INTRODUCTION
Maintaining satisfactory oral health and hygiene is one of the crucial factors affecting human well-being, both emotionally and physically. The most common oral/buccal cavity diseases include periodontal illnesses, oral infections, dental caries, oral cancers, and especially oral inflammations (World Health Organization, n.d.). Since these disorders occur locally in the oral/buccal cavity, local treatments gain numerous advantages, such as direct targeting and systemic-side-effects reduction (Holpuch et al., 2011; Sankar et al., 2011). To this end, numerous pharmaceutical products have been formulated, characterized, and commercially available, namely Actisite® (tetracycline-loaded polymeric fibers), Arestin® (minocycline-incorporated polymeric microspheres), and PerioChip® insert (chlorhexidine-loaded hydrolyzed-gelatinous matrix) (Song et al., 2014). Although these products demonstrate much potential for local oral diseases, optimal systems are limited due to the problems of inadequate drug effectiveness and short drug retention time at the targeted action sites (Nguyen and Hiorth, 2015).
In fact, one of the critical obstacles in oral drug delivery systems is the drug solubility, retention, and release properties (Huynh et al., 2022; Nguyen et al., 2021). To that end, carrier technology provides an innovative approach to oral drug delivery by incorporating the drugs with, for example, microparticulate delivery systems that modulate the drug’s release, retention, and solubility characteristics. These systems provide several advantages, such as enhanced drug efficacy, decreased drug toxicity, and increased patient compliance (Prajapat et al., 2013). In addition, the use of an in situ release drug delivery system in treating infections in the oral cavity has brought convenience to the use of the drug and, at the same time, supported special populations with difficulty swallowing (i.e., children, elders). Microspheres, defined as “monolithic spheres or therapeutic agents distributed throughout the matrix as molecular dispersions of particles,” are particles possessing diameters in the range of 1–1,000 μm (Dolma Gurung and Kakar, 2020). With the strong applications of nano-/microparticles in numerous biomedical areas (Chomchalao et al., 2020; Pham et al., 2019, 2020, 2022, 2023b; Pham and Tiyaboonchai, 2020, 2021), it is considered a new and superior oral drug delivery system with the following outstanding properties of (1) possessing large contact surface area and uniformly distribution capacity, (2) protecting the drug substances, (3) drug retention locally and prolonging the effect of the drug, and (4) reducing the risk of irritation and unwanted effects (Ramteke et al., 2012; Varde and Pack, 2004). Nevertheless, limited publications in the field of microparticles for oral cavity/buccal drug delivery have been reported, with only 13 related articles in the PubMed database (accessed 25 February 2023, keywords “microparticles buccal”). Therefore, it is crucial to develop novel products for the local drug delivery in the oral/buccal cavity.
One of the interesting approaches is to formulate swellable mucoadhesive systems. To this end, hydroxypropyl methylcellulose (HPMC), an alkyl hydroxyalkyl cellulose mixture containing a methoxy or hydroxy group that is widely applied to prepare controlled oral drug delivery systems (Li et al., 2005), has gained much potential. The hydration rate of HPMC, and it’s high swelling property that affects the drug release, increased with increasing hydroxypropyl content (Salsa et al., 2008). Previously, HPMC has been utilized in numerous controlled-release drug delivery systems. For instance, Tundisi et al. (2021) have applied HPMC in eye drops to increase the drug retention time in the eye and reduce drug side effects; Lee et al. (1999) successfully loaded melatonin into an HPMC-based tablet to control melatonin release; and Turkoglu and Ugurlu (2002), reported the development of 5-aminosalicylic tablet coated with a pectin-HPMC complex to transport/protect the 5-aminosalicylic to/in the intestine. Nevertheless, as far as we know, no study has considered the use of HPMC for the fabrication of a mucoadhesive local drug delivery system in the mouth.
Considering all aforementioned issues, this study developed and characterized the swellable mucoadhesive HPMC-based microparticles, incorporating ibuprofen, for treating inflammatory diseases in the oral cavity. Ibuprofen was chosen as a model drug because (1) it is a nonsteroidal anti-inflammatory drug generally used to alleviate the inflammation in the oral cavity (Nakagita et al., 2020) and (2) it belongs to the Biopharmaceutical Classification System class II, with high permeability and low solubility, which possibly needs a delivery system to enhance its solubility and dissolution profile (García-Arieta et al., 2015). Various formulation parameters were first screened for the optimal microparticle formula, including the amount of loaded ibuprofen, the stirring time and temperature, and the centrifugal time and speed. Then, the particles’ physicochemical properties were fully characterized. Finally, the in situ drug release rate and the mucoadhesiveness of the system were evaluated in simulated oral cavity conditions.
