INTRODUCTION
Marine bacteria have become a significant focus of interest as a promising source of novel bioactive molecules for pharmaceutical research, as they can produce a variety of compounds with potential therapeutic benefits [1,2]. In the past, the search for such molecules primarily targeted invertebrates, particularly sponges [3], but recent trends suggest that microorganisms such as fungi and bacteria are now the primary sources of marine natural products [4]. Marine bacteria can constitute a significant proportion of the biomass of sponges, and some of them are capable of producing compounds that are also found in their hosts [5,6]. Exploration of the anticancer compounds from marine bacteria also exhibited an exciting finding, as demonstrated by Salinospora sp. (actinomycetes), which produced salinosporamide A [7]. Moreover, marine bacteria generated unique and potent anti-infective compounds that could be developed as lead compounds [8].
Actinomycetes have many biosynthetic gene clusters (BGCs) [9], but some remain obscure [10]. On the other hand, the routine for exploring the bioactive compounds from them were usually done in lab condition using axenic cultures of the bacteria. Thus, there are many compounds still hidden. To overcome the challenges of producing such compounds by applying, for example, one strain many active compounds, co-culture, inducing stress responses, adding elicitors, or even through genetic engineering [11–13].
The co-culture technique involves growing two or more microorganisms in a solid or liquid medium [14,15]. When different species interact in a symbiotic relationship, it can activate cryptic biosynthetic genes and produce natural compounds not present in either organism when grown alone [16]. This phenomenon is demonstrated in studies on actinomycetes co-cultured with mycolic-acid-containing bacteria, which resulted in the activation of secondary metabolites that are not produced in single-species cultures [17].
There are reviews on marine microbial co-cultures by Caudal et al. [18] and Chen et al. [11]. Although there are existing reviews on co-cultures of marine microorganisms, there is still a need for reviews that specifically focus on co-cultured marine bacteria. Therefore, this review aims to explore how co-cultured bacteria impact the production of bioactive compounds and/or bioactivities. Publications between 2012 and 2022 about the co-culture of marine bacteria were collected using search engines such as Google Scholar, Pubmed, and Scopus.
THE RESULTS OF MARINE BACTERIA CO-CULTURE
The co-culture experiment aims to activate the cryptic BGCs and promote the production of novel bioactive compounds. However, we identified three distinct categories of results that emerge from co-culture between different marine bacteria strains based on the observed bioactivities and bioactive compounds. The three groups are as follows: 1) increased yield of bioactivities and/or bioactive compounds, 2) production of known compounds that were not present in single-strain cultures, and 3) production of previously undescribed compounds. The following paragraph describes each of these categories.
Increased yield of bioactivities and/or bioactive compounds
Co-culture of bacteria can involve intraspecies communication that is absent in single-species cultures. This communication may result in the induction of the production of certain metabolites in response to chemical signals or direct cell contact, which can also be detected by an increase in bioactivity. As exemplified by the study from Haque et al. [19] on the co-culture of two Actinomycetes, namely, Streptomyces sp. strain ANAM-5 and Streptomyces sp. strain AIAH-10 from the soil of Bangladesh’s Sundarbans mangrove forest. Both bacteria were cultured in yeast extract glucose broth media (0.25% w/v yeast extract and 0.5% w/v glucose) for a small-scale liquid culture (200 ml). The co-culture method involved the mixture of 1:1 (v/v) of each bacterium in yeast extract glucose broth media, then incubated for 7 days at 31°C with a rotation speed of 220 rpm. The minimum inhibitory concentration (MIC) values for the fungistatic activity of ethyl acetate extract from cell-free supernatant against Aspergillus niger, Candida albicans, and Saccharomyces cerevisiae were 64, 32, and 64 g/ml, respectively. Furthermore, the extract also inhibited the growth of ehrlich ascites carcinoma (EAC) cells in mice when administered intraperitoneally. Inhibition values were 42.49% and 75.75% when administered with doses 50 and 100 mg/kg, respectively. However, the anticancer activity of the extract was lower compared to bleomycin 0.3 mg/kg as the positive control, which inhibited 89.64% of the cell growth. The extract at 50 and 100 mg/kg increased the lifespan of the EAC-carrying mice with values of 49.00% and 71.79%, respectively [19]. These results showed the dose-dependent manner activities of the extract. Nonetheless, compounds responsible for the bioactivities were not isolated.
