INTRODUCTION
As per WHO, cancer is a large cluster of diseases where the abnormal cells of any organ or tissue grow extensively beyond their growth boundaries and invade the neighboring parts of the organs and tissues in the body (WHO, 2020). There are over 100 different types of cancer (Cooper, 2000). The treatment options available for cancer treatment includes radiation therapy, chemotherapy, immunotherapy, surgery, or stem cell transplant. Despite the adverse effects, chemotherapy is one of the most effective approaches (Huang et al., 2017). Due to their increased toxicity profiles, chemotherapy, and particularly chemotherapeutic agents, are known to produce either acute or chronic organ toxicities at varying levels (Chambers, 1987; Chan and Ismail, 2014; Nurgali et al., 2018).
Cyclophosphamide (CYP), also known as cytophosphane, is an oxazaphosphorine agent used in anticancer chemotherapy and as an immunosuppressive agent (Anderson et al., 1995; Mills et al., 2019). CYP is a type of nitrogen mustard drug, it metabolizes to an active form capable of inhibiting protein synthesis through DNA and RNA crosslinking (Colvin, 1999). Most of the antineoplastic effects of CYP are due to the phosphoramide mustard formed from the metabolism of the drug by liver enzymes such as cytochrome P-450 which converts CYP to hydroxy CYP and then subsequently metabolizes to aldophosphamide. It cleaved to the active alkylating agent phosphoramide mustard and acrolein. The phosphoramide metabolite forms cross-linkages within and between adjacent DNA strands at the guanine N-7 position and eventually leads to programmed cell death. Acrolein is a toxic metabolite that is responsible for organ toxicity by CYP (International Agency for Research on Cancer, 2012; Moghe et al., 2015).
Cardiotoxicity, reproductive toxicity, gastrointestinal toxicity, and hematotoxicity are the primary chronic CYP-induced toxicities. Uncertainty exists regarding the processes by which CYP therapy and associated significant malignancy led to organ damage (Poorvu et al., 2019). However, the proposed mechanisms by which toxicities are induced include inducing oxidative stress in the mitochondria that leads to DNA damage and peroxidation of membrane lipids, critical thiol functional groups of proteins that cause apoptotic changes and inflammation, as well as reduce the enzymes involved in the citric acid cycle (tricarboxylic acid), which disrupts cellular respiration and adenosine triphosphate production (Ghobadi et al., 2017). Therefore, it is of great importance to reduce toxicities with supplementary treatments that enhance antioxidant defense systems (Uyar et al., 2018).
Argyreia speciosa (AS) is a woody climber belonging to the Convolvulaceae family. The common name of AS includes Hawaiian Baby Woodrose and Elephant Creeper, and it is found throughout India (Galani et al., 2010). It is well documented for its various effects from antioxidant, hepatoprotective, immunomodulatory, and anti-inflammatory to antimicrobial, analgesic, aphrodisiac, and central nervous system depressant properties (Habbu et al., 2008). The extract from roots of AS contains 5,8-oxidotetracosan-10-one and tetradecanyl palmitate (Rani and Shukla, 1997), two aryl esters-hexadecanyl p-hydroxycinnamate and stigmasteryl p-hydroxycinnamate (Srivastava and Shukla, 1998), and coumarin scopoletin was also isolated from the roots along with several esters of fatty acids. Other isolated molecules from the root include coumarin glycosides called L-ester coumarin and 6-methoxy-7-o-alpha-D-glu (Chhavi et al., 2017; Galani et al., 2010; Joseph et al., 2011) and ergoline type of alkaloids and quercetin-type of flavonoids. A specific ergoline alkaloid N-methyl ergometrine was isolated from the alkaloidal fraction of the root of AS (Vyas et al., 2019). The present study is designed to investigate the protective effects of AS root extract against CYP-induced organ toxicities: cardiotoxicity, gonadal toxicity, gastrointestinal toxicity, and haematotoxicity. Amifostine was chosen as a standard protective agent for reference. The efficacy of AS root extract was evaluated using different biochemical assays, electrocardiogram measurements, and histopathology analysis.
MATERIALS AND METHODS
Chemicals
CYP (Cycloxan- Zydus Celexa), amifostine, methanol, toluene (Merck Lifescience Pvt. Ltd.), cardiac kinase—MB (CK-MB) kit, lactate dehydrogenase (LDH) kit (Arkray Healthcare Pvt. Ltd.), thiobarbituric acid, sodium dodecyl sulfate, acetic acid, potassium chloride, butanol, pyridine (HiMedia Laboratories Pvt. Ltd.), tetramethoxypropane, ethylenediamine tetra acetic acid (EDTA), phosphate buffer, hydrogen peroxide, Tris, Triton- X 100, and nitro blue tetrazolium, pyrogallol (TCI Chemicals Pvt. Ltd.)
Experimental animals
Male albino Wistar rats of age 8–10 weeks and weighing 150–200 g used in the present studies were procured from the animal house of the Sun Pharma Advanced Research Centre, Vadodara. All the animals were kept under the standard environment condition at 23°C ± 2°C (under well-ventilated room conditions with 12 hours light/12 hours dark cycle) and were allowed free access to a standard diet and clean drinking water. The rats were adapted to laboratory conditions for 7 days prior to experiments. The studies were performed with the approval of the Institutional Animal Ethics Committee (IAEC) at Ramanbhai Patel College of Pharmacy, CHARUSAT (RPCP/IAEC/2020-21/R132).