MATERIALS AND METHODS
Materials
HPMC, grade K100M (viscosity of 75,000–140,000 cps), and ibuprofen were imported by Sigma-Aldrich, Singapore. Dichloromethane (DCM) and liquid paraffin were bought from Xilong, China. The porcine buccal mucosa was supplied by the local slaughterhouse in Can Tho City, Vietnam. All other chemicals and reagents were of reagent grades or higher.
Blank HPMC microparticles formulation
The HPMC microparticles were prepared by simple desolvation method (Godbee et al., 2004), in which the HPMC was first dissolved in DCM at room temperature, followed by the dropwise addition of liquid paraffin into the solution with continuous stirring using a magnetic stirrer for 2 hours. Since HPMC is not soluble in liquid paraffin, the HPMC was dissolved from the DCM solution, and finely dispersed polymeric particles were spontaneously formed. Finally, the microparticles were filtered, centrifuged, and washed thrice with n-hexane and dried at ambient temperature (Raut et al., 2013).
To achieve the optimal formula with the smallest sizes and highest yield efficiencies, two main formulating parameters were surveyed, including (1) the HPMC:DCM ratio (1:10, 1:15, 1:20, 1:25, 1:30, 1:35, and 1:40 w/v), and (2) the liquid paraffin amount (30, 40, 50, and 60 ml). The best formula, in terms of particle sizes and yield efficiencies, was utilized to encapsulate the model drug, ibuprofen.
Ibuprofen-loaded HPMC microparticles formulation
Similar to the blank HPMC microparticle formulation, the desolvation method was used to fabricate the ibuprofen-loaded HPMC microparticles. For this, ibuprofen was dissolved in the DCM solution containing the HPMC with optimal HPMC:DCM ratio. Then, the liquid paraffin was slowly subjected to the solution, stirred, and the obtained particles were filtered, centrifuged, washed thrice with n-hexane, and air-dried.
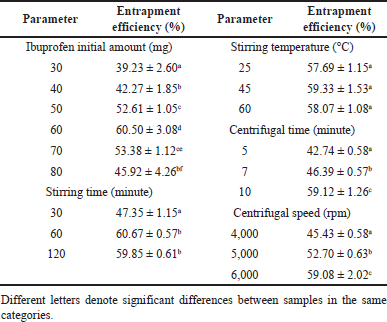
To obtain the optimal formula, various factors were considered, namely (1) the initial amount of ibuprofen (30, 40, 50, 60, 70, and 80 mg), (2) the stirring time (30, 60, and 120 minutes), (3) the stirring temperature (25, 45, and 60°C), (4) the centrifugal time (5, 7, and 10 minutes), and (5) the centrifugal speed (4,000, 5,000, and 6,000 rpm). The condition with the highest drug entrapment efficiency was utilized to formulate the optimal microparticles.
Microparticles’ physicochemical characterizations
The HPMC microparticles (blank and drug-loaded) were physicochemically characterized in terms of (1) the particle sizes and polydispersity indexes, (2) the particle shapes and morphology, (3) the drug entrapment efficiencies, and (4) the drug-material interactions.
For the particle sizes and polydispersity indexes, the laser diffraction technique was employed (Microtrac S3500 analyzer), utilizing the Mie principle (Fraunhofer theory) of light scattered from laser beams upon contacting a particle stream, which were measured with an array of optical detectors and analyzed using the Microtrac software.
The shapes and morphology of the HPMC microparticles, both in their dry solid state and in the wet swelling state after contact with water, were determined using the scanning electron microscopy (SEM) technique (Carl Zeiss Microscopy, 2.00 kV, Germany). The particle dispersions were dropped and immobilized on 100 nm plastic slices, followed by mounting on a metal base, coating with a 10 nm thick gold layer and being analyzed by the SEM.
