INTRODUCTION
Mutations that are inherited, generated by environmental factors, or occur from DNA replication errors cause cancer. In multi-cellular animal creatures, including humans, aging is the most important risk factor for carcinogenesis. Cancer is the first or second leading cause of death in 91 of the 172 nations studied, and third or fourth in another 22 [1]. In 2040, an estimated 28.4 million new cancer cases (including non-melanoma skin cancer, except basal cell carcinoma) are expected to be diagnosed worldwide, up to 47% from the 19.3 million cases diagnosed in 2020, assuming national rates remain constant [2]. In both urban and rural India, cancer is the second and fourth major cause of adult deaths, respectively. Cancer is currently the leading explanation for ruinous health payment, distress funding, and increasing expenditure before death in every country of the world [3]. From 1990 to 2016, India’s cancer death rate more than doubled. India’s cancer incidence was estimated to be 1.15 million new patients in 2018, and by 2040, it is expected to nearly triple due to demographic changes alone [4]. The northeast region of India has the highest cancer incidence rate [six population based cancer registries (PBCRs) for males and four PBCRs for females] compared to other parts of the country. The nasopharynx, hypopharynx, esophagus, stomach, liver, gallbladder, larynx, lung, breast, and cervix uteri were the most common cancer sites in northeast India. As seen by the low 5-year survival rates of breast, cervical, and head and neck cancer in the northeast compared to the rest of India, the region lacks the necessary infrastructure in terms of specialized treatment facilities and human resources. A significant number of cancer patients from the northeast travel beyond the region for treatment and care [5]. A lot of work has gone towards reducing the detrimental side effects of medications during cancer treatment, such as limiting side effects on adjacent cells and tissues, improving drug accumulation and efficacy in the lesion, and developing novel drug delivery and targeting systems [6]. Medicinal plants are a gift from nature to humanity, assisting them in their quest for improved health. Plants and their bioactive substances have been used in traditional medicine since the dawn of humanity. Phyto chemicals found in some medicinal plant species suppress the progression and development of cancer [7]. According to studies, the plant kingdom contains over 250,000 plant species, of which only about 10% have been explored for the treatment of various diseases and approximately 60%–80% of the world’s population still relies on traditional remedies for the treatment of common disorders and diseases [8]. Sikkim extends between 270 4′46? to 280 7′48? N and 880 58′00? to 880 55′25? E containing 4,000 flowering species [9]. The extraordinary geographical position and wide range of topography, high fertile soil, sufficient rainfall, and presence of a large number of perennial streams make the state of Sikkim one of the treasure houses of biodiversity in the country. Sikkim boasts an abundance of medicinal herbs and traditional medicine. About 550 medicinal plants are used by locals in the Sikkim Himalayas region for various ailments, of which only a few are commercially exploited. Plants are still not only important in health care, but they are also the finest and safest hope for future medicine. These local ethnomedicinal plants discovered in Sikkim have been scientifically studied, and the results have been widely disseminated so that people can learn more about effective drug treatments and improve their health.
RESULTS
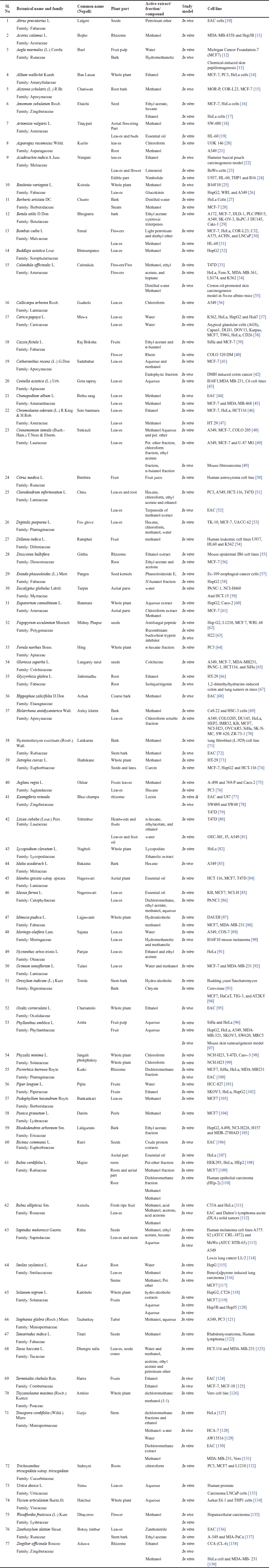 | Table 1. Plants found in Sikkim Himalayan region with reported anticancer activity. [Click here to view] |
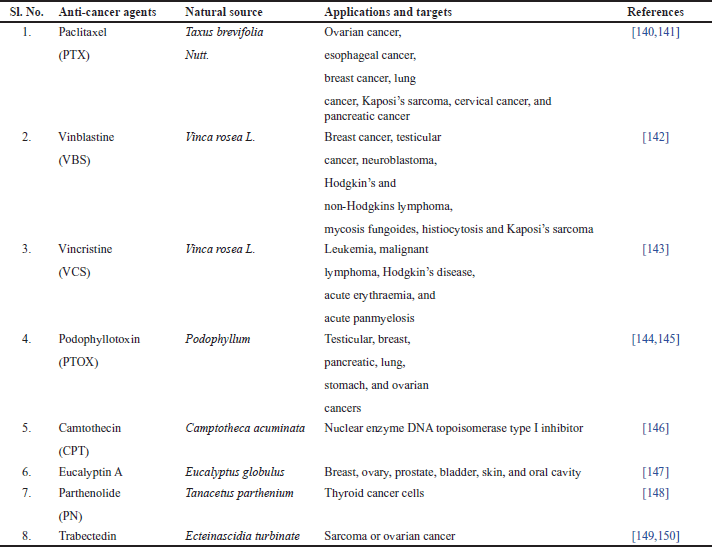 | Table 2. Plant based anti-cancer marketed drugs. [Click here to view] |
DISCUSSION
In drug development and therapy, particularly cancer research, plants and their secondary metabolites play a significant role. The goal of this review article is to list out the medicinal plants, their extracts, and metabolites that have recently attracted attention for their anticancer effects in vitro and in vivo from Sikkim Himalaya. Although the actual compounds isolated from the plant are frequently not used as medications, they provide clues for the creation of prospective novel agents [151]. Plants have been a major source of extremely successful conventional drugs for the treatment of many types of cancer [152]. Some of the drugs that failed earlier clinical tests are now igniting renewed interest as new technologies are created. The potential for attaching medications to carrier molecules targeted at certain cancers is demonstrated [153]. Natural products might be an important source of antitumor drugs for contemporary cancer treatment. It is anticipated that new anticancer phytopharmaceuticals made from medicinal plants will be useful for both cancer therapy and prevention [154]. The plant extracts having rich flavonoids have shown a chemopreventive role in cancer through their effects on signal transduction in cell proliferation and angiogenesis [155]. Some of the potential marketed plant-based drugs are highlighted in Table 2. As shown in Table 2, certain cancerous targets, such as ovarian cancer, esophageal cancer, breast cancer, lung cancer, Kaposi’s sarcoma, cervical cancer, and pancreatic cancer, are targeted by Paclitaxel (PTX) obtained from Taxus brevifolia Nutt., Vinblastine (VBS), and Vincristine (VCS) obtained from Vinca rosea Linn. used for breast cancer, testicular cancer, neuroblastoma, Hodgkin’s and non-Hodgkins lymphoma, mycosis fungoides, histiocytosis and Kaposi’s sarcoma, leukemia, malignant lymphoma, Hodgkin’s disease, acute erythraemia, and acute panmyelosis. Certain cancer like testicular, breast, pancreatic, lung, stomach, and ovarian cancers are treated by Podophyllotoxin (PTOX) obtained from Podophyllum spp. Camtothecin (CPT) obtained from Camptotheca acuminate is used for molecular targets such as nuclear enzyme DNA topoisomerase type I inhibitor. Eucalyptin A obtained from Eucalyptus globulus, Parthenolide (PN) obtained from Tanacetus parthenium, and Trabectedin obtained from Ecteinascidia turbinate have shown the potential for breast, ovary, prostate, bladder, skin, and oral cavity, thyroid cancer cells, and sarcoma or ovarian cancer. Most of the plant extracts were observed to have growth inhibition effects in the particular cell line, whereas other plant extracts inhibit DNA synthesis. The extract of Morus alba leaves, which includes several phenolic compounds in various solvents, inhibited the proliferation of the HepG2 cell line by stopping the cell cycle in the G2/M phase. This was accomplished by expressing the protein p27Kip1, activating caspases to cause cell death, and inhibiting topoisomerase II activity [156]. To investigate the impact of curcumin on the expression of COX-2, human HT-29 colon cancer cells were treated with various amounts of curcumin derived from Curcuma