Experimental protocol
The animals were divided into five groups. Group 1: Normal animals (6 rats) were considered as a control group. Group 2: CYP at a dose of 100 mg/kg was administered subcutaneously to 10 animals for 2 days. Group 3: CYP at a dose of 100 mg/kg was administered subcutaneously followed by amifostine at a dose of 200 mg/kg intraperitoneally to 8 animals for 14 days. Group 4: CYP at a dose of 100 mg/kg was administered subcutaneously followed by a low dose of AS root extract at a dose of 100 mg/kg orally to 8 animals for 14 days. Group 5: CYP at a dose of 100 mg/kg was administered subcutaneously followed by a high dose of AS root extract at a dose of 300 mg/kg orally to 8 animals for 14 days. A sixth group was included to standardize the CYP-induced toxicity model. The study lasted for 14 days, and on the 15th day, all the animals were euthanized by an overdose of ketamine (100 mg/kg) (CPCSEA, 2018; Stankiewicz and Skrzydlewska, 2005; Swamy et al., 2013).
Collection of plant materials
AS root powder was collected from a local herbal shop in Nadiad, Gujarat. The plant powder was identified in the Department of Pharmacognosy, RPCP.
Preparation of crude extract
Three different extracts of root powder of AS were prepared for the experimental purpose. The methanolic extract and aqueous extract were prepared by maceration method (for 4 days) and the toluene extract was prepared by using Soxhlet apparatus. From the results of preliminary phytochemical tests, it was observed that methanolic extract contains alkaloids, aqueous extract contains carbohydrates and toluene extract contains steroids and triterpenoids (Vyas et al., 2019).
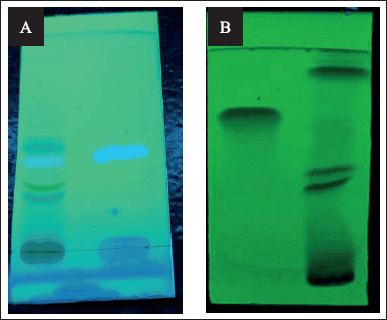 | Figure 1. TLC profile of active phytoconstituents; (A) Scopoletin at 366 nm, (B) Quercetin at 254 nm. [Click here to view] |
Thin layer chromatography (TLC)
For confirmation of Quercetin and Scopoletin, TLC was performed. ALUGRAMR Xtra SIL G/UV254 TLC-sheets were used for the stationary phase and the mobile phase used was Toluene: Ethyl acetate: Glacial acetic acid (5:4:0.5%V/V) for the quercetin and Toluene: Ethyl acetate: Formic acid (7:3:1%V/V) for scopoletin. With compared to standards, quercetin was identified at a short ultraviolet (UV) range (254 nm) and scopoletin was observed at a long UV range (366 nm) (Vyas et al., 2019).
Dose preparation
Methanol extract and Toluene extract each were dissolved in 3 ml of distilled water using Tween-80 as a dissolution aid. Water soluble extract was dissolved in 4 ml of distilled water. All the extracts were combined and stirred to form a dose with a final volume of 10 ml.
Electrocardiographic (ECG) studies
Cardiac recordings were made using a digital electrocardiograph (BIOPAC MP-36). The changes in heart rate and ST segment variations were observed and evaluated (Maheshkumar et al., 2016).
Blood collection and parameter estimation
After 24 hours of the last dose, all rats were anesthetized under ketamine (75 mg/kg) and xylazine (10 mg/kg) anesthesia intraperitoneally and blood was collected by the retro-orbital puncture. For the estimation of hematological parameters, the blood was given to CHARUSAT Hospital, Changa. The total blood count was estimated by the Mindray BC-5130 analyzer. The serum was separated through centrifugation at 5,000 rpm for 9 minutes and used for the estimation of marker enzymes (Ohaeri, 2001).
Determination of cardiac markers
The serum that was collected was analyzed for the activities of LDH and CK-MB. The estimation was done using commercial kits obtained from Arkray Healthcare Pvt. Ltd.
Sample collection of sperm
The animals were euthanized and the testis were exposed via a midline scrotal incision. The cauda epididymis was then identified and isolated carefully. The cauda epididymis was scraped in a petri dish with 10% phosphate buffer solution and left for around 10 minutes. The resulting milky suspension was filtered and used for further analysis (Srinivasulu and Changamma, 2017).
Sperm count
The filtered milky suspension of sperm was mixed with 1% aqueous Eosin Y in a ratio of 10:1 and kept aside for 15 minutes for the staining of sperm. Sperm count was performed in a white blood cell (WBC) counting chamber at 40× in the light microscope. The average number of sperm per chamber was calculated according to the number of counted cells and hemocytometer dimensions (Afsar et al., 2017).
Preparation of tissue homogenate—heart and testis
The animals were euthanized using an overdose of Ketamine. The same procedure was carried out for both the heart and testis. The animals were dissected, and the organs were isolated from an animal from each group. Portions of the organ’s tissue were cut and homogenized in a 10% phosphate buffer solution with pH 7.4. The homogenate was centrifuged at 4,000× g for 18 minutes. The supernatant was collected in an Eppendorf and was used for further analysis (Ali et al., 2020).
Oxidative stress estimation
Malondialdehyde (MDA)
0.2 ml of 8.1% sodium dodecyl sulfate, 1.5 ml of 20% acetic acid, and 1.5 ml of 0.81% thiobarbituric acid were added in succession. To this mixture, 0.2 ml of tissue homogenate was added. Then, the prepared mixture was heated over a water bath for 60 minutes and was then allowed to cool. After cooling to room temperature, 5 ml of butanol: pyridine(15:1 v/v) solution was added immediately. It was then kept in a centrifuge for 15 minutes at a speed of 5,000 rpm. On centrifugation, the organic layer that was formed above was separated and the intensity of the resulting pink color was read at 532 nm. In this method, tetramethoxypropane was used as an external standard and the lipid peroxide is expressed as nmol of MDA formed in µmol/g wet weight for lenses (Ohkawa et al., 1979).