The drug entrapment efficiencies were measured using the direct method with UV-Vis spectroscopy. Briefly, the ibuprofen-loaded HPMC microparticles were extracted with methanol, and the amount of ibuprofen incorporated in the particles was quantified by UV-Vis spectroscopy at the λmax of 272 nm and a standard curve of y = 907.94x – 13.217 (R2 = 0.9992, range: 0–1,000 ppm). The drug entrapment efficiencies were calculated based on Equation (1). The UV-Vis method was fully developed and validated following the ICH Q2(R1) validation of analytical procedures. The method showed high specificity, linearity with the correlation coefficient of R2 = 0.9992 (>0.995), precision with relative standard deviations (RSD%) of 0.415% ± 0.020% (<2%), and accuracy with recovery percentages of 100.79% ± 0.13% (in the range of 98.0%–102.0%). Furthermore, the method demonstrated a low limit of detection (LOD) of 0.5 ppb and a limit of quantitation of 2 ppb.
The particle structures and drug-material interactions were determined using Fourier-transformed infrared spectroscopy (FT-IR) (Jasco 6300 spectrophotometer, Japan) with the KBr pelleting technique. The spectra were obtained in a wavenumber range of 4,000–400 cm−1 at 4.0 cm−1 resolution.
In situ drug release test in simulated oral cavity condition
The ibuprofen release patterns from the HPMC microparticles were assessed in the in situ setting, utilizing the real unstimulated saliva from a human donor (Colombo et al., 1999; Siepmann et al., 1999). To this end, 5 mg of the ibuprofen-loaded HPMC microparticles was added to a shaker chamber containing an initial amount of 1 ml of saliva. The pure ibuprofen aqueous suspension (5 mg ibuprofen in 0.2 ml water) was used as a reference. To mimic the oral cavity, the chamber was shaken at 20 rpm, and the saliva flowed continuously in and out of the chamber at a rate of 0.25 ml/minute using a peristaltic pump. At each end of the saliva-filling lines, a 0.22-μm membrane was used to cover the lines opening and avoid particle loss. The whole chamber was put in a water bath with a set temperature of 37°C ± 0.5°C. At each time interval of 1, 3, 5, 15, 30, 60, 90, and 120 minutes, the sample was withdrawn, centrifuged (6,000 rpm, 20 seconds) to remove the particles, and the supernatant was diluted with methanol. Finally, the released ibuprofen was UV-Vis spectroscopic measured at λmax of 272 nm, and the released amount was calculated using the standard curve of ibuprofen in methanol. The cumulative release of ibuprofen was determined following Equation (2).
where Ct and Ci were the concentrations of released ibuprofen at the time points t and i, V0 was the total volume of the release buffer, V was the withdrawal volume, M0 was the ibuprofen initial amount, and Mi was the ibuprofen withdrawal amount at the time point i.
Ex vivo mucoadhesion study
The mucoadhesive property of the blank HPMC microparticles and the ibuprofen-loaded HPMC microparticles on the porcine buccal mucosa was determined by a texture analyzer, of which the required forces used to detach the particles from the mucosa were measured (Nafee et al., 2004). For this, 5 mg of the particles were mixed with 1 ml of unstimulated saliva and immediately applied on the upper probe of the texture analyzer to form a thin gel layer (due to the swellable property of the HPMC microparticles). The pure ibuprofen aqueous suspension (5 mg ibuprofen in 0.2 ml water) was used as a reference. Then, the porcine buccal mucosa (freshly obtained from the certified local slaughterhouse and kept in phosphate buffer until used) was placed onto the texture analyzer tissue holder, and 500 μl of the unstimulated saliva was added to the mucosa surface. Finally, the probe was lowered to initiate the contact between the HPMC particles and the tissue surface, and the force (N) required to separate the particles from the mucosa was reported (Nafee et al., 2004).
Statistical analysis
All experiments were performed in triplicate. The quantitative results were expressed as mean ± standard deviation (SD). Student’s t-test and ANOVA were used for statistical comparison between samples, and the p values were set at <0.05 for significant differences.
RESULTS AND DISCUSSIONS
Blank HPMC microparticles formulation
Prior to the formulation of ibuprofen-loaded HPMC microparticles, the optimal blank formula was determined by varying the HPMC/DCM ratio and the paraffin volume, taking the particle sizes and yield efficiencies into consideration (Table 1). For this, an increase in the DCM amount significantly reduced the particle sizes from ~36 μm (ratio 1:10) to ~12 μm (ratio 1:25 and higher). Since the desolvation method was employed for HPMC microparticle formulation, the dispersion viscosity strongly affected the resulting particle sizes. Thus, the more DCM, the less viscosity of the dispersion and the higher the shearing efficiency, consequently yielding smaller particles. These data were in accordance with a previous study (Raut et al., 2013). Additionally, an increase in liquid paraffin amount, up to 50 ml, gave more particles (i.e., higher yield efficiency). As paraffin was used as a desolvating agent, a low amount might not be sufficient to desolvate the HPMC; thus, some HPMC molecules remained in the DCM solution, reducing the particle amounts. Conclusively, to reduce harmful chemical usage and avoid environmental issues, the HPMC/DCM ratio of 1:25 w/v and the paraffin amount of 50 ml were chosen to formulate ibuprofen-loaded HPMC microparticles.