Another example was a study using marine Streptomyces sp. CGMCC4.7185, which generated bacillamides and tryptamine derivatives when co-cultured with Bacillus mycoides. The critical factors influencing compound production in the co-culture medium were optimized. The results showed that the optimum condition was a simple liquid medium containing glycerol 5 g, yeast extract 5 g, and 75% seawater with pH 8.0. The authors added 1% B. mycoides v/v to the 7 days old Streptomyces sp. and incubated the co-culture until 14 days in a static condition. Bacillamides and tryptamine derivatives were detected when both bacteria could grow in balance for a certain period without shaking incubation. From the optimum medium, the co-culture system resulted in the production of bacillamide A (1), bacillamide B (2), bacillamide C (3), N-acetyl tryptamine (4), N-propanoyltryptamine (5) (Fig. 1). Compounds 1–5 were barely seen in the chromatogram of each axenic culture extract. Moreover, the co-culture system’s reproducibility was in six out of seven samples. Therefore, the co-culture system provided a relatively simple technique to significantly increase bacillamides and tryptamine derivatives’ production [20].
Streptomyces sp. PTY087I2 was obtained from Styela canopus, a tunicate from Bastimentos Park, Panama. AntiSMASH analysis of the bacterium genome indicated that the bacterium has 37 clusters with percent similarity ranging from 4% to 100% to known BGCs. Extract from the axenic culture of the bacterium yielded only a fraction of the expected metabolites. The bacterium was then co-cultured in pair with pathogens bacteria such as methicillin-resistant Staphylococcus aureus (MRSA), methicillin-sensitive Staphylococcus aureus (MSSA), Bacillus subtilis, and Pseudomonas aeruginosa in a liquid medium for 10 days at 30°C. One liter of the medium contained peptone 1 g, yeast extract 2 g, starch 5 g, and Instant Ocean® 33 g [21].
Streptomyces sp. PTY087I2 showed increased antibacterial activity when co-cultured with B. subtilis, MSSA, and MRSA. Among the three, extract from the co-culture of Streptomyces sp. strain PTY087I2 with MRSA exhibited the highest antibacterial activity against MRSA, B. subtilis, and MSSA with MIC values of 6.25, 1.56, 12.5, and 12.5 μg/ml, respectively. Chromatogram analysis of the area under the curve (AUC) revealed a significant increase in the AUCs from the peaks of interest. However, the increased AUC values were not linear with the increased antibacterial activities. Further liquid chromatography tandem with mass spectrometers (LC-MS/MS) analysis using molecular networking showed that chemical constituents were not identical in all extracts. Naphthoquinone derivatives such as granatomycin D (6) and granaticin (7) (Fig. 1) were found in all extracts. However, dihydrogranaticin B (8) was found only in the co-culture system with MSSA and MRSA. LC-MS/MS analysis results also showed the difference of naphthoquinone derivatives occurrence in the MRSA co-culture system, which is highly possible to affect the resulting extract’s bioactivity [21].