Catalase (CAT)
1 ml of supernatant was added to the cuvette containing 1.9 ml of 50 mM phosphate buffer with a pH of 7.0. This reaction was initiated by the addition of 1.0 ml of freshly prepared 30 mM H2O2. The rate of degradation of H2O2 was assessed spectrophotometrically by recording the changes in absorbance at 240 nm. The activity of CAT is generally expressed as units/mg protein (Hugo and Lester, 1984).
Superoxide dismutase
0.25 ml of supernatant was added to the reaction mixture containing 0.5 ml tris EDTA buffer, 0.1 ml of Triton-X 100, and 0.1 ml of nitro blue tetrazolium, and the reaction was initiated by adding pyrogallol. The changes were recorded in absorbance spectrophotometrically at 546 nm every 30 seconds for about 40 minutes. The activity of superoxide dismutase (SOD) is generally expressed in units/mg protein (Masayasu and Hiroshi, 1979).
Preparation of tissue homogenate— seminal vesicles
After dissection, seminal vesicles were isolated from animals of each group and homogenized in distilled water. Homogenate was centrifuged at 4,000× g for 18 minutes. The supernatant was collected in an Eppendorf and was used for fructose content estimation (Ali et al., 2020).
Estimation of fructose content in seminal vesicles
The supernatant fluid collected after homogenization and centrifugation of seminal vesicles was taken in a test tube. To 1 ml of supernatant fluid, 0.5 ml of resorcinol and 1.5 ml of HCL were added. The resulting mixture was heated at 80°C for 12 minutes in a water bath. The resorcinol in the mixture reacts with fructose to form a rosy color, which was measured at 500 nm using a spectrophotometer (Chauhan et al., 2011).
Histopathological examination
After the last treatment, the animals were euthanized using an overdose of Ketamine. The animals were dissected, and organ samples (heart, testis, tongue, duodenum, and colon) were collected. The separated organs were washed in saline solution before being kept in a 40% of formalin solution. The samples were subsequently sent to the Vadodara Clinical Laboratory for pathological analysis (Maynard and Downes, 2019).
Statistical analysis
Data is stated as mean ± SEM, for various parameters that were carried out. Statistical comparison between different groups was carried out using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests. The values in the table and graph are expressed as mean ± SEM of 6 animals. A p-value of 0.05 or less was taken as a criterion for a statistically significant difference with a confidence level of 95%. Statistical analysis was carried out using GraphPad Prism 8.4.3 software.
RESULTS AND DISCUSSION
CYP is a potent anticancer, immunosuppressive, and immunomodulatory chemotherapeutic drug, which is classified as an alkylating agent. The anticancer effect of CYP is dose-dependent, and higher doses of CYP lead to the induction of various toxicities like cardiotoxicity, testicular toxicity, gastrointestinal toxicity, and haematotoxicity (Taniguchi, 2005). Plant sources have always been a major source of free radical quenching active principles and these plant-based therapies are a part of our vast repository of traditional medicines (Henkel et al., 2005). AS is a native Indian medicinal plant with a variety of uses in traditional medicines out of which its antioxidant, anti-inflammatory, and spermatogenic properties are highly appreciated. Therefore, the main objective of this study was to investigate the effect of AS plant extract against CYP-induced testicular toxicity, cardiotoxicity, gastrointestinal toxicity, and haematotoxicity.
Preliminary phytochemical tests
For preliminary phytochemical screening, various tests were performed. Dragendroff’s test was performed for alkaloids, Molisch’s test was performed for carbohydrates, for flavonoids, Shinoda’s test was performed, Froth formation test was performed for saponins, Libermann-Burchard test was performed for steroids, Ferric chloride test was performed for tannin and protein Biuret test was performed (Kokate et al., 2021). Based on the results obtained from preliminary tests, it was found that in the plant extract alkaloids, steroids, carbohydrates, and flavonoids were present while saponin, tannin, and protein were absent.
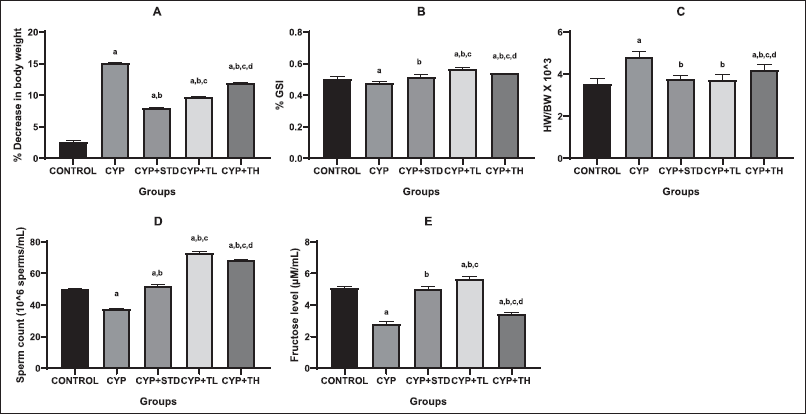 | Figure 2. Effect of treatments on various physiological parameters. (A): % Decrease in BW, (B): % GSI, (C): heart weight/BW ratio, (D): Sperm count, (E): Fructose level. All values are expressed as mean ± SEM of six animals. a, p < 0.05 versus control; b, p < 0.05 versus CYP treated; c, p < 0.05 versus CYP + Amifostine treated; d, p < 0.05 versus CYP + Test low dose treated; e, p < 0.05 versus CYP + high dose treated. One-way ANOVA followed by Tukey’s multiple comparison tests using GraphPad Prism software. [Click here to view] |
Thin layer chromatography
After placing standard and test marker at the respective places, the existence of a specific phytoconstituent was established by ascending of mobile phase at to maximum height and comparing of Retention factor (Fig. 1).