longa. Curcumin reduced the proliferation of HT-29 cells in a concentration- and time-dependent manner. Although COX-2’s mRNA and protein expression were inhibited by curcumin, COX-1 was not similarly altered [157]. In addition, lactate dehydrogenase and 3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide tests to measure cytotoxicity and cell viability were used to evaluate the anticancer effects of curcumin on human breast cancer cell lines (MCF-7) [158]. HeLa and AGS cell lines were examined with the Artemisia annua extracts. In comparison to leaf extracts, stem extracts had less effectiveness inhibiting cell proliferation. At a dosage of 500 mg/ml, the ethanolic extract of leaves causes growth inhibitions in HeLa and AGS cells (57.24% and 67.07%), respectively [159]. In terms of toxicity studies, many plants showed some extent of toxicity, Albizia coriaria (Welw. ex) Oliver is used to treat skin conditions, jaundice, cough, sore eyes, postpartum hemorrhage, menorrhagia, threatening abortion, and venereal illnesses-syphilis, HIV, and gonorrhea [160]. Catharanthus roseus L. is an example, as it contains alkaloids that are neurotoxic, particularly VCS [161]. Highly toxic antimitotics-VCS and VBS prevent mitosis in metaphase after attaching to microtubules [162]. It is obvious that adverse symptoms such myelosuppression, alopecia, abdominal pains, constipation, nausea, paralytic ileus, mouth ulcers, hepatocellular damage, kidney impairment, pulmonary fibrosis, urine retention, amenorrhea, azoospermia, orthostatic hypotension, and hypertension might occur. These plants have been documented for the commercial medications made from this plant, VCS and VBS. In essence, meticulous monitoring of these medications’ administration is required to minimise their negative effects [163–166]. From one study, luteolin obtained from Daucus carota, Salvia rosmarinus, shielded breast cancer cells from doxorubicin-induced toxicity by decreasing reactive oxygen species production [167]. Colchicine was previously considered as a cancer treatment, but it has a few disadvantages: it is highly toxic and exhibits little tumor cell specificity, which causes it to target normal cells. Colchicine hence has a limited role in treating cancer [168]. Cucurbitacins I and D undergo acetylation, which increases their hydrophobicity and cytotoxicity, to produce cucurbitacins E and B. In addition to reducing tumor size and weight, cucurbitacin E and doxorubicin are effectively cytotoxic for tumor cells in culture and in vivo [169–171]. A once-daily intraperitoneal injection of 1,100 mg/kg of Withania somnifera extract did not cause any death within 24 hours in Swiss albino mice. However, an acute toxicity investigation found that a small dose increase results in death, with an LD50 of 1,260 mg/kg/body weight. The components of peripheral blood did not alter. But the weights of the spleen, thymus, and adrenal glands were significantly decreased [172]. Artemia salina larvae were poisonous to the ethanolic leaf extract of Hyptis capitata Jacq., with the greatest toxicity value being 196,772.7 g/ml [173]. Although an analysis of the literature for Astragalus bruguieri revealed no records of acute toxicity, an acute toxicity test conducted on Astragalus membranaceus on Wister rats demonstrated the plant’s safety up to 1,200 mg/kg bw/day [174]. Total 77 medicinal plants found in the Sikkim Himalayan region have proven anticancer activity in diverse cell lines. From this short review work, authors have highlighted the ethno medicinal plants found in Sikkim Himalaya region having potent anti-cancer activity summarized in Table 1 by considering their local name (Nepali), part used for the treatment procedure, active extract/study models (both in-vitro and in-vitro) and cell culture assay (diverse cell lines studies). Out of 77 selected ethno-medicinal plants, 27 plants were active in the in-vivo model and the remaining 50 plants were active in the in-vitro model. As per the activity found in the active extracts, activity was highest in alcohol (methanol and ethanol extracts), followed by aqueous and ethyl acetate, chloroform, etc.
CONCLUSION
Mother Nature has given humans a gift in the form of plants. Most of today’s medicines are derived from plant sources. However, whether the effect of a plant and its extract revealed in experimental animals and in vitro research can be expected in humans is a significant concern. Alkaloids, terpenoids, and flavonoids, which are found in certain plants from the Sikkim Himalayan region, are essential for fighting various ailments. Total 77 medicinal plants found in the Sikkim Himalayan region have proven anticancer activity in diverse cell lines. Further research can be conducted on those plants that have shown the most promising anti-cancer efficacy in previous studies, perhaps leading to low-cost plant-derived drugs from the Sikkim Himalayan region to combat the expanding cancer epidemic. Therefore, we are hopeful that in the near future, the therapeutic benefits of medicinal plants will be useful in treating sickness and displacing chemotherapy.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The authors did not receive any financial support from any organization for the submitted work.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–53. doi: https://doi.org/10.1002/ijc.31937
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. doi: https://doi.org/10.3322/caac.21660
3. Smith RD, Mallath MK. History of the growing burden of cancer in India: from antiquity to the 21st century. J Global Oncol. 2019;5:1–15. doi: https://doi.org/10.1200/JGO.19.00048
4. WHO: global cancer observatory. International Agency for Research on Cancer. Lyon, France: IARC. Available from: https://gco.iarc.fr/
5. Mathur P, Sathishkumar K, Chaturvedi M, Das P, Sudarshan L, Roseland FS, et al. Cancer statistics, 2020: report from national cancer registry programme, India. JCO Glob Oncol. 2020;6:1063–75. doi: https://doi.org/10.1200/GO.20.00122
6. Vinogradov S, Wei X. Cancer stem cells and drug resistance: the potential of nanomedicine. Nanomedicine. 2012;7(4):597–615. doi: https://doi.org/10.2217/nnm.12.22
7. Aung TN, Qu Z, Kortschak RD, Adelson DL. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int J Mol Sci. 2017;18(3):656. doi: https://doi.org/10.3390/ijms18030656
8. Libman A, Bouamanivong S, Southavong B, Sydara K, Soejarto DD. Medicinal plants: an important asset to health care in a region of Central Laos. J Ethnopharmacol. 2006;106(3):303–11. doi: https://doi.org/10.1016/j.jep.2005.11.034
9. Rai SC, Sundriyal RC. Tourism and biodiversity conservation: the Sikkim Himalaya. Amboi. 1997;26(4):235–42. Available from: https://www.jstor.org/stable/4314593
10. Anbu J, Ravichandiran V, Sumithra M, Chowgary BS, Kumar SSLVVSN, Kannadhasan R, et al. Anticancer activity of petroleum ether extract of Abrus precatorius on ehrlich ascitis carcinoma in mice. Int J Pharm Bio Sci. 2011;2(3):24–31.
11. Raj Kumar V, Gunjan G, Ashok Kumar R, Lazar M. Evaluation of cytotoxic potential of Acorus calamus rhizome. Ethnobot Leafl. 2009;13(7):832–9.