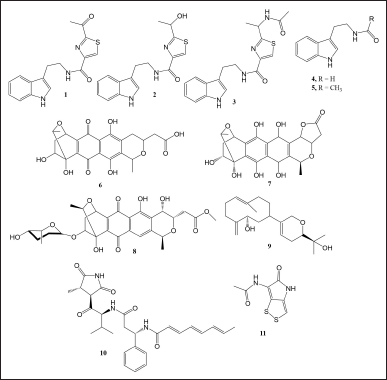 | Figure 1. Compounds 1–11. [Click here to view] |
Streptomyces cinnabarinus PK209 was able to produce lobocompactol (9), an antifouling diterpene, through co-culture with Alteromonas sp. KNS-16, which is resistant to lobocompactol. The addition of KNS-16 to PK209 culture led to a 10.4-fold increase in lobocompactol production compared to when PK209 was cultured alone. Lobocompactol demonstrated an EC50 of 0.18 and 0.43 μg/ml in inhibiting the spore settlement of macroalga Ulva pertusa and the accumulation of the diatom Navicula annexa, respectively. Furthermore, lobocompactol exhibited MIC values of 66 and 112 μg/ml when tested against the biofouling bacteria KNP-5 and KNP-8, respectively. Notably, lobocompactol was first isolated from Lobophytum compactum, a soft coral with cytotoxic activity. Despite lobocompactol having lower antifouling activity than irgarol (the positive control), the increase in lobocompactol production is a crucial step toward optimizing its structure and bioactivity [22].
The technique of co-culturing two marine bacteria, namely Marinirhabdus sp. strain HTHA1 and Marinobacter hydrocarbonoclasticus strain HTHA2, resulted in an increase in their biomass and protease activity. These bacteria were obtained from a seawater sample collected in Port Philip Bay, Australia. Compared to their individual cultures, the co-culture method led to a 1.6–2.0 times increase in biomass and a 1.8–2.4 times increase in protease activity. The co-culture was achieved by preventing one bacterium from overgrowing the other and maintaining direct cell contact. However, M. hydrocarbonoclasticus was the dominant bacterium in the co-culture system, with a population percentage of 64% [23].
Co-culture of two co-occurred marine Vibrionaceae, namely, Vibrio coralliilyticus strain S2052 and Photobacterium galatheae strain S2753 resulted in the increased production of antibiotic andrimid (10) by V. coralliilyticus and holomycin (11) by P. galatheae by 4.3 and 2.7 times higher compared to single-species cultures. The increased production of these antibiotics was assumed to be the signal molecules for communication in competition for the niche because the phenomenon did not occur when co-cultured with one of the mutants that do not produce antibiotics [24].
Production of known compounds that were not present in single-strain cultures
Interaction with different bacterial species that occurred in the co-culture system can induce the production of certain compounds. These compounds are only produced when in the co-culture and undetected in the axenic culture of each bacterium. Nevertheless, some of these compounds are not novel compounds. Two sponge-associated actinobacteria Actinokineospora sp. EG49 and Nocardiopsis sp. RV16 were obtained from the Red Sea sponge, Spheciospongia vagabunda, and a Mediterranean sponge, Dysidea avara, respectively. EtOAc extracts from the axenic and the co-culture were monitored using 1H-NMR and liquid chromatography-photodiode array fingerprinting techniques to improve the detection of the compounds, especially the unionized ones when detected using LC-MS. The results showed that N-(2-hydroxyphenyl)-acetamide (12), 1,6-dihydroxy phenazine (13), 5a,6,11a,12-tetrahydro-5a,11a-dimethyl-1,4-benzoxazino[3,2-b][1,4]benzoxazine (14) occurred only in the medium from the co-culture (Fig. 2). The structure of compounds was obtained from references based on the ultraviolet absorption, exact mass, and 1H-NMR spectra of the isolated compounds from the extracts. Those compounds were tested for their bioactivities. However, only compound 13 showed antibacterial activity against Bacillus sp. P25 and interestingly against Actinokineospora sp. EG49 itself with diameter zone of 11 and 15 mm, respectively. Compound 13 also exhibited antiparasitic activity against Trypanosoma brucei with an IC50 value of 19 μM [25].
Production of previously undescribed compounds
The most common motivation for applying the co-culture technique is to obtain novel and potent compounds. The multiomics approach to finding natural antimicrobial products lead to the biosynthesis of keyicin (15) (Fig. 3). The compound was an undescribed antibiotic obtained from co-cultured Rhodococcus sp. and Micromonospora sp. extracts undetected in the axenic culture extract. The chemical structure of compound 15 was determined from Fourier/transform ion cyclotron resonance-mass spectrometry data and the extensive analysis of 1D and 2D of 13C and 1H-NMR. Compound 15 inhibited B. subtilis and MSSA with MIC values of 8 and 2 μg/ml, respectively. Results of the culture experiment showed that compound 15 was produced only when both bacteria were cultured together for some time without an overgrowth from one species. Therefore, the co-culture system was designed using unique flasks and a semipermeable membrane to inhibit direct contact or mixture of interspecies but allow interaction of substances in the liquid medium without mixing the species [26].