Total blood count
The study results of hematological parameters show that both doses of the test drug were not able to improve blood counts which were disturbed due to the toxicity of CYP (Table 1). The reason behind this can be that the test drug protects vital organ tissue cells against CYP-induced toxicities but are unable to protect blood cells.
Physiological parameters
Toxicological studies greatly rely upon the weight of animals and specific organs, and they constitute an essential benchmark for the studies (Desai et al., 2016). AS root extract administration prevented weight reduction in rats up to an extent as compared to the CYP-treated group (Fig. 2). The retardation in the body weight (BW) of rats can be attributed to various factors that include anorectic properties of the CYP (Merwid-L?d et al., 2015) or the toxicities induced by CYP which includes diarrhea, gastro-intestinal ulceration, or cell growth disruption.
Primarily, the effect of AS against testicular toxicity was observed and evaluated using spermatogenic and biochemical parameters along with histopathological changes. Several effects of chemotherapeutic drugs on testicular damage have been established (Uyar et al., 2018). From the observations, it is observed that AS is found to decrease lipid peroxidation levels along with the reduction in the MDA content and thereby, increasing the antioxidant capacity. Reduction in testicular weight may be due to the decreased number of germ cells and elongated spermatids (Katoh et al., 2002). Results of gonadosomatic index (GSI) revealed that a low dose of AS root extract had high GSI as compared to the CYP + amifostine treated group and CYP + high dose treated group of the test drug.
Semen analysis plays a foremost role in the evaluation of male factor infertility. The epididymal sperm concentration in CYP-treated rats was less as compared to the control rats. The reduced sperm count clearly demonstrates the removal of sperm cells at various stages of development and points to the harmful reactive oxygen species (ROS) produced by CYP metabolism. Furthermore, at some stages of the germinal cycle, CYP triggers an increase in apoptosis (Cai et al., 1997). The increase in sperm concentration on root extract administration can be presumed to be because of an increase in the concentration of hormones that affect metabolism and steroid secretion in testis (McLachlan et al., 2002).
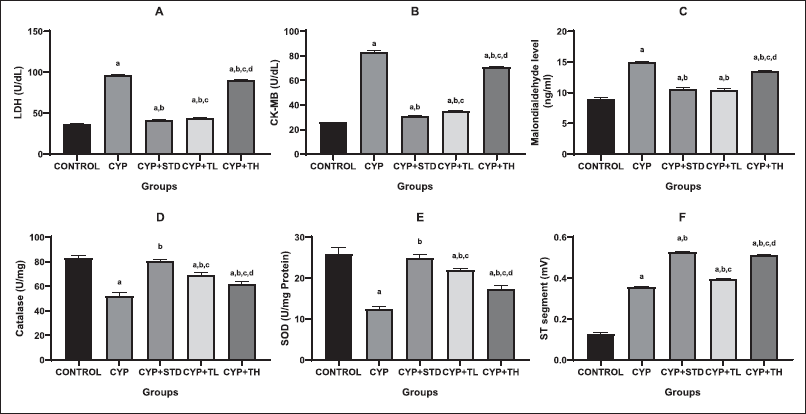 | Figure 3. Effect of treatments on oxidative stress and ECG parameters. (A): LDH level, (B): CK-MB level, (C): MDA level, (D): Catalase, (E): SOD, (F): ST segment. All values are expressed as mean ± SEM of six animals. a, p < 0.05 versus control; b, p < 0.05 versus CYP treated; c, p < 0.05 versus CYP + Amifostine treated; d, p < 0.05 versus CYP + Test low dose treated; e, p < 0.05 versus CYP + high dose treated. One-way ANOVA followed by Tukey’s multiple comparison tests using GraphPad Prism software. [Click here to view] |
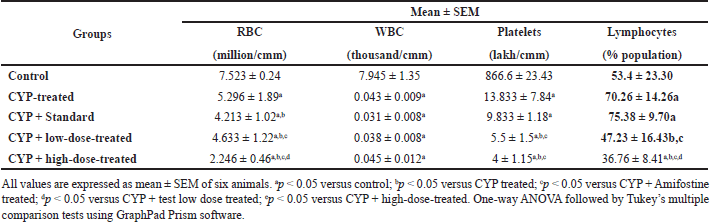 | Table 1. Total blood count parameters. [Click here to view] |
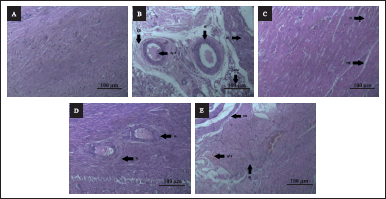 | Figure 4. A–F: Effect of AS on histology of heart. (A): Hematoxylin and Eosin (H & E) stained microscopic section (100 µm) of control rat’s heart shows normal cardiac muscle bundle. (B): H & E stained microscopic section (100 µm) of CYP treated (100 mg/kg) rat’s heart shows extensive cardiac injuries with necrotic damage. (C): H & E stained microscopic section (100 µm) of CYP (100 mg/kg) + amifostine (200 mg/kg) group rat’s heart shows necrosis and interstitial edema in minimal areas along with contraction band necrosis in myocardial fibers. (D): (H & E) stained microscopic section (100 µm) of CYP (100 mg/kg) + low dose (100 mg/kg) treated group rat’s heart shows mild necrosis and interstitial edema. (E): (H & E) stained microscopic section (100 µm) of CYP (100 mg/kg) + high dose (300 mg/kg) treated group rat’s heart shows reactive swollen viable myocardium, intravascular thrombus, contraction band necrosis; N-necrosis, CB-contraction band necrosis, IV-T-intravascular thrombus, SVM-swollen viable myocardium, and IE-interstitial edema. [Click here to view] |
ECG studies
CYP was observed to induce changes in ECG patterns such as a decrease in the heart rate, P wave inversion, prolongation of QT interval, PR interval, ST interval difference, and decrease in R wave amplitude (Fig. 3). The CYP + high dose treated group of test drug showed changes identical to that of CYP + amifostine treated group. The CYP + low dose treated group of the test drug would not completely normalize the ST segment but was found to reduce the amplitude of ST segment.