 | Table 1. Effects of the HPMC and DCM ratio (w/v) and the liquid paraffin volume, on the blank HPMC microparticle sizes and yield efficiencies, respectively (n = 3). [Click here to view] |
 | Table 2. Effects of formulation factors of (1) the initial amount of ibuprofen, (2) the stirring time, (3) the stirring temperature, (4) the centrifugal time, and (5) the centrifugal speed on the entrapment efficiencies of the ibuprofen-loaded HPMC microparticles (n = 3). [Click here to view] |
Ibuprofen-loaded HPMC microparticles formulation
From the optimal blank formula, the ibuprofen-loaded HPMC microparticles were fabricated. To this end, various factors were additionally investigated (Table 2). First, the amount of active ingredient ibuprofen was varied, in which the initial amount of 60 mg ibuprofen resulted in the highest entrapment efficiency of 60.50% ± 3.08%. This amount was considered the “saturation point” (i.e., the point at which the drug concentrations inside and outside the particles are equilibrated) since both lower amounts and higher amounts reduced the drug encapsulations (Pham et al., 2023a). Second, the stirring time of 60 minutes increased the entrapment efficiency compared to that of 30 minutes, and this value did not increase at 120 minutes, suggesting that 60 minutes was the minimal formulation time to adequately form HPMC particles. Third, the optimal preparation temperature was 25°C (i.e., room temperature) since a higher temperature did not yield significant differences in drug entrapment efficiencies. Theoretically, an increase in temperature reduces the solution viscosity, making it easier to form particles and encapsulate the drugs (Seeton, 2006). However, this was not the case in our results, possibly due to the high DCM volume that considerably made the solution less viscous. Finally, the centrifugal time and speed of 10 minutes and 6,000 rpm, respectively, could completely recover most particles, leading to the highest drug entrapment efficiency values. In summary, the optimal condition included (1) the initial ibuprofen of 60 mg, (2) the stirring time of 60 minutes, (3) the stirring temperature of 25°C, (4) the centrifugal time of 10 minutes, and (5) the centrifugal speed of 6,000 rpm.
Microparticles physicochemical characterizations
The optimal ibuprofen-loaded HPMC microparticles were evaluated for their physicochemical properties of particle size, morphology, drug entrapment efficiency, and drug-material interaction. For this, the particle size was 13.47 ± 0.32 μm (Fig. 1A), slightly bigger than the blank counterpart (12.24 ± 1.11 μm), possibly due to the encapsulation of ibuprofen on the particle surfaces. Moreover, the particle polydispersity index was 0.218 ± 0.025, suggesting narrow size distributions. Regarding the particle morphology, the SEM images revealed the pellet aggregated HPMC microparticles in the dry solid state (Fig. 1B), which rapidly swelled and formed gel upon contact with water (Fig. 1C). The ibuprofen entrapment efficiency was 61.23% ± 1.04%, which was acceptable for the microparticles in loading drugs (Pham et al., 2022, 2019). Lastly, the drug-material interactions were investigated using the FT-IR method (Fig. 2). Obviously, the characterized ibuprofen peaks, such as 1,721 cm−1 (C=O), 1,506 cm−1 (C=C), and 1,184 cm−1 (C–OH), appeared in the spectrum of ibuprofen-loaded HPMC microparticles, indicating that ibuprofen was successfully incorporated in the systems and the formulation process did not alter the drug structure. Moreover, no observable changes/shifts in these ibuprofen peaks were noted, suggesting that the interactions between the drug ibuprofen and HPMC were mainly weak bonding (i.e., van der Waals interactions and hydrogen bonding). This fact explains the relatively fast release of the drug in the in situ environment, discussed in the next section.