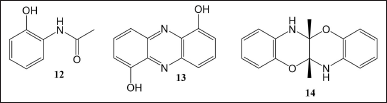 | Figure 2. Compounds 12–14. [Click here to view] |
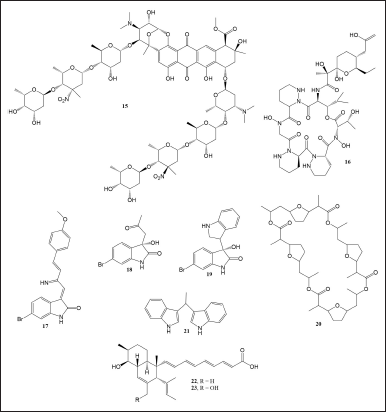 | Figure 3. Compounds 15–23. [Click here to view] |
Dentigerumycin E (16) was isolated from Streptomyces sp. JB5 that co-cultured with marine Bacillus sp. GN1 (Fig. 3). Both bacteria were isolated from intertidal mud. The extract of the liquid medium resulting from the co-culture was observed using LC-MS and compared to the extracts of axenic culture. After an incubation period of 6 days at 30°C, one peak was detected only in the co-culture medium (4 g of glucose, 4 g of yeast extract, and 10 g of malt extract per 1 l of artificial seawater). The peak was further isolated, and the structure was determined using HR-FAB-MS, 1D and 2D of 13C and 1H-NMR analysis. The compound showed moderate cytotoxic activity toward cancer cell lines, namely, A549, HCT116, MDA-MB-231, SK-HEP-1, and SNU638, representing cancer in the lung, colorectal, breast, liver, and stomach, respectively. Further assay on MDA-MB-231 (a breast cancer cell line) showed that dentigerumycin E reduced cell migration by up to 48% in the wound healing experiment at 40 M. While in the cell invasion assay, it inhibited up to 34% at 40 μM [27].
Previously undescribed oxindole alkaloids saccharomonosporine A (17) and convolutamydine F (18) together with three known compounds (S) 6-bromo-3-hydroxy-3-(1H-indole -3-yl) (19), nonactin (20), and vibrindole (21) were produced from the co-culture medium of Saccharomonospora sp. UR22 and Dietzia sp. UR66 (Fig. 3). Both bacteria were isolated from the sponge Callyspongia siphonella in the Red Sea. By thoroughly analyzing HR-ESI-MS, 1D, and 2D NMR spectral data, the structures of compounds 14–18 were discovered. The isolated compounds were tested against various cell lines such as H-1650 (lung adenocarcinoma), HL-60 (human promyelocytic leukemia), and HT-29 (human colon adenocarcinoma). The results showed that compounds 17 and 19 strongly inhibited the proliferation of HL-60 and HT-29 cells with IC50 values of 2.8, 3.6, 4.2, and 3.7 μM, respectively. In addition, both compounds also inhibited the activity of Pim-1 kinase, an enzyme mediating tumor cell growth, with IC50 values of 0.3 ± 0.02 and 0.95 ± 0.01μM, respectively [28].
Two novel polyketides, janthinopolyenemycins A and B (22-23), have been isolated from the marine bacterium Janthinobacterium sp. ZZ145, which was co-cultured with another bacterium in the same genus, Janthinobacterium sp. ZZ148 (Fig. 3). Both bacteria were obtained from marine soil and were tested against MRSA, Escherichia coli, and C. albicans. Various co-culture media were tested for bioactivity, and the results showed that rice solid medium produced superior bioactivity. Isolation of active compounds yielded compounds 22-23 with antifungal activity against C. albicans, with a MIC value of 15.6 μg/ml for each compound. The structures of the compounds were determined based on HR-ESI-MS, 1D, and 2D NMR spectra. Furthermore, the absolute configuration of both compounds was identified based on a comparison of the experimental and calculated electronic circular dichroism spectra. These findings could be valuable in developing novel antifungal agents for treating C. albicans infections [29].
