INTRODUCTION
One of the three main gynecological cancers, ovarian cancer (OC), is the fourth most frequent malignancy in females and is associated with mortality among gynecological cancers worldwide. As a result of the ovary’s position deeper within the pelvis, the illness, which is currently in stage I or II, is sneaky and asymptomatic. The 5-year survival rate for occurrence of OC is less than 30% when they are advanced, or stage III or IV, although it can increase exponentially to 92.7% in the early stages of the disease. Thus, the earlier a precise diagnosis is made, the better the disease will be managed in its early phases. To improve overall patient survival, sensitive and accurate medical diagnostics or molecular markers for the early diagnosis of OC are urgently required. The most recent treatment involves chemotherapy with platinum and taxane, which is administered after a surgical procedure. Although individuals gradually become resistant to platinum-based chemotherapy, the majority of recurrences are often the most platinum-sensitive. The main challenge in treating OC is resistance to chemo, either first-class (i.e., intrinsic) or second-class (i.e., acquired). Based on their first-class reaction to the therapy, subjects are classified as (i) responsive to platinum, (ii) refractory to platinum, and (iii) resistant to platinum. Patients who are platinum-responsive first react to platinum-based treatment, and their response may endure anywhere between 6 months and many years. The group refractory to platinum experiences disease progression and does not respond to treatment [1].
Numerous phytochemicals, including curcumin, epigallocatechin-3-gallate, vitexin, epigallocatechin, ginsenosides, lycopene, salidroside, aviscumine, resveratrol, and homoharringtonine, have been demonstrated to have promising antitumor effects in recent years in addition to minimizing chemotherapy-induced toxin. While normal (noncancer) cells are spared, numerous in vitro and in vivo research studies have shown that micronutrients as well as nutritional supplements derived from plants, such as flavonoids, flavones, and other plant-derived antioxidants, may inhibit cancer cell growth of cancer cell, trigger cell death, and cause toxicity to cells. Due to these qualities, natural products are excellent choices for OC chemotherapy adjuvant therapy and chemoprevention. Here, we examine the most recent research on natural OC chemoprevention agents [2].
There are four stages of OC and based on that survival rate of 5 years is being measured:
1) Stage 1
Stage 1 is up to the ovaries or the fallopian tubes.
The 5-year relative survival rate is about 93%.
2) Stage 2
Stage 2 is considered to be in one or both the ovaries or fallopian tubes and further spreads to other organs in the pelvis.
The 5-year relative survival rate is about 74%.
3) Stage 3
Stage 2 is considered to be in one or both the ovaries or fallopian tubes and spreads outside the pelvis to other parts of the abdomen and/or nearby lymph nodes. Sometimes on the surface of the liver.
The 5-year relative survival rate is about 41%.
4) Stage 4
At this stage, cancer spreads to the lungs or the inner part of the liver or distant sites.
The 5-year relative survival rate is about 31% [3].
poly (ADP-ribose) polymerase-1 (PARP-1)
An important enzyme called PARP is involved in the repair of DNA as well as many other cellular functions like transcription and chromatin shape modification. The healing of DNA damage brought on by chemotherapeutic and alkylating chemicals is made possible by PARP, which is essential for nucleotide excision repair and base excision repair . The PARP family includes at least 18 members that are encoded by several genes and have similarities in a conserved catalytic site. Although several iso-forms, including PARP1 and PARP2, are well known for their function in the DNA fixing processes. The PARP1 gene in humans produces PARP-1, also known as poly (ADP-ribose) synthase-1 or NAD ADP-ribosyltransferase-1 [4].
Individuals with BRCA1 or BRCA2 mutations are at a high risk of developing cancer. Ovarian and breast cancer are among the most common cancers observed but there is also a high risk of developing other cancers, such as pancreatic and fallopian tubes, which have also been documented. Approximately 40%–50% of the risk is due to the mutation of the BRCA1 mutation carrier in females, and approximately 10–20% are BRCA2 mutation carriers. Autosomal dominance of any genetic transmission is defined as both male and female children of the carrier having a 50% chance of inheriting the mutation. BRCA1 or BRCA2 mutations are sufficient to cause cancer, but loss of a second allele in cancer cells consistently confirms the classic Knudson 2 hit hypothesis for tumor suppressor genes [5].
Patients with BRCA 1/2 mutations in OC display distinctive clinical behavior patterns and react to therapy in various ways. A mutation in the BRCA gene causes a lack of homologous recombination (HR), a process that is used to repair DNA.