Cardiac markers estimation and oxidative stress estimation
Cardiotoxicity caused by CYP is due to impaired mitochondrial metabolism (Swamy et al., 2013). Cardiotoxicity caused by the generation of free radicals (Gunes et al., 2017) causes significant damage to a cell’s membrane integrity accompanied by endothelial and vascular damage to the myocardium. According to some studies, the estimation of cardiac-specific biomarkers such as CK-MB, LDH, troponin, and myoglobin has been observed to evaluate the cardiac injury in a more precise manner (Benstoem et al., 2015; Gunes et al., 2017). The data demonstrated here shows that CYP causes an increase in the levels of enzymes like LDH, CK-MB, and MDA, which are associated with certain types of cardiac damage. On one hand, CYP-induced cardiotoxicity successfully elevates the biomarker enzyme levels but on the other hand, AS was not able to stop the rupture and extra leakage but helped in reducing the levels of lipid peroxidation, LDH and CK-MB. So, this confirms that AS cannot restrict the leakage of biochemical markers, but it was observed that the rupture and leakage were controlled up to an extent in low doses due to its antioxidant and membrane stabilizing properties. In the CYP + low dose treated group of the test drug, inflammation was controlled while in the CYP-treated group, inflammation was observed. As a result of changes in antioxidant parameters, it was demonstrated that CYP increased lipid peroxidation in rat blood (Alhumaidha et al., 2016). For oxidative stress estimation, a fall in CAT and superoxide dismutase levels was seen but the low dose of AS displayed a control in the fall of oxidative stress level which shows the antioxidant properties of AS.
Histopathological studies
Heart
The tissue in the control group appears normal and stable. The CYP + amifostine-treated group shows necrosis and interstitial edema in minimal areas along with contraction band necrosis in myocardial fibers. The CYP-treated group had extensive cardiac injuries with almost 80% necrotic damage such as inflammation, necrosis, interstitial edema, reactive swollen viable myocardium, intravascular thrombus along with interstitial fibrin deposition and contraction band necrosis in myocardial fibers. The CYP + low-dose-treated group has some necrosis of just around 5%–10% with interstitial edema. The CYP + high dose treated group showed 25%–30% of necrotic damage. The section of the heart was observed to have reactive swollen viable myocardium, intravascular thrombus, contraction band necrosis, and necrosis of 20%–25%. Some areas of the heart were observed to have inflammation even on treatment. Also, the CYP + low dose treated group of test drugs showed changes identical to that of the CYP + amifostine treated group. Therefore, it can be inferred that AS root extract shows anti-inflammatory and cardioprotective effects at low doses and it is able to reduce cardiac inflammation as well as resist extensive cardiac damage (Fig. 4).
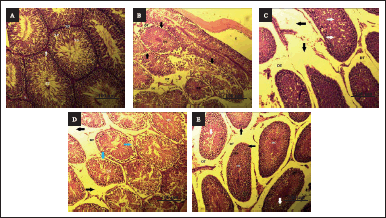 | Figure 5. A–F: Effect of AS on histology of testis. (A): H & E stained microscopic section (100 µm) of control rat’s testis shows a normal structure of testis. (B): H & E stained microscopic section (100 µm) of CYP treated (100 mg/kg) rat’s testis shows interstitial edema, detachment of the spermatogonial cells, high vacuolization in the spermatogenic cells, and desquamation can be seen in the lumen of the spermatogenic cells. (C): H & E stained microscopic section (100 µm) of CYP (100 mg/kg) + Amifostine (200 mg/kg) group rat’s testis shows intertubular edema, detachment of the spermatogonial cells and mild vacuolization in the spermatogenic cells. (D): (H & E) stained microscopic section (100 µm) of CYP (100 mg/kg) + low dose (100 mg/kg) treated group rat’s testis shows interstitial edema, detachment of the spermatogonial cells and vacuolization in the spermatogenic cells and desquamation in the lumen of the spermatogenic cells. (E): (H & E) stained microscopic section (100 µm) of CYP (100 mg/kg) + high dose (300 mg/kg) treated group rat’s testis shows detachment of the spermatogonial cells and vacuolization in the spermatogenic cells; SZ-spermatozoa, SG-spermatogonia, SP-primary spermatocytes, SD-spermatids, L-interstitial cells of Leydig, DT-detachment, E-edema, and DS-desquamation. [Click here to view] |
Testis
In the control group, no histopathological changes were observed. The CYP + amifostine-treated group showed intertubular edema, detachment of the spermatogonial cells, and mild vacuolization in the spermatogenic cells. Testis of the CYP-treated group shows interstitial edema, detachment of the spermatogonial cells, high vacuolization in the spermatogenic cells, and desquamation can be seen in the lumen of the spermatogenic cells. The CYP + low dose treated group of the test drug displayed interstitial edema, detachment of the spermatogonial cells and vacuolization in the spermatogenic cells and desquamation in the lumen of the spermatogenic cells. The CYP + high dose treated group of the test drug showed detachment of the spermatogonial cells and vacuolization in the spermatogenic cells. Desquamation can be seen in the lumen of the spermatogenic cells, immature germ cell proliferation, interstitial edema as well as detachment of the spermatogenic cells with desquamated cells in the lumen and vacuolization of the spermatogonial cells are observed. It can, therefore, be deduced that AS root extract in low dose is able to resist histological changes induced by CYP. However, in CYP + high-dose-treated group, it is not as effective to counteract the histological distortions (Fig. 5).