In situ drug release test in simulated oral cavity condition
The main purpose of this study was to develop swellable mucoadhesive HPMC microparticles for local drug delivery to the oral/buccal cavity, using ibuprofen as a model drug. Hence, we conducted the drug release experiments in in situ conditions, simulating the oral cavity, taking the mouth movements, and saliva flow into consideration. To this end, the pure ibuprofen aqueous suspension demonstrated a fast “release” at 5 minutes (~25%) due to its inherent low solubility in the dissolution medium (<1 mg/ml), and no additional dissolve/release drugs were noted for the remaining test duration (Fig. 3). This was because of the crystalline polymorph of the pure ibuprofen powder, which significantly hinders its solubility. On the other hand, the ibuprofen-loaded HPMC microparticles could partly sustain the drug release rate up to 2 hours (Fig. 3). The release rate of HPMC-based platforms is dependent on their viscosity, in which the high-viscosity (>4,000 cps) systems sustain the drug release and the low-viscosity (≤100 cps) ones rapidly release the encapsulated drugs via diffusion mechanism (Gao et al., 1996). Upon contact with the in situ oral cavity, the particles immediately form a solid gel due to the inherent swelling property of HPMC (Fig. 1C), making it harder for the saliva flow and mouth movement to wash off the particles. Moreover, the newly formed gel slowly released the encapsulated ibuprofen into the medium, which is ideal for extended treatments in the local oral cavity.
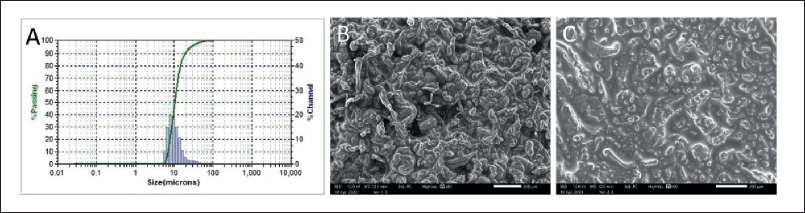 | Figure 1. The particle size graph (A) and SEM micrographs of the ibuprofen-loaded HPMC microparticles in the dry state (B) and after contact with water (C). Scale bar: 200 μm. [Click here to view] |
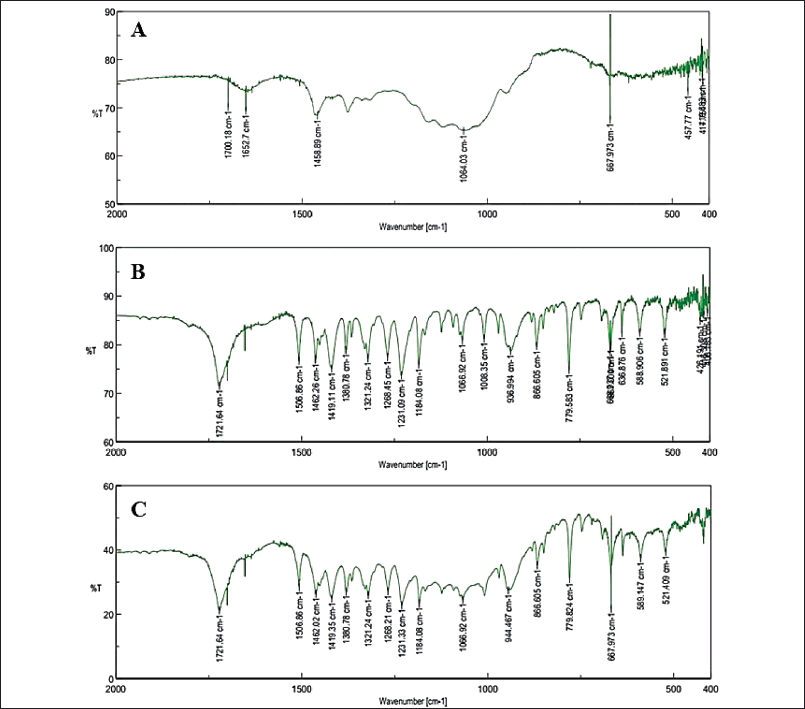 | Figure 2. FT-IR spectrum of (A) the blank HPMC microparticles, (B) the pure ibuprofen powder, and (C) the ibuprofen-loaded HPMC microparticles. [Click here to view] |
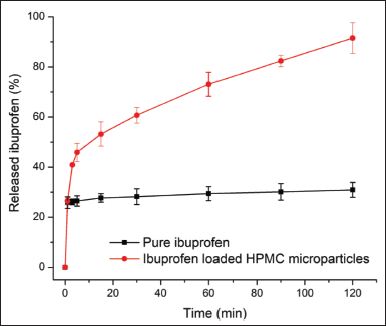 | Figure 3. The ibuprofen release patterns of the pure ibuprofen aqueous suspension and ibuprofen-loaded HPMC microparticles in in situ experiments, simulating the local oral cavity condition (n = 3). [Click here to view] |
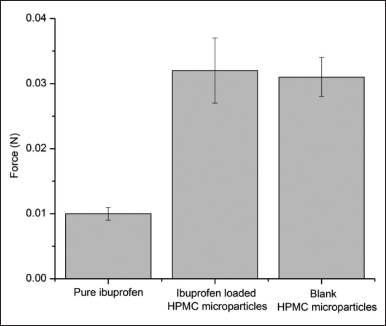 | Figure 4. The mucoadhesive force (N) of the pure ibuprofen aqueous suspension, the blank HPMC microparticles, and the ibuprofen-loaded HPMC microparticles in the ex vivo tests with the porcine buccal mucosa (n = 3). [Click here to view] |
Ex vivo mucoadhesion study
To further confirm the ability of the ibuprofen-loaded HPMC microparticles to be retained in the oral cavity, the ex vivo mucoadhesive test was conducted, utilizing the porcine buccal mucosa. For this, the minimal required force to separate the particles that formed gel upon contacting the mucosa was approximately three times higher than that of the pure ibuprofen aqueous suspension (Fig. 4). Additionally, no significant difference in the mucoadhesive force was noted