Other causes, such as germline mutations, somatic mutations, and epigenetic changes to other genes implicated in the HR pathway, can also cause HRD. The “BRCAness” phenotype refers to the behavior of ovarian tumors with these changes, which resembles that of tumors with BRCA mutations [6].
Inhibiting PARP affects two DNA repair mechanisms in HRD patients, leading to synthetic lethality. Current research has demonstrated that PARP inhibitors are more effective not just in ovarian malignancies with germline or somatic BRCA mutations but also in tumors where HRD is induced by different underlying etiologies [7].
MATERIALS AND METHOD
Protein preparation
PARP1 was the primary target chosen for the OC experiment. The protein data bank was used to obtain a three dimensional (3-D) structure of the protein that is the target for OC (PDB ID: 5DSY).
Dataset preparation
According to their “within the lab” and “within the living organism” studies, the reported anti-OC substances were used. Considering information from the naturally occurring plant-based anti-cancerous compound-activity-target database, 114 compounds were tested against the OC target [8]. There is information about the database’s in vitro and in vivo activity, simplified molecular-input line-entry system and SMiles ARbritrary Target Specification notation, structure, and drug-likeness. The Protein Data Bank was used to download proteins. For the 3-D structure of ligand and energy minimization, ChemDraw and Chem3D were used. Ligands and proteins were prepared using Molecular Graphics Laboratory (MGL) tools. ChemDraw was used to create ligand structures. Under the MMFF94 force field, an energy reduction gradient of 0.000001 kcal/mol/A was applied.
Docking-based virtual screening
AutoDockVina was used for docking tests. The graphic user interface for AutoDockVina is MGL tools 1.5.7. It was opened to view the protein structure in the MGL tools window. The Kollman charges were added, polar hydrogen was added, missing atoms were fixed, and water molecules were removed. Then, the ligands were docked into the constructed enzyme’s binding area. For each molecule, nine alternative conformations were created using the default docking settings. The binding affinities for each conformation were estimated using the AutoDockVina instructions. The output file from AutoDockVina findings was then utilized to visualize interactions using Discovery Studio Visualizer.
Drug-likeness evaluation
The Lipinski Rule was applied to determine the 114 compounds’ drug-likeness properties. Out of which 48 compounds that passed the Lipinski Rule were filtered out. SwissADME (http://www.swissadme.ch/) was utilized for this. The five factors listed by the Lipinski Rule of Five are molecular weight (about 500 Da), average log P (about 4.15), number of rotatable bonds (about 5), hydrogen donating bonds number (about 5), and hydrogen accepting bonds number (about 10). The molar mass should be ≤500 Daltons for oral delivery. Analysis of the total polar surface areas is crucial for figuring out bioavailability.
RESULTS AND DISCUSSION
In-silico docking results
OC is a common cancer that is now the third leading root of cancer-related deaths in females worldwide. Several medications have received United States Food and Drug Administration approval for ovarian carcinoma treatment [9]. Although costly, these treatments can have a wide range of negative effects. Patients typically experience adverse effects such as weariness, headaches, musculoskeletal issues, blood clots, lymphedema, reproductive issues, memory loss, and other symptoms that necessitate the use of alternative medicines derived from plant sources [10]. New phytochemical anticancer medicines can help overcome some of these problems. Several well-known plant-based substances have been identified. Although many are currently undergoing preclinical and clinical research, only a small number of substances and their derivatives have been granted licenses for commercial use. The objective of the current study was to find alternative natural chemicals that can combat OC by targeting PARP1. Chemotherapy for OC is less successful without PARP1 inhibitors than it is with them [11]. For virtual screening, the X-ray-crystallography-based structure of PARP-1 in combination with EB-47 (PDB code: 5DSY) was chosen.
Validation of docking protocol
We thoroughly validated the AutoDockVina docking procedure before conducting the dataset’s virtual screening using docking. Co-crystallized ligand EB-47 was removed and docked back into the active site of 5DSY for this purpose, and root mean square deviation (RMSD) was determined as shown in Table 3 [12].