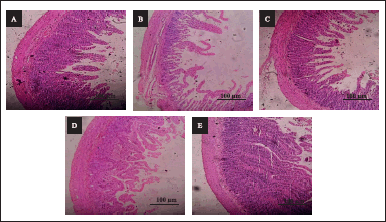 | Figure 6. A–F: Effect of AS on histology of duodenum. (A): H & E stained microscopic section (100 µm) of control rat’s duodenum shows a normal architecture of villi and epithelial cells of the intestinal mucosa. (B): H & E stained microscopic section (100 µm) of CYP treated (100 mg/kg) rat’s duodenum shows shortening of villi and damage at the mucosal level. (C): H & E stained microscopic section (100 µm) of CYP (100 mg/kg) + Amifostine (200 mg/kg) group rat’s duodenum shows mild lamina inflammation. (D): (H & E) stained microscopic section (100 µm) of CYP (100 mg/kg) + low dose (100 mg/kg) treated group rat’s duodenum shows improvement in villi and mucosa structure and mild focal mucositis. (E): (H & E) stained microscopic section (100 µm) of CYP (100 mg/kg) + high dose (300 mg/kg) treated group rat’s duodenum shows a large number of inflammatory cells infiltration in villi along with crypts deformation. [Click here to view] |
Duodenum
The effect of AS against gastrointestinal toxicity was evaluated as it is a major obstacle to the absorption of nutrition from food. There was no indication of histological impairment found in the control for tongue, duodenum, and colon. For the CYP-treated group, severe keratosis on the mucosal layer of the tongue, major destruction in the duodenum which includes extensive damage in lamina propria and cryptitis, and colon, major destruction in the mucosal and submucosal layer of tissue which include damage to the lamina propria and decreased goblet cells was observed. In the CYP + amifostine-treated group, mild keratosis on the tongue, mild lamina inflammation in the duodenum and mild mucositis in the colon was observed. In the CYP + low-dose-treated group of the test drug, keratosis only on focal areas of the tongue, mild focal mucositis in the duodenum and the tissue of the colon were similar to the control group (Fig. 6). In the CYP + high-dose-treated group of the test drug, moderate keratosis in the tongue, extensive lamina propria damage along with crypts and crypts deformation in the duodenum and extensive mucositis along with ulceration was seen in the colon.
Despite CYP being known to produce diarrhea, there are relatively very few research studies available to understand the underlying process (Siber et al., 1980). However, few experts claim that CYP is the causal agent for the mitotic arrest of intestinal crypt cells, which reduces the relative percentage of villous enterocytes and decrease the surface area available for resorption (Cao et al., 1998). The results clearly suggest that a low dose of AS shows a great effect on diarrhea.
However, the exact mechanism of AS is unclear, it is proposed that the presence of flavonoids and triterpenes possesses an anti-inflammatory effect, because of which AS tends to reduce ROS levels and inflammation which leads to healing in a low dose of AS root extract (Bachhav et al., 2009). For the high dose of AS root extract, we did not observe any sign of toxicities at the high dose (300 mg/kg) of the test drug in normal rats (test drug alone, without induction of CYP toxicities). So, it seems that the combination of CYP with a high dose of the test drug aggravates the CYP-induced toxicity. It may be due to the excessive metabolic burden on vital organs like the liver, lungs, and kidneys and high oxidative stress.
CONCLUSION
In conclusion, our study results showed that AS root extract prevented the development of cardiotoxicity, testicular toxicity, and gastrointestinal toxicity of CYP and alleviated the damage occurring in the organs due to its antioxidant, anti-inflammatory, and cytoprotective effects. However, AS was found ineffective in the prevention of haematotoxicity. The conclusion is supported by the histopathological and biochemical findings. Based on these results, we concluded that the AS root extract can alleviate CYP-induced toxicities but among the two doses low dose (100 mg/kg) is proven to be more effective.
ACKNOWLEDGMENTS
The authors are thankful to the Ramanbhai Patel College of Pharmacy for providing the necessary facilities for conducting the study.