12. Vardhini SP, Sivaraj C, Arumugam P, Himanshu R, Kumaran T, Baskar M. Antioxidant, anticancer, antibacterial activities and GC-MS analysis of aqueous extract of pulps of Aegle marmelos (L.) Correa. J Phytopharmacol. 2018;7(1):72–8.
13. Gupta N, Agrawal RC, Sharma P, Narwariya A. Anticancer potential of Aegle marmelos bark extract against DMBA induced skin papillomagenesis with reference to oxidative stress. Eur J Pharm Med Res. 2016;3(4):309–14.
14. Bhandari J, Muhammad BT, Thapa P, Shrestha BG. Study of phytochemical, anti-microbial, anti-oxidant, and anti-cancer properties of Allium wallichii. BMC Complement Altern Med. 2017;17(1):102–11. doi: https://doi.org/10.1186/s12906-017-1622-6
15. Khyade MS, Kasote DM, Vaikos NP. Alstonia scholaris (L.) R. Br. and Alstonia macrophylla Wall. ex G. Don: a comparative review on traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2014;153(1):01–18. doi: https://doi.org/10.1016/j.jep.2014.1.025
16. Sharma V, Lohia N, Handa V, Baranwal M. Amomum subulatum seed extract exhibit antioxidant, cytotoxic and immune-suppressive effect. Indian J Biochem Biophys. 2017;54:135–9.
17. Ramakrishnana P, Neducheziyeanb R. Apoptosis induction of ethanol extract of Amomum subulatum Roxb. in HeLa cell lines. Indian Drugs. 2021;58(03):41–7. doi: https://doi.org/10.53879/id.58.03.11626
18. Jakovljevic MR, Grujicic D, Vukajlovic JT, Markovic A, Milutinovica M, Stankovica M, et al. In vitro study of genotoxic and cytotoxic activities of methanol extracts of Artemisia vulgaris L. and Artemisia alba Turra. S Afr J Bot. 2020;132:117–26. doi: https://doi.org/10.1016/j.sajb.2020.04.016
19. Saleh AM, Aljada A, Rizvi SAA, Nasr A, Alaskar AS, Williams JD. In vitro cytotoxicity of Artemisia vulgaris L. essential oil is mediated by a mitochondria-dependent apoptosis in HL-60 leukemic cell line. BMC Complement Altern Med. 2014;14:226–40. doi: https://doi.org/10.1186/1472-6882-14-226
20. Verma SP, Tripathi VC, Das P. Asparagus racemosus leaf extract inhibits growth of UOK 146 renal cell carcinoma cell line: simultaneous oncogenic PRCCTFE3 fusion transcript inhibition and apoptosis independent cell death. Asian Pac J Cancer Prev. 2014;15:1937–41. doi: https://doi.org/10.7314/apjcp.2014.15.5.1937
21. Biswas D, Mathur M, Bhargava S, Malhotra H, Malhotra B. Anticancer activity of Asparagus racemosus root extracts in non-small cell lung cancer A549 cells. Asian J Pharm Pharmacol. 2018;4(6):764–70. doi: https://doi.org/10.31024/ajpp.2018.4.6.7
22. Subapriya R, Bhuvaneswari V, Nagini S. Ethanolic neem (Azadirachta indica) leaf extract induces apoptosis in the hamster buccal pouch carcinogenesis model by modulation of Bcl-2, Bim, caspase 8 and caspase 3. Asian Pac J Cancer Prev. 2005;6(4):515–20.
23. Harish Kumar G, Mohan CKV, Rao JA, Nagini S. Nimbolide a limonoid from Azadirachta indica inhibits proliferation and induces apoptosis of human choriocarcinoma (BeWo) cells. Invest New Drugs. 2009;27(3):246–52. doi: https://doi.org/10.1007/s10637-008-9170-z
24. Roy MK, Kobori M, Takenaka M, Nakahara K, Shinmoto H, Isobe S, et al. Antiproliferative effect on human cancer cell lines after treatment with nimbolide extracted from an edible part of the neem tree (Azadirachta indica). Phytother Res. 2007;21(3):245–50. doi: https://doi.org/10.1002/ptr.2058
25. Pandey S. In vivo antitumor potential of extracts from different parts of Bauhinia variegata linn. Against b16f10 melanoma tumour model in c57bl/6 mice. Appl Cancer Res. 2017;37(33):1–14. doi: https://doi.org/10.1186/s41241-017-0039-3
26. Shamran DJ, Alwan Al-Jumaili EF, Tawfeeq AT. Cytotoxicity effect of glucokinin isolated from Bauhinia variegata against several cancer cell lines. Iraqi J Biotechnol. 2020;19(1):69–74.
27. Sharmila KJ, Monisha SM, Akila Beevi A, Deebarathi V. Antibacterial, antioxidant, anticancer effects and GCMS analysis of Berberis aristata. Biomedicine. 2020;40(3):286–93. doi: https://doi.org/10.51248/.v40i3.10
28. Serasanambati M, Chilakapati SR, Manikonda PK, Reddy Kanala J. Anticancer activity of methanolic extract of Berberis aristata in MCF-7 human breast cancer cell lines. Int J Life Sci Biotech Pharm Res. 2015;4(1):31–5.
29. Mishra T, Arya RK, Meena S, Joshi P, Pal M, Meena B, et al. Isolation, characterization and anticancer potential of cytotoxic triterpenes from Betula utilis Bark. PLoS One. 2016;11(7):1–14. doi: https://doi.org/10.1371/journal.pone.0159430
30. Tundis R, Rashed K, Said A, Menichinia F, Loizzoa MR. In vitro cancer cell growth inhibition and antioxidant activity of Bombax ceiba (Bombacaceae) flower extracts. Nat Prod Commun. 2014;9(5):691–4.
31. Sharma N, Kispotta S, Mazumder PM. Immunomodulatory and anticancer activity of Bombax ceiba Linn leaf extract. Asian Pac J Trop Biomed. 2020;10(9):426–32. doi: https://doi.org/10.4103/2221-1691.290134
32. Mohamed M, Abdou A, Saad A, Ibrahim M. Cytotoxic activity of Buddleja asiatica. Life Sci J. 2013;10(1):2773–7. doi: https://doi.org/10.7537/marslsj100113.333
33. Matysik G, Wójciak-Kosior M, Paduch R. The influence of Calendulae officinalis flos extracts on cell cultures, and the chromatographic analysis of extracts. J Pharm Biomed Anal. 2005;38:285–92. doi: https://doi.org/10.1016/j.jpba.2004.12.034
34. Mati? IZ, Jurani? Z, Savikin K, Zduni? G, Na?vinski N, Go?evac D. Chamomile and marigold tea: chemical characterization and evaluation of anticancer activity. Phytother Res. 2013;27(6):852–8. doi: https://doi.org/10.1002/ptr.4807
35. Ali F, Khan R, Khan AQ, Lateef MA, Maqbool T, Sultana S. Assessment of augmented immune surveillance and tumor cell death by cytoplasmic stabilization of p53 as a chemopreventive strategy of 3 promising medicinal herbs in murine 2-stage skin carcinogenesis. Integr Cancer Ther. 2014;13:351–67. doi: https://doi.org/10.1177/1534735413513831
36. Nghakliana F, Fanai JL, Tochhawng F, Balachandar V, Zothansiama. Anticancer activity of Callicarpa arborea Roxb. extracts against type-II human lung adenocarcinoma cell line, A549. J Environ Biol. 2020;41:901–7. doi: https://doi.org/10.22438/jeb/4(SI)/MS_1916
37. Otsuki N, Dang NH, Kumagai E, Kondo A. Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J Ethno Pharmacol. 2010;127:760–7. doi: https://doi.org/10.1016/j.jep.2009.11.024
38. Marotta F, Weksler M, Naito Y, Yoshida C, Yoshioka M, Marandola P. Nutracuitical supplementation, effect of a fermented papaya prepration on rodex status and DNA damage in healthy elderly individuals and relationships with GSTM1 genotype, a randomized, placebocontroll, cross-over study. Ann N Y Acad Sci. 2006;1067(1):400–7. doi: https://doi.org/10.1196/annals.1354.057
39. Irshad M, Mehdi SJ, Al-Fatlawi AA, Zafaryab M, Ali A, Ahmad I, et al. Phytochemical composition of Cassia fistula fruit extracts and its anticancer activity against human cancer cell lines. J Biol Act Prod Nat. 2014;4(3):158–70. doi: https://doi.org/10.1080/22311866.2014.933084
40. Duraipandiyan V, Baskar AA, Ignacimuthu S, Muthukumar C, Al-Harbi NA. Anticancer activity of rhein isolated from Cassia fistula L. flower. Asian Pac J Trop Dis. 2012;2(1):S517–23. doi: https://doi.org/10.1016/S2222-1808(12)60213-8
41. Suderan S, Paramasivam R, Sunder S, Amathy AR, Rincy R, Ramasamy V. Evolution of metabolic compounds of Catharanthus roseus and its anticancer activity. Eur J Pharm Med Res. 2017;4(9):282–90.