CONCLUSION
Co-culture is an effective technique for enhancing the discovery of bioactive molecules from marine bacteria, including marine Actinomycetes. It enables the production of hidden secondary metabolites that are not present in single-strain cultures, increasing the yield of active compounds and bioactivity of the extracts. To optimize the co-culture, it is important to maintain the occurrence of each group of bacteria during the incubation period. The co-culture system needs to be personalized based on the characteristics needed to induce intraspecies communication. Factors such as direct cell contact, initial volume of each group of bacteria, and incubation period need to be optimized to prevent overdomination of one group in the co-culture system to maximize interaction and generate the desired outcome.
ACKNOWLEDGMENTS
This study was supported by the Research Organization for Health National Research and Innovation Agency of Indonesia through Rumah Program Vaksin dan Obat TA. 2023 and Research Institutions and Community Service of STIKes Widya Dharma Husada Tangerang, which has facilitated research collaboration activities and publication funding from the Yayasan Widya Dharma Husada.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
Funding from National Research and Innovation Agency (BRIN) Rumah Program Vaksin dan Obat Nomor 23/III.9/HK/2023 and Research Institutions and Community Service of STIKes Widya Dharma Husada Tangerang.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Stincone P, Brandelli A. Marine bacteria as source of antimicrobial compounds. Crit Rev Biotechnol. 2020;40(3):306–19.
2. Tipre DR, Purohit MS, Dave SR. Marine bacteria—a treasure house of valuable products and functions. In: Nathani NM, Mootapally C, Gadhvi IR, Maitreya B, Joshi CG, editors. Marine niche: applications in pharmaceutical sciences: translational research. Gateway East, Singapore: Springer; 2020. pp. 415–36.
3. Santos JD, Vitorino I, Reyes F, Vicente F, Lage OM. From ocean to medicine: pharmaceutical applications of metabolites from marine bacteria. Antibiotics. 2020;9(8):455.
4. Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR. Marine natural products. Nat Prod Rep. 2023;40:275–325.
5. Jime?nez C. Marine natural products in medicinal chemistry. ACS Med Chem Lett. 2018;9(10):959–61.
6. Newman DJ, Cragg GM. Drugs and drug candidates from marine sources: an assessment of the current “state of play”. Planta Med. 2016;82(09/10):775–89.
7. Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew Chem Int Ed. 2023;42(3):355–7.
8. Schinke C, Martins T, Queiroz SCN, Melo IS, Reyes FGR. Antibacterial compounds from marine bacteria, 2010–2015. J Nat Prod. 2017;80(4):1215–28.
9. Niu G. Genomics-driven natural product discovery in actinomycetes. Trends Biotechnol. 2018;36(3):238–41.
10. Reen FJ, Romano S, Dobson AD, O’Gara F. The sound of silence: activating silent biosynthetic gene clusters in marine microorganisms. Mar Drugs. 2015;13(8):4754–83.
11. Chen J, Zhang P, Ye X, Wei B, Emam M, Zhang H, et al. The structural diversity of marine microbial secondary metabolites based on co-culture strategy: 2009–2019. Mar Drugs. 2020;18(9):449.
12. Manteca Á, Yagüe P. Streptomyces differentiation in liquid cultures as a trigger of secondary metabolism. Antibiotics. 2018;7(2):41.
13. Peng XY, Wu JT, Shao CL, Li ZY, Chen M, Wang CY. Co-culture: stimulate the metabolic potential and explore the molecular diversity of natural products from microorganisms. Mar Life Sci Technol. 2021;3:363–74
14. Arora D, Gupta P, Jaglan S, Roullier C, Grovel O, Bertrand S. Expanding the chemical diversity through microorganisms co-culture: current status and outlook. Biotechnol Adv. 2020;40:107521.