LIST OF ABBREVIATIONS
AS, Argyreia speciosa; BW, Body weight; CAT, Catalase; CK-MB, Creatine kinase-MB; CYP, Cyclophosphamide; ECG, Electrocardiograph; EDTA, Ethylenediamine tetra acetic acid; GSI, Gonadosomatic index; LDH; Lactate dehydrogenase; MDA, Malondialdehyde; SOD, Superoxide dismutase; ROS, Reactive oxygen species; UV, Ultraviolet; WBC, White blood cell.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST
The authors declare no conflict of interest
ETHICAL APPROVAL
Necessary approval and ethical clearances were taken from the Institutional Ethical Committee Ramanbhai Patel College of Pharmacy, CHARUSAT (RPCP/IAEC/2020-21/R132), on the use and care of experimental animals before conducting any of the experiments. All experiments performed on animals complied with the Guide for the Care and Use of Laboratory Animals and CPCSEA guidelines.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral regarding jurisdictional claims in published institutional affiliation.
REFERENCES
Afsar T, Razak S, Almajwal A. Acacia hydaspica ethyl acetate extract protects against cisplatin-induced DNA damage, oxidative stress and testicular injuries in adult male rats. BMC Cancer, 2017; 17:1–14.
Alhumaidha KA, Saleh DO, Abd El Fattah MA, El-Eraky WI, Moawad H. Cardiorenal protective effect of taurine against cyclophosphamide-induced toxicity in albino rats. Can J Physiol Pharmacol, 2016; 94:131–9.
Ali BH, Al-Salam S, Al Balushi KA, Al Za’abi M, Adham SA, Beegam S, Yuvaraju P, Manoj P, Nemmar A. Ameliorative effect of gum acacia on hookah smoke-induced testicular impairment in mice. Biomolecules, 2020; 10:762.
Anderson D, Bishop JB, Garner RC, Ostrosky-Wegman P, Selby PB. Cyclophosphamide: review of its mutagenicity for an assessment of potential germ cell risks. Mutat Res Fund Mol Mech Mutagen, 1995; 330:115–81.
Bachhav RS, Gulecha VS, Upasani C. Analgesic and anti-inflammatory activity of Argyreia speciosa root. Indian J Pharmacol, 2009; 41:158.
Benstoem C, Goetzenich A, Kraemer S, Borosch S, Manzanares W, Hardy G, Stoppe C. Selenium and its supplementation in cardiovascular disease—what do we know? Nutrients, 2015; 7:3094–118.
Cai L, Hales BF, Robaire B. Induction of apoptosis in the germ cells of adult male rats after exposure to cyclophosphamide. Biol Reprod, 1997; 56:1490–7.
Cao S, Troutt AB, Rustum YM. Interleukin 15 protects against toxicity and potentiates antitumor activity of 5-fluorouracil alone and in combination with leucovorin in rats bearing colorectal cancer. Cancer Res, 1998; 58:1695–9.
Chambers FL. Organ toxicity: a textbook of modern toxicology. In: Hodgson E, Levi PE (eds.). Elsevier, Amsterdam, The Netherlands, 1987.
Chan HK, Ismail S. Side effects of chemotherapy among cancer patients in a Malaysian General Hospital: experiences, perceptions and informational needs from clinical pharmacists. Asian Pac J Cancer Prev, 2014; 15:5305–9.
Chauhan NS, Sharma V, Dixit VK. Effect of Asteracantha longifolia seeds on the sexual behaviour of male rats. Nat Prod Res, 2011; 25:1423–31.
Chhavi Y, Suresh C, Tejbeer S. Review on Argyreia speciosa (l. F.) Sweet.(vrdhhadaru): plant of Indian medical lexicons. Int J Ayu Pharma Res, 2017; 5 :66–72.
Colvin OM. An overview of cyclophosphamide development and clinical applications. Curr Pharm Des, 1999; 5:555–60.
Cooper GM. The cell: a molecular approach. 2nd edition, Sinauer Associates, Sunderland, MA, 2000.
CPCSEA. Compendium of CPCSEA 2018. Committee for the purpose of control and supervision of experiments on animals , Government of India, New Delhi, India, 2018.
Desai KR, Moid N, Patel PB, Highland HN. Evaluation of deltamethrin induced reproductive toxicity in male Swiss albino mice. Asian Pac J Reprod, 2016; 5:24–30.
Galani VJ, Patel BG, Patel NB. Argyreia speciosa (Linn. f.) sweet: a comprehensive review. Pharmacogn Rev, 2010; 4:172.
Ghobadi E, Moloudizargari M, Asghari MH, Abdollahi M. The mechanisms of cyclophosphamide-induced testicular toxicity and the protective agents. Expert Opin Drug Metab Toxicol, 2017; 13:525–36.
Gunes S, Sahinturk V, Karasati P, Sahin IK, Ayhanci A. Cardioprotective effect of selenium against cyclophosphamide-induced cardiotoxicity in rats. Biol Trace Elem Res, 2017; 177:107–14.
Habbu PV, Shastry RA, Mahadevan KM, Joshi H, Das SK. Hepatoprotective and antioxidant effects of Argyreia speciosa in rats. Afr J Tradit Complement Altern Med, 2008; 5:158–64.
Henkel R, Maaß G, Bödeker R, Scheibelhut C, Stalf T, Mehnert C, Schuppe HC, Jung A, Schill WB. Sperm function and assisted reproduction technology. Reprod Med Biol, 2005; 4:7–30.
Huang CY, Ju DT, Chang CF, Reddy PM, Velmurugan BK. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. Biomedicine (Taipei), 2017;7:23.
Hugo A, Lester P. Catalase in vitro. Methods Enzymol, 1984; 105:121–6.