42. Shukla ST, Rudani MG, Gohil KJ, Patel NA, Habbu PV, Mishra RK, et al. Antioxidant and anticancer activities of endophytic-crude fraction of Sadabahar [Catharanthus roseus (L.) G.Don] in rats. Int J Unani Integr Med. 2018;2(4):26–34.
43. Pittella F, Dutra RC, Dalton Junior D, Lopes MTP, Barbosa NR. Antioxidant and cytotoxic activities of Centella asiatica (L) Urb. Int J Mol Sci. 2009;10:3713–21. doi: https://doi.org/10.3390/ijms10093713
44. Rana S, Rahman S, Sana S, Biswas TK, Md. Hashem AK, Parvin S, et al. Anticancer potential of Chenopodium album leaf extract against ehrlich ascites carcinoma cells in Swiss albino mice. Future J Pharm Sci. 2020;6(65):1–9. doi: https://doi.org/10.1186/s43094-020-00080-8
45. Khoobchandani M, Ojeswi BK, Sharma B, Srivastava MM. Chenopodium album prevents progression of cell growth and enhances cell toxicity in human breast cancer cell lines. Oxid Med Cell Longev. 2009;2(3):160–5. doi: https://doi.org/10.4161/oxim.2.3.8837
46. Selvanathan J, Sundaresan S. Cytotoxicity effects on negative breast and colon cancer cell lines of Chromolaena odorata. J Compos Theory. 2020;XIII(I):223–43.
47. Adedapo AA, Oyagbemi AA, Fagbohun OA, Omobowale TO, Yakubu MA. Evaluation of the anticancer properties of the methanol leaf extract of Chromolaena odorata on HT-29 cell line. J Pharmacogn Phytochem. 2016;5(2):52–7. doi: https://doi.org/10.1096/fasebj.30.1_supplement.1193.6
48. Thanekar DR, Dhodi JB, Juvekar AR. Evaluation of in vitro cytotoxic activity of petroleum ether, methanol and aqueous extracts of Indian bay leaf, Cinnamomum tamala (Buch.—ham.) T. Nees & Eberm. On cancer cells. World J Pharm Pharm Sci. 2013;3(1):519–33.
49. Thanekar D, Dhodi J, Gawali N, Raju A, Deshpande P, Degani M, et al. Evaluation of antitumor and anti-angiogenic activity of bioactive compounds from Cinnamomum tamala: in vitro, in vivo and in silico approach. S Afr J Bot. 2016;104:6–14. doi: https://doi.org/10.1016/j.sajb.2015.09.014
50. Entezari M, Majd A, Falahian F, Mehrabian S, Hashemi M, Lajimi AA. Antimutagenicity and anticancer effects of Citrus medica fruit juice. Acta Med Iran. 2009;47(5):373–7.
51. Haris M, Mahmood R, Rahman H, Rahman N. In vitro cytotoxic activity of Clerodendrum infortunatum against T47D, PC-3, A549 and HCT-116 human cancer cell lines and its phytochemical screening. Int J Pharm Pharm Sci. 2016;8(1):439–44.
52. Sannigrahi S, Mazumder UK, Pal D, Mishra SL. Terpenoids of methanol extract of Clerodendrum infortunatum exhibit anticancer activity against ehrlich’s ascites carcinoma (EAC) in mice. Pharm Biol. 2012;50(3):304–9. doi: https://doi.org/10.3109/13880209.2011.604089
53. Lazaro ML, De La Pene NP, Pastor N, Martin Cordero C, Navarro E, Cortes F, et al. Anti-tumour activity of Digitalis purpurea L. subsp. Heywoodii. Planta Med. 2003;69(8):701–4. doi: https://doi.org/10.1055/s-2003-42789
54. Kumar D, Mallick S, Vedasiromoni JR, Pal BC. Anti-leukemic activity of Dillenia indica L. fruit extract and quantification of betulinic acid by HPLC. Phytomedicine. 2010;17(6):431–5. doi: https://doi.org/10.1016/j.phymed.2009.07.010
55. Huiyuan G, Masanori K, Lijun WU, Nobuo K, Takeaki Y, Yoshiyuki N. Antitumor-promoting constituents from Dioscorea bulbifera L. in JB6 mouse epidermal cells. Biol Pharm Bull. 2002;25(9):1241–3. doi: https://doi.org/10.1248/bpb.25.1241
56. Rachaiah K, Keshavan D, Salimath BP. Anti-neoplastic activity of Dioscorea bulbifera root extracts by targeting MTA1. RJLBPCS. 2019;5(1):549–63.
57. Mo S, Xiong H, Shu G, Yang X, Wang J, Zheng C, et al. Phaseoloideside E, a novel natural triterpenoid saponin identified from Entada phaseoloides, induces apoptosis in Ec-109 esophageal cancer cells through reactive oxygen apecies generation. J Pharmacol Sci. 2013;122(3):1–13. doi: https://doi.org/10.1254/jphs.12193fp
58. Zhang L, Huang L, Liu Q, Kuang S, Xu Q, Qin X, et al. N-butanol fraction of Entada phaseoloides ethanol extract inhibits hepatocellular carcinoma HepG2 cell proliferation by inducing apoptosis. JBUON. 2014;19(2):406–11.
