitaningsih E, Dahesihdewi A. Amikasin: Profil Penggunaan pada Pasien Dewasa Rawat Inap di RSUP Dr. Sardjito Yogyakarta berdasarkan Fungsi Ginjal. J Farm Indones. 2021;13(1):1–12.
42. Germovsek E, Barker CI, Sharland M. What do I need to know about aminoglycoside antibiotics? Arch Dis Child Educ Pract Ed. 2017;102(2):89–93.
43. Sudoyo AW, Setiyohadi B, Alwi I, Simadibrata M. Buku Ajar: Ilmu Penyakit Dalam. Jakarta, Indonesia: FKUI; 2006.
44. Deck DH, Winston LG. Aminoglycosides & spectinomycin. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic and clinical pharmacology. 12th ed. Singapore: McGraw-Hill; 2012. pp 821–7.
45. Mahmoudi L, Mohammadpour AH, Ahmadi A, Niknam R, Mojtaheddzadeh M. Influence of sepsis on higher daily dose of amikacin pharmacokinetics in critically ill patients. Eur Rev Med Pharmacol Sci. 2013;17(3):285–91.
46. Tamma PD, Putcha N, Suh YD, Van Arendonk KJ, Rinke ML. Does prolonged beta-lactam infusion improve clinical outcomes compared to intermittent infusions? A meta-analysis and systematic review of randomized, controlled trials. BMC Infect Dis. 2011;11(1):1–13.
47. American Thoracic Society, Infectious Disease Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.
48. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228.
49. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519–27.
50. Marsot A, Guilhaumou R, Riff C, Blin O. Amikacin in critically ill patients: a review of population pharmacokinetic studies. Clin Pharmacokinet. 2017;56:127–38.
51. Potel G, Caillon J, Fantin B, Raza J, Le Gallou F, Lepage J, et al. Impact of dosage schedule on the efficacy of gentamicin,tobramycin, or amikacin in an experimental model of serratia marcescens endocarditis: in vitro-in vivo correlation. Antimicrob Agents Chemother. 1991;35(1):111–6.
52. Yates RA, Mitcbard M, Wise R. Disposition studies with amikacin after rapid intravenous and intramuscular administration to human volunteers. J Antimicrob Chemother. 1978;4(4):335–41.
53. Nema S, Ludwig JD. Pharmaceutical dosage forms: parenteral medications. 3rd ed. Volume 1 Formulation and Packaging. New York, NY: Informa Healthcare; 2010.
54. Lingga HN, Hakim L, Pramantara IDP. Evaluasi Dosis Asam Valproat pada Pasien Epilepsi Anak. J Manaj Pelayanan Farm. 2013;3(2):137–43.
55. Rahmatullah SW, Hakim L, Pramantara DP. Perkiraan Kadar Fenitoin dalam Darah dan Hasil Terapi pada Pasien Epilepsi. J Manaj Pelayanan Farm. 2013;3(2):132–6.
56. Husnasya D, Ihsan M. Tingkat Rasionalitas Pendosisan Obat Berdasarkan Persamaan Jelliffe pada Pasien dengan Acute Kidney Injury. J Manaj Pelayanan Farm. 2018;8(4):175–88.