International Agency for Research on Cancer. IARC Working Group on the evaluation of carcinogenic risks to humans. Pharmaceuticals. (IARC monographs on the evaluation of carcinogenic risks to humans, No. 100A.) Cyclophosphamide. International Agency for Research on Cancer, Lyon, France, 2012. Available via https://www.ncbi.nlm.nih.gov/books/NBK304336/
Joseph A, Mathew S, Skaria BP, Sheeja EC. Medicinal uses and biological activities of Argyreia speciosa sweet (Hawaiian baby woodrose)-an overview. 2011. Available via http://nopr.niscpr.res.in/handle/123456789/12731
Katoh C, Kitajima S, Saga Y, Kanno J, Horii I, Inoue T. Assessment of quantitative dual-parameter flow cytometric analysis for the evaluation of testicular toxicity using cyclophosphamide-and ethinylestradiol-treated rats. J Toxicol Sci, 2002; 27:87–96.
Kokate CK, Purohit CK, Gokhale SB. “Phytochemical tests.” In: Pharmacognosy. Nirali Prakashan, Pune, India, vol. 35, 2021.
Maheshkumar K, Dilara K, Maruthy KN, Sundareswaren L. Validation of PC-based sound card with biopac for digitalization of ECG recording in short-term HRV analysis. N Am J Med Sci, 2016; 8:307.
Masayasu M, Hiroshi Y. A simplified assay method of superoxide dismutase activity for clinical use. Clin Chim Acta, 1979; 92:337–42.
Maynard RL, Downes N. Anatomy and histology of the laboratory rat in toxicology and biomedical research. Academic Press, Cambridge, MA, 2019.
McLachlan RI, O’donnell L, Meachem SJ, Stanton PG, de Kretser DM, Pratis K, Robertson DM. Hormonal regulation of spermatogenesis in primates and man: insights for development of the male hormonal contraceptive. J Androl, 2002; 23:149–62.
Merwid-L?d A, Ksi?dzyna D, Ha?o? A, Chlebda-Sieragowska E, Trocha M, Szandruk M, Soza?ski T, Magdalan J, Kopacz M, Ku?niar A, Nowak D, Pie?niewska M, Szel?g A. Impact of morin-5′-sulfonic acid sodium salt on cyclophosphamide-induced gastrointestinal toxicity in rats. Pharmacol Rep, 2015; 67:1259–63.
Mills KA, Chess-Williams R, McDermott C. Novel insights into the mechanism of cyclophosphamide-induced bladder toxicity: chloroacetaldehyde’s contribution to urothelial dysfunction in vitro. Arch Toxicol, 2019; 93:3291–303.
Moghe A, Ghare S, Lamoreau B, Mohammad M, Barve S, McClain C, Joshi-Barve S. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci, 2015; 143:242–55.
Nurgali K, Jagoe RT, Abalo R. Adverse effects of cancer chemotherapy: anything new to improve tolerance and reduce sequelae? Front Pharmacol, 2018; 9:245.
Ohaeri OC. Effect of garlic oil on the levels of various enzymes in the serum and tissue of streptozotocin diabetic rats. Biosci Rep, 2001; 21:19–24.
Ohkawa H, Ohishi W, Yagi K. Colorimetric method for determination of MDA activity. Biochemistry, 1979; 95:351.
Poorvu PD, Frazier AL, Feraco AM, Manley PE, Ginsburg ES, Laufer MR, LaCasce AS, Diller LR, Partridge AH. Cancer treatment-related infertility: a critical review of the evidence. JNCI Cancer Spectr, 2019; 3:008.
Rani A, Shukla YN. Disubstituted tetrahydrofuran and an ester from Algyreia speciosa. Indian J Chem B Org Chem Incl Med Chem, 1997; 36:299–300.
Siber GR, Mayer RJ, Levin MJ. Increased gastrointestinal absorption of large molecules in patients after 5-fluorouracil therapy for metastatic colon carcinoma. Cancer Res, 1980; 40:3430–6.
Srinivasulu K, Changamma C. A study on the effect of Ocimum sanctum (Linn.) leaf extract and ursolic acid on spermatogenesis in male rats. Indian J Pharm Sci, 2017; 79:158–63.
Srivastava A, Shukla YN. Aryl esters and A coumarin from Aygyreia speciosa. Indian J Chem B Org Chem Incl Med Chem, 1998; 37:192–4.
Stankiewicz A, Skrzydlewska E. Amifostine antioxidant effect on serum of rats treated with cyclophosphodamide. Polish J Environ Stud, 2005; 14:341–6.
Swamy AHMV, Patel UM, Koti BC, Gadad PC, Patel NL, Thippeswamy AHM. Cardioprotective effect of Saraca indica against cyclophosphamide induced cardiotoxicity in rats: a biochemical, electrocardiographic and histopathological study. Indian J Pharmacol, 2013; 45:44.
Taniguchi I. Clinical significance of cyclophosphamide-induced cardiotoxicity. Intern Med, 2005; 44:89–90.
Uyar A, Yaman T, Dogan A, Uslu S, Keles ÖF, Yener Z, Celik I. Is methotrexateinduced testicular damage preventable using nettle seed extract: a histopathological, immunohistochemical, biochemical and spermatological examination. Fresenius Environ Bull, 2018; 27:2320–31.
Vyas N, Gamit K, Raval M, Patel SG. Isolation and chemical characterization of bioactive alkaloid from Argyreia speciosa Linn. having action on isolated rat Leydig cells. Asian J Pharm Clin Res, 2019; 12:276–80.
World Health Organization. WHO report on cancer: setting priorities, investing wisely and providing care for all. WHO, Geneva, Switzerland, 2020.