59. Teixeira A, DaCunha DC, Barros L, Caires HR, Xavier CPR, Ferreira ICFR, et al. Eucalyptus globulus Labill. decoction extract inhibits the growth of NCI-H460 cells by increasing the p53 levels and altering the cell cycle profile. Food Funct. 2019;10(6):3188–97. doi: https://doi.org/10.1039/c8fo02466a
60. Alice G, Georgeta N, Nicoleta D, Adrian A, Lucian I, Carmen I, et al. Evaluation of antiproliferative and protective effects of Eupatorium cannabinum L. extracts. Turk J Biol. 2018;42(4):341–51. doi: https://doi.org/10.3906/biy-1803-72
61. Abuali M, Shams Ardekani MR, Rezadoost H, Vazirian M, Balaei-Kahnamoei M, Hamzeloo-Moghadam M. Cytotoxic effects of Eupatorium cannabinum on MCF-7 human breast cancer cell line. Res J Pharmacogn. 2021;8(2):69–75. doi: https://doi.org/10.22127/RJP.2021.263706.1654
62. Leung EH, Ng TB. A relatively stable antifungal peptide from buckwheat seeds with antiproliferative activity toward cancer cells. J Pept Sci. 2007;13(11):762–7. doi: https://doi.org/10.1002/psc.891. PMID: 17828793
63. Bai CZ, Feng ML, Hao XL, Zhao ZJ, Li YY, Wang ZH. Anti-tumoral effects of a trypsin inhibitor derived from buckwheat in vitro and in vivo. Mol Med Rep. 2015;12(2):1777–82. doi: https://doi.org/10.3892/mmr.2015.3649
64. Alam M, Khan A, Wadood A, Khan A, Bashir S, Aman A, et al. Bioassay-guided isolation of sesquiterpene coumarins from Ferula narthex Bioss: a new anticancer agent. Front Pharmacol. 2016;7(26):1–6. doi: https://doi.org/10.3389/fphar.2016.00026
65. Balkrishna A, Das SK, Pokhrel S, Joshi A, Laxmi, Verma S, et al. Colchicine: isolation, LC–MS QTof screening, and anticancer activity study of Gloriosa superba seeds. Molecules. 2019;24(2772):1–15. doi: https://doi.org/10.3390/molecules24152772
66. Nourazarian SM, Nourazarian A, Majidinia M, Roshaniasl E. Effect of root extracts of medicinal herb Glycyrrhiza glabra on HSP90 gene expression and apoptosis in the HT-29 colon cancer cell line. Asian Pac J Cancer Prev. 2015;16:8563–6. doi: https://doi.org/10.7314/apjcp.2015.16.18.8563
67. Chin YW, Jung HA, Liu Y, Su BN, Castoro JA, Keller WJ, et al. Anti-oxidant constituents of the roots and stolons of licorice (Glycyrrhiza glabra ). J Agric Food Chem. 2007;55(12):4691–7. doi: https://doi.org/10.1021/jf0703553
68. Chakraborty M, Karmakar I, Haldar S, Nepal A, Haldar PK. Anticancer and antioxidant activity of methanol extract of Hippophae salicifolia in EAC induced Swiss albino mice. Int J Pharm Pharm Sci. 2015;7(8):180–4.
69. Yoon H, Park J, Park KK, Kim J, Bandara NC, Bandara BMR, et al. Methanol extract of Holarrhena antidysenterica inhibits the growth of human oral squamous cell carcinoma cells and osteoclastogenesis of bone marrow macrophages. Evid-Based Complement Altern Med. 2017;2017:1–9. doi: https://doi.org/10.1155/2017/7272947
70. Sharma V, Hussain S, Bakshi M, Bhat N, Saxena AK. In vitro cytotoxic activity of leaves extract of Holarrhena antidysenterica against some human cancer cell lines. Indian J Biochem Biophys. 2014;51(1):46–51.
71. Khairunnisa K, Karthik D. Evaluation of in-vitro apoptosis induction, cytotoxic activity of Hymenodictyon excelsum (Roxb) Wall in Dalton’s lymphoma ascites (DLA) and lung fibroblast—muse L929 cell lines. J Appl Pharm Sci. 2014;4(08):11–7. doi: https://doi.org/10.7324/JAPS.2014.40803
72. Nepal A, Chakraborty M, Karmakar I, Bala A, Haldar PK. Cytotoxic and anti proliferative activity of Hymenodictyon excelsum in ehrlich ascites carcinoma beating mice: in vitro and in vivo studies. Int J Chem Pharm Anal. 2016;3(2):1–7.
73. Oskoueian E, Abdullah N, Saad WZ, Omar AR, Ahmad S, Kuan WB, et al. Antioxidant, anti-inflammatory and anticancer activities of methanolic extracts from Jatropha curcas Linn. J Med Plant Res. 2011;5(1):49–57. doi: https://doi.org/10.5897/JMPR.9000229
74. Ahmed AM, Ibrahim MM, E El-said MAA, Elsadek BEM. Anti-cancer sctivity of curcin and latex isolated from Jatropha plant (Jatropha Curcas L.). J Agric Chem Biotechnol. 2020;11(11):339–44. doi: https://doi.org/10.21608/jacb.2020.128902
75. Carvalho M, Ferreira PJ, Mendes VS, Silva R, Pereira JA, Jerónimo C, et al. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem Toxicol. 2010;48(1):441–7. doi: https://doi.org/10.1016/j.fct.2009.10.043
76. Li W, Li DY, Wang HD, Zheng ZJ, Hu J, Li ZZ. Juglans regia hexane extract exerts antitumor effect, apoptosis induction and cell circle arrest in prostate cancer cells in vitro. Trop J Pharm Res. 2015;14(3):399–405. doi: https://doi.org/10.4314/tjpr.v14i3.7
77. Ahmed FRS, Amin R, Hasan I, Asaduzzaman AKM, Kabir SR. Antitumor properties of a methyl-beta-d-galactopyranoside specificlectin from Kaempferia rotunda against ehrlich ascites carcinoma cells. Int J Biol Macromol. 2017;102:952–9. doi: https://doi.org/10.1016/j.ijbiomac.2017.04.109
78. Islam F, Gopalan V, Lam AKY, Kabir SR. Kaempferia rotunda tuberous rhizome lectin induces apoptosis and growth inhibition of colon cancer cells in vitro. Int J Biol Macromol. 2019;141:775–82. doi: https://doi.org/10.1016/j.ijbiomac.2019.09.051
79. Atun S, Arianingrum R. Anticancer activity of bioactive compounds from Kaempferia rotunda rhizome against human breast cancer. Int J Pharmacogn Phytochem Res. 2015;7(2):262–9.
80. Dalimunthe A, Hasibuan PAZ, Satria D. Cell cycle arrest activity of Litsea cubeba lour: heartwood and fruit extracts against T47D breast cancer cells. Asian J Pharm Clin Res. 2017;10(11):404–6. doi: https://doi.org/10.22159/ajpcr.2017.v10i11.20204
81. Ho CL, Jie-Ping O, Liud YC, Hunge CP, Chene YL, Su YC. Compositions and in vitro anticancer activities of the leaf and fruit oils of Litsea cubeba from Taiwan. Nat Prod Commun. 2010;5(4):617–20. doi: https://doi.org/10.1177/1934578X1000500425
82. Mandal SK, Biswas R, Bhattacharyya SS, Paul S, Dutta S, Pathak S, et al. Lycopodine from Lycopodium clavatum extract inhibits proliferation of HeLa cells through induction of apoptosis via caspase-3 activation. Eur J Pharmacol. 2010;626:115–22. doi: https://doi.org/10.1016/j.ejphar.2009.09.033
83. Kim HW, Kang SC. The toxicity and anti-cancer activity of the hexane layer of Melia azedarach L. var. japonica Makino’s bark extract. Toxicol Res. 2012;28(1):57–65. doi: https://doi.org/10.5487/TR.2012.28.1.057
84. Bardaweel SK, Bakchiche B, ALSalamat HA, Rezzoug M, Gherib A, Flamini G. Chemical composition, antioxidant, antimicrobial and antiproliferative activities of essential oil of Mentha spicata L. (Lamiaceae) from Algerian Saharan atlas. BMC Complement Altern Med. 2018;18(201):1–7. doi: https://doi.org/10.1186/s12906-018-2274-x
85. Keawsa-ard S, Kongtaweelert S. Antioxidant, antibacterial, anticancer activities and chemical constituents of the essential oil from Mesua ferrea leaves. Chiang Mai J Sci. 2012;39(3):455–63.
86. Rajendran K, Reddy EV, Khanna A. Anticancer effect of Mesua ferrea extracts on human pancreatic cancer cell line. Int J Life Sci Sci Res. 2016;2(2):198–205.
87. Parmer F, Kushawaha N, Highland H, George L. In vitro antioxidant and anticancer activity of Mimosa pudica Linn extract and L-mimosine on lymphoma daudi cells. Int J Pharm Pharm Sci. 2015;7(12):100–4.