Virtual screening based on structure
Virtual screening based on the structure of this dataset of compounds was done following the validation of the docking procedure. The ranking of the compounds was done using the binding affinity score (in kcal/mol) [13]. The best compounds for inhibition were those with binding energy values less than −7.5 kcal/mol. The top seven structurally varied scaffolds were chosen for additional examination (Fig. 2). Reissantins-E (1e), 23-Hydroxy-3-beta-[(O- beta-D-glucopyranosyl-(1->4)-alpha-L-arabinopyranosyl) oxy] and 7?-methylagathisflavone (1c) were found to have the best docking conformation with a binding affinity value of −8.5 kcal/mol. Alpha-lapachone (1f), lup-20-[29]-en-28-oic acid (1a), 6-oxo-pristimerol (1b), Ananixanthone (1d), and 1′,2′,3′,4′-tetradehydrotubulosine (1g) are some examples of these compounds (Fig. 1). Table 1 displays computed values for binding affinities. After that, we looked at how the identified seven chemicals bind to the 5DSY binding site. Four functional domains (A–D) make up PARP-1. The main amino acid residues lining the active sites, according to the analysis of the 5DSY, are HIS A:381, ASP A:383, ASN A:459, and ALA A:463. Figure 3 displays the discovered compounds’ two-dimensional binding interaction graphs. Table 1 lists the significant amino acid residues that interact and demonstrate these interactions.
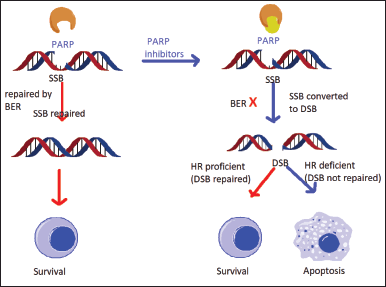 | Figure 1. Mechanism of action of PARP-1. [Click here to view] |
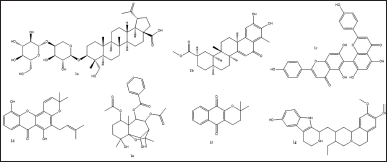 | Figure 2. Structures of top seven structurally different scaffolds from virtual screening of phytochemical compounds. [Click here to view] |
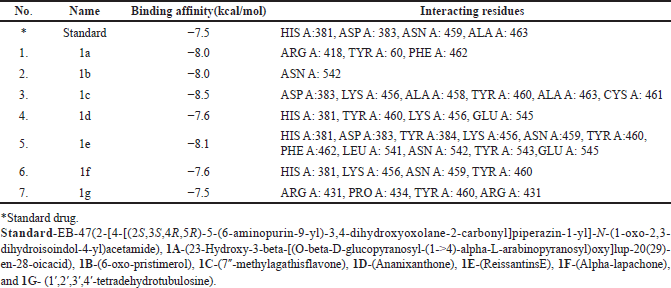 | Table 1. Compounds with their binding affinities and interacting amino-acid residues (AutoDock Vina and Discovery Studio). [Click here to view] |
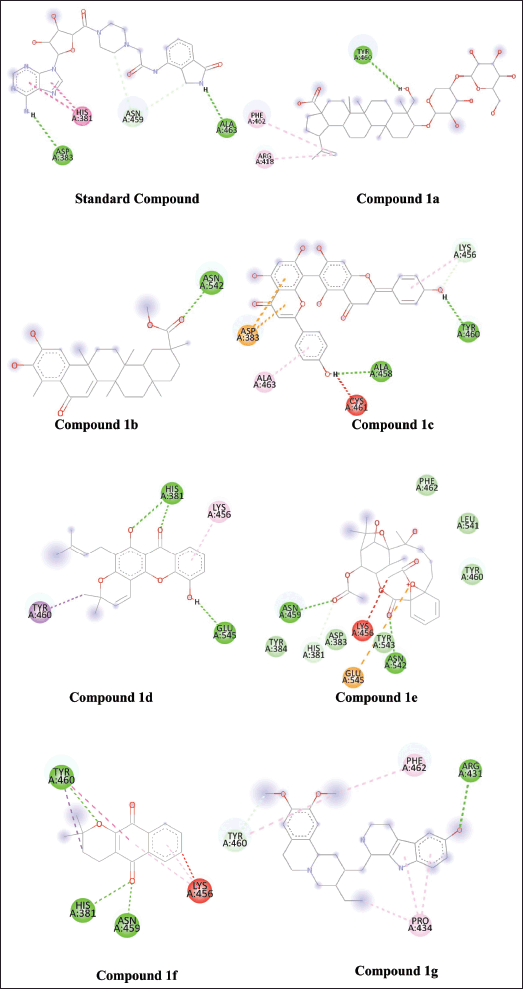 | Figure 3. Intramolecular interaction of standard compound and compounds-1a, 1b, 1c, 1d, 1e, 1f, and 1g with the active site of PARP-1 protein. [Click here to view] |
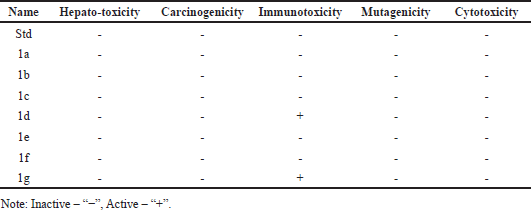 | Table 2. The phytochemical compounds and their toxicities (Protox-II). [Click here to view] |
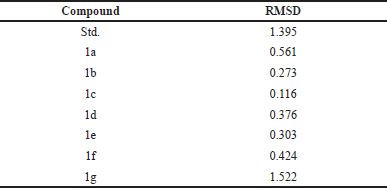 | Table 3. RMSD value of the compounds. [Click here to view] |
In-silico toxicity prediction
We calculated the toxicity of the discovered chemicals using the web server Protox-II. The only chemicals, 1a, 1d, and 1g exhibited the immunotoxicity. Table 2 lists the in-silico toxicity results for the identified chemicals.