between the blank HPMC microparticles and the ibuprofen-loaded HPMC microparticles, indicating that the encapsulated drug did not affect the particle mucoadhesiveness. The mucoadhesive property of HPMC has been exploited in various biomedical platforms, such as tablets and films (da Silva et al., 2021; Joshi and Chen, 2009; Kraisit et al., 2017), mainly due to its swelling property. Additionally, HPMC swelling property is proportionally correlated with its inherent viscosity (Mohamed et al., 2019). In our work, the HPMC K100M, with high viscosity, was employed. Thus, the HPMC microparticles could easily swell upon contacting the buccal tissue and provide mucoadhesiveness. To the best of our knowledge, the HPMC microparticles’ mucoadhesiveness was first investigated in this study, which reconfirmed this property of another HPMC platform.
CONCLUSION
The present work successfully developed and physicochemically characterized the swellable mucoadhesive ibuprofen-loaded HPMC microparticles for local inflammatory treatment in the oral cavity. Utilizing the simple desolvation method, the optimal formulation condition included (1) the initial ibuprofen amount of 60 mg, (2) the stirring time of 60 minutes, (3) the stirring temperature of 25°C, (4) the centrifugal time of 10 minutes, and (5) the centrifugal speed of 6,000 rpm. This condition yielded particles with a size of 13.47 ± 0.32 μm, an ibuprofen entrapment efficiency of 61.23% ± 1.04%, and weak interactions between the drug ibuprofen and HPMC. Compared to the free drug aqueous suspension, the drug-loaded HPMC microparticles possessed an outstanding sustained release pattern and higher mucoadhesive properties. As confirmed by the positive results, the HPMC microparticles could be further investigated to become a potential mucoadhesive drug delivery system.
ACKNOWLEDGMENTS
The authors sincerely thank Can Tho University and Can Tho University of Medicine and Pharmacy for supporting this work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors declare there are no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author Van De Tran.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Chomchalao P, Nimtrakul P, Pham DT, Tiyaboonchai W. Development of amphotericin B-loaded fibroin nanoparticles: a novel approach for topical ocular application. J Mater Sci, 2020; 55:5268–79; https://doi.org/10.1007/s10853-020-04350-x
Colombo P, Bettini R, Peppas NA. Observation of swelling process and diffusion front position during swelling in hydroxypropyl methyl cellulose (HPMC) matrices containing a soluble drug. J Control Release, 1999; 61:83–91; https://doi.org/10.1016/S0168-3659(99)00104-2
da Silva JB, dos Santos RS, da Silva MB, Braga G, Cook MT, Bruschi ML. Interaction between mucoadhesive cellulose derivatives and pluronic F127: investigation on the micelle structure and mucoadhesive performance. Mater Sci Eng C, 2021; 119:111643; https://doi.org/10.1016/J.MSEC.2020.111643
Dolma Gurung B, Kakar S. An overview on microspheres. Gurung Kakar Int J Heal Clin Res, 2020; 3:11–24.