88. John R, Kariyil BJ, Usha PTA, Surya S, Anu G, John P, et al. In vitro antitumor potential of methanol extract of Mimosa pudica in human breast cancer cell lines. Phcog Mag. 2020;16:S396–403. doi: https://doi.org/10.4103/pm.pm_527_19
89. Jung IL. Soluble extract from Moringa oleifera leaves with a new anticancer ativity. PLoS One. 2014;9(4):e95492. doi: https://doi.org/10.1371/journal.pone.0095492
90. Purwal L, Pathak AK, Jain UK. In vivo anticancer activity of the leaves and fruits of Moringa oleifera on mouse melanoma. Pharmacologyonline. 2010;1:655–65.
91. Benefit D. Anticancer activity of Nyctanthes arbortristis. Int J Adv Res Ideas Innov Technol. 2019;5(3):84–7.
92. Lam SN, Neda GD, Rabeta MS. The anticancer effect of Ocimum tenuiflorum leaves. Food Res. 2018;2(2):154–62. doi: https://doi.org/10.26656/fr.2017.2(2).251
93. Shrivastava V, Sijoria R, Dey YN, Pandey NK, Jadhav A, Wanjari M. Antimitotic and antiproliferative activity of stem bark of Oroxylum indicum. JOBARI. 2016;13(3):147–54.
94. Nagasaka M, Hashimoto R, Inoue Y, Ishiuchi K, Matsuno M, Itoh Y, et al. Anti-tumorigenic activity of chrysin from Oroxylum indicum via non-genotoxic p53 activation through the ATM-Chk2 pathway. Molecules. 2018;23:1394. doi: https://doi.org/10.3390/molecules23061394
95. Kathiriya AK, Das K, Kumar E, Mathai K. Evaluation of antitumor and antioxidant activity of Oxalis corniculata Linn. Against ehrlich ascites carcinoma on mice. Iran J Cancer Prev. 2010;3(4):157–65.
96. Mahata S, Pandey A, Shukla S, Tyagi A, Husain SA, Das BC, et al. Anticancer activity of Phyllanthus emblica Linn. (Indian Gooseberry): inhibition of transcription factor AP-1 and HPV gene expression in cervical ancer cells. Nutr Cancer. 2013;65(S1):88–97. doi: https://doi.org/10.1080/01635581.2013.785008
97. Ngamkitidechakul C, Jaijoy K, Hansakul P, Soonthornchareonnon N, Sireeratawong S. Antitumour effects of Phyllanthus emblica L.: induction of cancer cell apoptosis and inhibition of in vivo tumour promotion and in vitro invasion of human cancer cells. Phytother Res. 2010;24:1405–13. doi: https://doi.org/10.1002/ptr.3127
98. Chothani DL, Vaghasiya HU. A phyto-pharmacological overview on Physalis minima Linn. Indian J Nat Prod Resour. 2012;3(4):477–82.
99. Leong OK, Muhammad TST, Sulaiman SF. Cytotoxic activities of Physalis minima L. Chloroform extract on human lung adenocarcinoma NCI-H23 cell lines by induction of apoptosis. Evid-Based Complement Altern Med. 2011;2011:185064. doi: https://doi.org/10.1093/ecam/nep057
100. Mallick NM, Singh M, Parveen R, Khan W, Ahmad S, Zeeshan Najm M, et al. HPTLC analysis of bioactivity guided anticancer enriched fraction of hydroalcoholic extract of Picrorhiza kurroa. BioMed Res Int. 2015;2015:513875. doi: https://doi.org/10.1155/2015/513875
101. Sawhney SS, Painuli RM, Chauhan N. Evaluation of bactericidal and anticancer properties of fruits of Piper longum. Int J Pharm Pharm Sci. 2011;3(5):282–7.
102. Guo Z, Xu J, Xia J, Wu Z, Lei J, Yu J. Anti-inflammatory and antitumour activity of various extracts and compounds from the fruits of Piper longum L. J Pharm Pharmacol. 2019;71(7):1162–71. doi: https://doi.org/10.1111/jphp.13099
103. Javaid A, Ejaz Aziz Asad Z, Mubeen TN, Aamir S, Muhammad Q. Study of anticancer and antibacterial activities of Podophyllum hexandrum as natural curatives. Adv Complement Alt Med. 2020;5(5):482–5. doi: https://doi.org/10.31031/ACAM.2020.05.000621
104. Rani J, Giri RS. Screening of bio-active compounds and anticancer activity of Punica granatum L. World J Sci Res. 2016;1(3):06–13.
105. Ali S, Nisar M, Qaisar M, Khan A, Khan AA. Evaluation of the cytotoxic potential of a new pentacyclic triterpene from Rhododendron arboreum Sm stem bark. Pharm Biol. 2017;55(1):1927–30. doi: https://doi.org/10.1080/13880209.2017.1343359.
106. Al-Mamun MA, Akter Z, Uddin MJ, Ferdaus KMKB, Hoque KMF, Ferdousi Z, et al. Characterization and evaluation of antibacterial and antiproliferative activities of crude protein extracts isolated from the seed of Ricinus communis in Bangladesh. BMC Complement Altern Med. 2016;16:211. doi: https://doi.org/10.1186/s12906-016-1185-y
107. Zarai Z, Chobba IB, Mansour RB, Békir A, Gharsallah N, Kadri A. Essential oil of the leaves of Ricinus communis L.: in vitro cytotoxicity and antimicrobial properties. Lipids Health Dis. 2012;11(102):1–7. doi: https://doi.org/10.1186/1476-511X-11-102
108. Patel PG, Nagar AA, Patel RC, Rathod DK, Patel VR. In vitro anticancer activity of Rubia cordifolla against HeLa and Hep2 cell line. Int J Pharm Pharm Sci. 2011;3(2):70–1.
109. Mughees M, Sharma Y, Ahmad J, Ahmad A. Comparative analysis of anticancer activity of Rubia cordifolla L & adulterant on MCF-7 and SCAR marker development. Int J Plant Anim Environ Sci. 2017;7(1):70–9. doi: https://doi.org/10.21276/Ijpaes
110. Shilpa PN, Sivaramakrishnan V, Devaraj SN. Induction of apoptosis by methanolic extract of Rubia cordifolia Linn in HEp-2 cell line is mediated by reactive oxygen species. Asian Pac J Cancer Prev. 2012;13(6):2753–8. doi: https://doi.org/10.7314/apjcp.2012.13.6.2753
111. Saini R, Dangwal K, Singh H, Garg V. Antioxidant and antiproliferative activities of phenolics isolated from fruits of Himalayan yellow raspberry (Rubus ellipticus). J Food Sci Technol. 2014;51(11):3369–75. doi: https://doi.org/10.1007/s13197-012-0836-3
112. George BP, Parimelazhagan T, Kumar YT, Sajeesh T. Antitumor and wound healing properties of Rubus ellipticus Smith. J Acupunct Meridian Stud. 2015;8(3):134–41. doi: https://doi.org/10.1016/j.jams.2013.10.002
113. Chen CY, Kuo PL, Chen YH, Huang JC, Ho ML, Lin RJ, et al. Tyrosinase inhibition, free radical scavenging, antimicroorganism and anticancer proliferation activities of Sapindus mukorossi extracts. J Taiwan Inst Chem Eng. 2010;41(2):129–35. doi: https://doi.org/10.1016/j.jtice.2009.08.005
114. Liu M, Chen YL, Kuo YH, Lu MK, Liao CC. Aqueous extract of Sapindus mukorossi induced cell death of A549 cells and exhibited antitumor property in vivo. Sci Rep. 2018;8(4831):1–15. doi: https://doi.org/10.1038/s41598-018-23096-w.