15. Bertrand S, Bohni N, Schnee S, Schumpp O, Gindro K, Wolfender JL. Metabolite induction via microorganism co-culture: a potential way to enhance chemical diversity for drug discovery. Biotechnol Adv. 2014;32(6):1180–204.
16. Adnani N, Rajski SR, Bugni TS. Symbiosis-inspired approaches to antibiotic discovery. Nat Prod Rep. 2017;34(7):784–814.
17. Hoshino S, Onaka H, Abe I. Activation of silent biosynthetic pathways and discovery of novel secondary metabolites in actinomycetes by co-culture with mycolic acid-containing bacteria. J Ind Microbiol Biotechnol. 2019;46(3–4):363–74.
18. Caudal F, Tapissier-Bontemps N, Edrada-Ebel RA. Impact of co-culture on the metabolism of marine microorganisms. Mar Drugs. 2022;20(2):153.
19. Haque MU, Rahman MA, Haque MA, Sarker AK, Islam MAU. Antimicrobial and anticancer activities of ethyl acetate extract of co-culture of Streptomyces sp. ANAM-5 and AIAH-10 isolated from Mangrove Forest of Sundarbans, Bangladesh. J Appl Pharm Sci. 2016;6(2):51–5.
20. Yu L, Hu Z, Ma Z. Production of bioactive tryptamine derivatives by co-culture of marine Streptomyces with Bacillus mycoides. Nat Prod Res. 2015;29(22):2087–91.
21. Sung AA, Gromek SM, Balunas MJ. Upregulation and identification of antibiotic activity of a marine-derived Streptomyces sp. via co-cultures with human pathogens. Mar Drugs. 2017;15(8):250.
22. Cho JY, Kim MS. Induction of antifouling diterpene production by Streptomyces cinnabarinus PK209 in co-culture with marine-derived Alteromonas sp. KNS-16. Biosci Biotechnol Biochem. 2012;76(10):1849–54.
23. Anh HTH, Shahsavari E, Bott NJ, Ball AS. Application of co-culture technology to enhance protease production by two halophilic bacteria, Marinirhabdus sp. and Marinobacter hydrocarbonoclasticus. Molecules. 2021;26(11):3141.
24. Buijs Y, Zhang SD, Jørgensen KM, Isbrandt T, Larsen TO, Gram L. Enhancement of antibiotic production by co-cultivation of two antibiotic producing marine Vibrionaceae strains. FEMS Microbiol Ecol. 2021;97(4):fiab041.
25. Dashti Y, Grkovic T, Abdelmohsen UR, Hentschel U, Quinn RJ. Production of induced secondary metabolites by a co-culture of sponge-associated actinomycetes, Actinokineospora sp. EG49 and Nocardiopsis sp. RV163. Mar Drugs. 2014;12(5):3046–59.
26. Adnani N, Chevrette MG, Adibhatla SN, Zhang F, Yu Q, Braun DR, et al. Coculture of marine invertebrate-associated bacteria and interdisciplinary technologies enable biosynthesis and discovery of a new antibiotic, keyicin. ACS Chem Biol. 2017;12(12):3093–102.
27. Shin D, Byun WS, Moon K, Kwon Y, Bae M, Um S, et al. Coculture of marine Streptomyces sp. with Bacillus sp. produces a new piperazic acid-bearing cyclic peptide. Front Chem. 2018;6:498.
28. El-Hawary SS, Sayed AM, Mohammed R, Khanfar MA, Rateb ME, Mohammed TA, et al. New Pim-1 kinase inhibitor from the co-culture of two sponge-associated actinomycetes. Front Chem. 2018;6:538.
29. Anjum K, Sadiq I, Chen L, Kaleem S, Li XC, Zhang Z, et al. Novel antifungal janthinopolyenemycins A and B from a co-culture of marine-associated Janthinobacterium spp. ZZ145 and ZZ148. Tetrahedron Lett. 2018;59(38):3490–4.