CONCLUSION
One of the most prevalent malignancies in women worldwide is OC. Drug development and the identification of multi-targeted inhibitors of many elevated proteins in OC have both made extensive use of computational approaches. Four phytochemical medicines with potent binding affinities against the most prominent target proteins connected to OC are identified by this study. These virtual hits with excellent pharmacokinetics and pharmacodynamics characteristics may be taken into consideration for early therapeutic development against carcinoma of the ovary after “within the lab” and “within the living organism” studies. The seven phytochemicals had the best docking scores, indicating that they are effective anti-ovary cancer substances. These substances are intriguing candidates for more experimental research efforts due to their bioactive potential and possible drug-like characteristics. The discovered compounds demonstrated their potential for use as pharmaceuticals or as useful food additives with a potential role in developing drugs and nutraceuticals. Through in vivo testing, lead optimization, and bioavailability, it was mechanistically demonstrated that the development of new functional ingredients/foods was effective.
AUTHORS CONTRIBUTION
All the authors listed in this article have made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work and drafting the work or revising it critically for important intellectual content and final approval of the version to be published. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJEs) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTERESTS
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Women’s Health. 2019;30:287–99.
2. Pistollato F, Iglesias RC, Ruiz R, Aparicio S, Crespo J, Lopez LD, et al. The use of natural compounds for the targeting and chemoprevention of ovarian cancer. Cancer Lett. 2017;411:191–200.
3. [cited 2022 Sep 25]. Available from: https://ocrahope.org/get-the-facts/staging
4. Zheng F, Zhang Y, Chen S, Weng X, Rao Y, Fang H. Mechanism and current progress of poly ADP-ribose polymerase (PARP) inhibitors in the treatment of ovarian cancer. Biomed Pharmacother. 2020;123:109661.
5. Drew Y, Calvert H. The potential of PARP inhibitors in genetic breast and ovarian cancers. Ann N Y Acad Sci. 2008;1138(1):136–45.
6. Ko HL, Ren EC. Functional aspects of PARP1 in DNA repair and transcription. Biomolecules. 2012;2(4):524–48.
7. da Cunha Colombo Bonadio RR, Fogace RN, Miranda VC, Diz MD. Homologous recombination deficiency in ovarian cancer: a review of its epidemiology and management. Clinics. 2018;20:73.
8. Mangal M, Sagar P, Singh H, Raghava GP, Agarwal SM. NPACT: naturally occurring plant-based anti-cancer compound-activity-target database. Nucleic Acids Res. 2013;41(D1):D1124–9.
9. Berek JS, Renz M, Kehoe S, Kumar L, Friedlander M. Cancer of the vary, fallopian tube, and peritoneum: 2021 update. Int J Gynaecol Obstet. 2021;155:61–85.1
10. Gomide LB, Matheus JP, Candido dos Reis FJ. Morbidity after breast cancer treatment and physiotherapeutic performance. Int J Clin Pract. 2007;61(6):972–82.
11. Cragg GM, Pezzuto JM. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med Princ Pract. 2016;25(2):41–59.
12. Cosconati S, Forli S, Perryman AL, Harris R, Goodsell DS, Olson AJ. Virtual screening with AutoDock: theory and practice. Expert Opin Drug Discov. 2010;5(6):597–607.
13. Hevener KE, Zhao W, Ball DM, Babaoglu K, Qi J, White SW, et al. Validation of molecular docking programs for virtual screening against dihydropteroate synthase. J Chem Inf Model. 2009;49(2):444–60.