115. Malge NR, Bandara AMPW, Keerthirathna WLR, Dissanayake DMI, Perera PK, Witharana C, et al. Antioxidant and antiproliferative activities of Smilax zeylanica root and rhizome extract against liver carcinoma cells, J Herbs Spices Med Plants. 2021;1:1–13. doi: https://doi.org/10.1080/10496475.2021.1891178
116. Rajesh V, Perumal P. Cytoprotective effect of Smilax zeylanica Linn. leaves against Benzo[a]pyrene induced lung cancer with reference to lipid peroxidation and antioxidant system in Swiss albino mice. Orient Pharm Exp Med. 2013;13:267–77. doi: https://doi.org/10.1007/s13596-013-0114-6
117. Uddin MN, Ahmed T, Pathan S, Al-Amin MM, Rana MS. Antioxidant and cytotoxic activity of stems of Smilax zeylanica in vitro. J Basic Clin Physiol Pharmacol. 2015;26(5):453–63. doi: https://doi.org/10.1515/jbcpp-2014-0114
118. Shokrzadeh M, Azadbakht M, Ahangar N, Hashemi A, Saeedi Saravi SS. Cytotoxicity of hydro-alcoholic extracts of Cucurbita pepo and Solanum nigrum on HepG2 and CT26 cancer cell lines. Pharmacogn Mag. 2010;6(23):176–9. doi: https://doi.org/10.4103/0973-1296.66931
119. Lai YL, Tai CJ, Wang CW, Choong CY, Lee BH, Shi YC, et al. Anti-cancer activity of Solanum nigrum (AESN) through suppression of mitochondrial function and epithelial-mesenchymal transition (EMT) in breast cancer cells. Molecules. 2016;21(5):553. doi: https://doi.org/10.3390/molecules21050553
120. Wang C, Lin Y, Tai C, Wang C, Chang Y, Choong C, et al. Integrated treatment of aqueous extract of Solanum nigrum-potentiated cisplatin-and doxorubicin-induced cytotoxicity in human hepatocellular carcinoma cells. Evid-Based Complement Altern Med. 2015;2015:1–10. doi: https://doi.org/10.1155/2015/675270
121. Vaid P, Saini AK, Saini RV. Evaluation of antioxidant, anti-cancer and antimicrobial activity of different extract and fraction of Stephania glabra. Plant Archiv. 2021;21(1):579–91. doi: https://doi.org/10.51470/PLANTARCHIVES.2021.v21.no1.081
122. Hussein SI, Yaseen NY, Jawad SQ, Abd ST. Seeds of Tamarindus indica as anti-cancer in some cell line. IJABR. 2017;7(2):360–2.
123. Milutinovi? MG, Stankovi? MS, Cvetkovi? DM, Topuzovi? MD, Mihailovi? VB, Markovi? SD. Antioxidant and anticancer properties of leaves and seed cones from European Yew (Taxus Baccata L.). Arch Biol Sci. 2015;67(2):525–34. doi: https://doi.org/10.2298/ABS141006015M
124. Ahuja R, Agrawal N, Mukerjee A. Evaluation of anticancer potential of Terminalia chebula fruits against ehrlich ascites carcinoma induced cancer in mice. JSIR. 2013;2(3):549–54.
125. Henry DP, Ranjan J, Murugan RK, Sivanantham A, Alagumuthu M. Exploration of anti-breast cancer effects of Terminalia chebula extract on DMBA induced mammary carcinoma in Sprague Dawley rats. Future J Pharm Sci. 2020;6(108):1–13. doi: https://doi.org/10.1186/s43094-020-00124-z
126. Hoque N, Sohrab MH, Afroz F, Rony SR, Sharmin S, Moni F, et al. Cytotoxic metabolites from Thysanolaena maxima Roxb. available in Bangladesh. Clin Phytosci. 2020;6(89):1–10. doi: https://doi.org/10.1186/s40816-020-00226-4
127. Polu PR, Nayanbhirama U, Khan S, Maheswari R. Assessment of free radical scavenging and anti-proliferative activities of Tinospora cordifolia Miers (Willd). BMC Complement Altern Med. 2017;17(1):457. doi: https://doi.org/10.1186/s12906-017-1953-3
128. Palmieri A, Scapoli L, Iapichino A, Mercolini L, Mandrone M, Poli F, et al. Berberine and Tinospora cordifolia exert a potential anticancer effect on colon cancer cells by acting on specific pathways. Int J Immunopathol Pharmacol. 2019;33:1–10. doi: https://doi.org/10.1177/2058738419855567
129. Patil S, Ashi H, Hosmani J, Almalki AY, Alhazmi YA, Mushtaq S, et al. Tinospora cordifolia (Thunb.) Miers (Giloy) inhibits oral cancer cells in a dose-dependent manner by inducing apoptosis and attenuating epithelial-mesenchymal transition. Saudi J Biol Sci. 2021;28:4553–9. doi: https://doi.org/10.1016/j.sjbs.2021.04.056
130. Jagetia GC, Rao SK. Evaluation of the antineoplastic activity of Guduchi (Tinospora cordifolia) in ehrlich ascites carcinoma bearing mice. Biol Pharm Bull. 2006;29(3):460–6. doi: https://doi.org/10.1248/bpb..29.460
131. Ahmad R, Srivastava AN, Khan MA. Evaluation of in vitro anticancer activity of stem of Tinospora cordifolia against human breast cancer and Vero cell lines. JMPS. 2015;3(4):33–7.
132. Saboo SS, Thorat PK, Tapadiya GG, Khadabadi SS. Evaluation of phytochemical and anticancer potential of chloroform extract of Trichosanthes tricuspidata Lour roots (Cucurbitaceae) using in-vitro models. Int J Pharm Pharm Sci. 2013;5(4):203–8.
133. Levy A, Sivanesan D, Murugan R, Jornadal J, Quinonez Y, Jaffe M, et al. Urtica dioica induces cytotoxicity in human prostate carcinoma LNCaP cells: involvement of oxidative stress, mitochondrial depolarization and apoptosis. Trop J Pharm Res. 2014;13(5):711–7. doi: https://doi.org/10.4314/tjpr.v13i5.9
134. Mishra R, Sharma S, Sharma RS, Singh S, Sardesai MM, Sharma S, et al. Viscum articulatum Burm. f. aqueous extract exerts antiproliferative effect and induces cell cycle arrest and apoptosis in leukemia cells. J Ethnopharmacol. 2018;219:91–102. doi: https://doi.org/10.1016/j.jep.2018.03.005
135. Nitha A, Prabha SP, Ansil PN, Latha MS. Antiproliferative effect of Woodfordia fruticosa Kurz flowers on experimentally induced hepatocellular carcinoma in rats and in human hepatoma cell line. J Pharm Res. 2013;6(2):239–48. doi: https://doi.org/10.1016/j.jopr.2013.02.003
136. Karmakar I, Haldar S, Chakraborty M, Dewanjee S, Haldar PK. In vitro antioxidant and cytotoxic activity of Zanthonitrile isolated from Zanthoxylum alatum. J Appl Pharm Sci. 2016; 6(06):119–22. doi: https://doi.org/10.7324/JAPS.2016.60621
137. Mukhija M, Singh MP, Dhar KL, Kalia AN. Cytotoxic and antioxidant activity of Zanthoxylum alatum stem bark and its flavonoid constituents. J Pharmacogn Phytochem. 2015;4(4):86–92.
138. Plengsuriyakarn T, Viyanant V, Eursitthichai V, Tesana S, Chaijaroenkul W, Itharat A, et al. Cytotoxicity, toxicity, and anticancer activity of Zingiber officinale Roscoe against cholangiocarcinoma. Asian Pac J Cancer Prev. 2012;13:4597–606. doi: https://doi.org/10.7314/APJCP.2012.13.9.4597
139. Ansari JA, Ahmad MK, Khan AR, Fatima N, Khan HJ, Mahdi AA. Anticancer and antioxidant activity of Zingiber officinale Roscoe rhizome. Indian J Experiment Biol. 2016;54(11):767–73.