Gao P, Skoug JW, Nixon PR, Ju TR, Stemm NL, Sung KC. Swelling of hydroxypropyl methylcellulose matrix tablets. 2. Mechanistic study of the influence of formulation variables on matrix performance and drug release. J Pharm Sci, 1996; 85:732–40; https://doi.org/10.1021/JS9504595
García-Arieta A, Gordon J, Potthast H. On the biopharmaceutics classification system biowaiver of ibuprofen. J Pharm Sci, 2015; 104:2429–32; https://doi.org/10.1002/JPS.24519
Godbee J, Scott E, Pattamunuch P, Chen S, Mathiowitz E. Role of solvent/non-solvent ratio on microsphere formation using the solvent removal method. J Microencapsul, 2004; 21:151–60; https://doi.org/10.1080/02652040310001637875
Holpuch A, Desai KG, Schwendeman S, Mallery S. Optimizing therapeutic efficacy of chemopreventive agents: a critical review of delivery strategies in oral cancer chemoprevention clinical trials. J Carcinog, 2011; 10:23; https://doi.org/10.4103/1477-3163.85185
Huynh DTM, Tran VH, Le MNT, Huynh VH, Pham DT. Floating tablets incorporating curcumin solid dispersion as a potential pharmaceutical dosage form for stomach cancer treatment. J Appl Pharm Sci, 2022; 13(04):240–50; https://doi.org/10.7324/JAPS.2023.114417
Joshi SC, Chen B. Swelling, dissolution and disintegration of HPMC in aqueous media. IFMBE Proc, 2009; 23:1244–7; https://doi.org/10.1007/978-3-540-92841-6_305/COVER
Kraisit P, Limmatvapirat S, Nunthanid J, Sriamornsak P, Luangtana-Anan M. Preparation and characterization of hydroxypropyl methylcellulose/polycarbophil mucoadhesive blend films using a mixture design approach. Chem Pharm Bull, 2017; 65:284–94; https://doi.org/10.1248/CPB.C16-00849
Lee BJ, Ryu SG, Cui JH. Controlled release of dual drug-loaded hydroxypropyl methylcellulose matrix tablet using drug-containing polymeric coatings. Int J Pharm, 1999; 188:71–80; https://doi.org/10.1016/S0378-5173(99)00204-5
Li CL, Martini LG, Ford JL, Roberts M. The use of hypromellose in oral drug delivery. J Pharm Pharmacol, 2005; 57:533–46; https://doi.org/10.1211/0022357055957
Mohamed D, Nacéra C, Fatiha B, Noureddine A,. Effervescent floating tablets of metformin HCl developed by melt granulation. Part I: effect of hydrophilic polymer on biopharmaceutical properties. GSC Biol Pharm Sci, 2019; 6:52–67; https://doi.org/10.30574/GSCBPS.2019.6.2.0014
Nafee NA, Ismail FA, Boraie NA, Mortada LM. Mucoadhesive delivery systems. I. Evaluation of mucoadhesive polymers for buccal tablet formulation. Drug Dev Ind Pharm, 2004; 30:985–93; https://doi.org/10.1081/DDC-200037245
Nakagita T, Taketani C, Narukawa M, Hirokawa T, Kobayashi T, Misaka T. Ibuprofen, a nonsteroidal anti-inflammatory drug, is a potent inhibitor of the human sweet taste receptor. Chem Senses, 2020; 45:667–73; https://doi.org/10.1093/CHEMSE/BJAA057
Nguyen NNT, Pham DT, Nguyen DT, Trinh TTL. Bilayer tablets with sustained-release metformin and immediate-release sitagliptin: preparation and in vitro/in vivo evaluation. J Pharm Investig, 2021; 51:579–86; https://doi.org/10.1007/S40005-021-00533-Z
Nguyen S, Hiorth M. Advanced drug delivery systems for local treatment of the oral cavity. Ther Deliv, 2015; 6:197–210; https://doi.org/10.4155/TDE.15.5
Pham DT, Chokamonsirikun A, Phattaravorakarn V, Tiyaboonchai W. Polymeric micelles for pulmonary drug delivery: a comprehensive review. J Mater Sci, 2020; 56:2016–36; https://doi.org/10.1007/S10853-020-05361-4