140. Falih SMJ, Al-Saray ST, Alfaris AA, Al-Ali AA. The synergistic effect of eucalyptus oil and retinoic acid on human esophagus cancer cell line SK-GT-4. Egypt J Med Hum Genet. 2022;23:70. doi: https://doi.org/10.1186/s43042-022-00259-z
141. Cui M, Wang Z, Huang LT, Wang JH. Parthenolide leads to proteomic differences in thyroid cancer cells and promotes apoptosis. BMC Complement Med Ther. 2022;22:99. doi: https://doi.org/10.1186/s12906-022-03579-0
142. Shaik BB, Katari NK, Jonnalagadda SB. Role of natural products in developing novel anticancer agents: a perspective. Chem Biodivers. 2022;19(11):e202200535.
143. Bohannon RA, Miller DG, Diamond HD. Vincristine in the treatment of lymphomas and leukemias. Cancer Res. 1963;23(4_Part_1):613–21.
144. Regassa H, Sourirajan A, Kumar V, Pandey S, Kumar D, Dev K. A review of medicinal plants of the Himalayas with anti-proliferative activity for the treatment of various cancers. Cancers. 2022;14(16):3898.
145. Saeidnia S. New approaches to natural anticancer drugs (No. 12036). Cham, Switzerland: Springer International Publishing; 2015.
146. Basili S, Moro S. Novel camptothecin derivatives as topoisomerase I inhibitors. Expert Opin Ther Pat. 2009;19(5):555–74.
147. Cui M, Wang Z, Huang LT, Wang JH. Parthenolide leads to proteomic differences in thyroid cancer cells and promotes apoptosis. BMC Complement Med Ther. 2022;22(1):1–14.
148. Yuan L, Wang Z, Zhang D, Wang J. Metabonomic study of the intervention effects of parthenolide on anti-thyroid cancer activity. J Chromatogr B. 2020;1150:122179.
149. Carter NJ, Keam SJ. Trabectedin: a review of its use in the management of soft tissue sarcoma and ovarian cancer. Drugs. 2007;67:2257–76.
150. Carter NJ, Keam SJ. Trabectedin: a review of its use in soft tissue sarcoma and ovarian cancer. Drugs. 2010;70:355–76.
151. Suffness M, Douros J. Current status of the NCI plant and animal product program. J Nat Prod. 1982;45(1):1–14.
152. Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100(1–2):72–9.
153. Mumtaz M, Hussain N, Salam S, Bilal M. Multifunctional nanodiamonds as emerging platforms for cancer treatment, and targeted delivery of genetic factors and protein medications—a review. J Mater Sci. 2022;57(17):8064–99.
154. Tan G, Gyllenhaal C, Soejarto DD. Biodiversity as a source of anticancer drugs. Curr Drug Targets. 2006;7(3):265–77.
155. Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H. Flavonoids, dietary-derived, inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916–21.
156. Naowaratwattana W, De-Eknamkul W, De Mejia EG. Phenolic-containing organic extracts of mulberry (Morus alba L.) leaves inhibit HepG2 hepatoma cells through G2/M phase arrest, induction of apoptosis, and inhibition of topoisomerase IIα activity. J Med Food. 2010;13:1045–56. doi: https://doi.org/10.1089/jmf.2010.1021
157. Goel A, Boland CR, Chauhan DP. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001;172:111–8. doi: https://doi.org/10.1016/S0304-3835(01)00655-3
158. Wang X, Hang Y, Liu J, Hou Y, Wang N, Wang M. Anticancer effect of curcumin inhibits cell growth through miR-21/PTEN/Akt pathway in breast cancer cell. Oncol Lett. 2017;13:4825–31. doi: https://doi.org/10.3892/ol.2017.6053
159. Ryu JH, Lee SJ, Kim MJ, Shin JH, Kang SK, Cho KM, et al. Antioxidant and anticancer activities of Artemisia annua L. and determination of functional compounds. J Korean Soc Food Sci Nutr. 2011;40:509–16. doi: https://doi.org/10.3746/jkfn.2011.40.4.509
160. Omara T, Kiprop AK, Ramkat RC, Cherutoi J, Kagoya S, Moraa Nyangena D, et al. Medicinal plants used in traditional management of cancer in Uganda: a review of ethnobotanical surveys, phytochemistry, and anticancer studies. Evid-Based Complement Alternat Med. 2020;2020:1–26. doi: https://doi.org/10.1002/jsfa.12892
161. Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E, et al. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59(6):365–78. doi: https://doi.org/10.1016/j.phrs.2009.01.017
162. Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–65. doi: https://doi.org/10.1016/j.ejcb.2020.151075
163. Annan K, Dickson R. Evaluation of wound healing actions of Hoslundia opposita vahl, Anthocleista nobilis G. Don. and Balanites aegyptiaca L. J Sci Technol. 2008;28(2):26–35. doi: https://doi.org/10.4314/just.v28i2.33091
164. Levˆeque D, Jehl F. Molecular pharmacokinetics of Catharanthus (vinca) alkaloids. J Clin Pharmacol. 2007;47(5):579–88. doi: https://doi.org/10.1177/0091270007299430
165. James SA, Bilbiss L, Muhammad BY. The effects of Catharanthus roseus (L) G. Don 1838 aqueous leaf extract on some liver enzymes, serum proteins and vital organs. Sci World J. 2007;2(1):5–9. doi: https://doi.org/10.4314/swj.v2i1.51700
166. Sherines RJ, Howard SS. Male infertility. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, Novick AC., editors. Campbells urology. 4th ed. Philadelphia, PA: WB Saunders; 1978. vol. 1, 715 p.
167. Ganai SA, Sheikh FA, Baba ZA, Mir MA, Mantoo MA, Yatoo MA. Anticancer activity of the plant flavonoid luteolin against preclinical models of various cancers and insights on different signalling mechanisms modulated. Phytother Res. 2021;35(7):3509–32. doi: https://doi.org/10.1002/ptr.7044. Epub 2021 Feb 13
168. Garcia-Oliveira P, Otero P, Pereira AG, Chamorro F, Carpena M, Echave J, et al. Status and challenges of plant-anticancer compounds in cancer treatment. Pharmaceuticals. 2021;14(2):157. doi: https://doi.org/10.3390/ph14020157
169. Alghasham AA. Cucurbitacins–a promising target for cancer therapy. Int J Health Sci. 2013;7(1):77. doi: https://doi.org/10.12816/0006025
170. Bartalis J, Halaweish FT. Relationship between cucurbitacins reversed-phase high performance liquid chromatography hydrophobicity index and basal cytotoxicity on HepG2 cells. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;818:159–66. doi: https://doi.org/10.1016/j.jchromb.2004.12.020
171. Sadzuka Y, Hatakeyama H, Daimon T, Sonobe T. Screening of biochemical modulator by tumor cell permeability of doxorubicin. Int J Pharm. 2008;354:63–9. doi: https://doi.org/10.1016/j.ijpharm.2007.10.015
172. Mandlik DS, Namdeo AG. Pharmacological evaluation of Ashwagandha highlighting its healthcare claims, safety, and toxicity aspects. J Diet Suppl. 2021;18(2):183–226. doi: https://doi.org/10.1080/19390211.2020.1741484
173. To’bungan N, Jati WN, Zahida F. Acute toxicity and anticancer potential of knobweed (Hyptis capitata) ethanolic leaf extract and fraction. Plant Sci Today. 2022;9(4):955–62. doi: https://doi.org/10.14719/pst.1847
174. Jabbar AA, Abdulrahman KK, Abdulsamad P, Mojarrad S, Mehmetçik G, Sardar AS. Phytochemical profile, antioxidant, enzyme inhibitory and acute toxicity activity of Astragalus bruguieri. Baghdad Sci J. 2023;20(1):0157. doi: https://doi.org/10.21123/bsj.2022.6769