Pham DT, Ha TKQ, Nguyen MQ, Tran V De, Nguyen VB, Quyen TTB. Silk fibroin nanoparticles as a versatile oral delivery system for drugs of different biopharmaceutics classification system (BCS) classes: a comprehensive comparison. J Mater Res, 2022; 37:4169–81; https://doi.org/ 10.1557/S43578-022-00782-0
Pham DT, Huynh QC, Lieu R, Nguyen VB, Tran V, Thuy BTP. Controlled-release Wedelia trilobata L. flower extract loaded fibroin microparticles as potential anti-aging preparations for cosmetic trade commercialization. Clin Cosmet Investig Dermatol, 2023a; 16:1109–21; https://doi.org/10.2147/CCID.S405464
Pham DT, Nguyen DXT, Lieu R, Huynh QC, Nguyen NY, Quyen TTB, Tran V. Silk nanoparticles for the protection and delivery of guava leaf (Psidium guajava L.) extract for cosmetic industry, a new approach for an old herb. Drug Deliv, 2023b; 30:2168793. https://doi.org/10.1080/10717544.2023.2168793
Pham DT, Tetyczka C, Hartl S, Absenger-Novak M, Fröhlich E, Tiyaboonchai W, Roblegg E. Comprehensive investigations of fibroin and poly(ethylenimine) functionalized fibroin nanoparticles for ulcerative colitis treatment. J Drug Deliv Sci Technol, 2019:101484; https://doi.org/10.1016/j.jddst.2019.101484
Pham DT, Tiyaboonchai W. Fibroin-coated poly(ethylenimine)-docusate nanoparticles as a novel drug delivery system. Curr Sci, 2021; 121:775–80; https://doi.org/10.18520/CS/V121/I6/775-780
Pham DT, Tiyaboonchai W. Fibroin nanoparticles: a promising drug delivery system. Drug Deliv, 2020; 27:431; https://doi.org/10.1080/10717544.2020.1736208
Prajapat P, Agrawal D, Bhaduka G. Sustained release oral drug delivery system-an overview. Int J Pharma Res Rev, 2013; 2:11–21.
Ramteke KH, Jadhav VB, Dhole SN. Microspheres: as carrieres used for novel drug delivery system. IOSR J Pharm, 2012; 2:44–8.
Raut NS, Somvanshi S, Jumde AB, Khandelwal HM, Umekar MJ, Kotagale NR. Ethyl cellulose and hydroxypropyl methyl cellulose buoyant microspheres of metoprolol succinate: influence of pH modifiers. Int J Pharm Investig, 2013; 3:163; https://doi.org/10.4103/2230-973X.119235
Salsa T, Veiga F, Pina ME. Oral controlled-release dosage forms. I. Cellulose ether polymers in hydrophilic matrices. Drug Dev Ind Pharm, 2008; 23:929–38; https://doi.org/10.3109/03639049709148697
Sankar V, Hearnden V, Hull K, Juras DV, Greenberg MS, Kerr AR, Lockhart PB, Patton LL, Porter S, Thornhill M. Local drug delivery for oral mucosal diseases: challenges and opportunities. Oral Dis, 2011; 17:73–84; https://doi.org/10.1111/J.1601-0825.2011.01793.X
Seeton CJ. Viscosity-temperature correlation for liquids. Tribol Lett, 2006; 22:67–78; https://doi.org/10.1007/S11249-006-9071-2
Siepmann J, Podual K, Sriwongjanya M, Peppas NA, Bodmeier R. A new model describing the swelling and drug release kinetics from hydroxypropyl methylcellulose tablets. J Pharm Sci, 1999; 88:65–72; https://doi.org/10.1021/JS9802291
Song X, Yaskell T, Klepac-Ceraj V, Lynch MC, Soukos NS. Antimicrobial action of minocycline microspheres versus 810-nm diode laser on human dental plaque microcosm biofilms. J Periodontol, 2014; 85:335–42; https://doi.org/10.1902/JOP.2013.130007
Tundisi LL, Mostaço GB, Carricondo PC, Petri DFS. Hydroxypropyl methylcellulose: physicochemical properties and ocular drug delivery formulations. Eur J Pharm Sci, 2021; 159:105736; https://doi.org/10.1016/J.EJPS.2021.105736
Turkoglu M, Ugurlu T. In vitro evaluation of pectin-HPMC compression coated 5-aminosalicylic acid tablets for colonic delivery. Eur J Pharm Biopharm, 2002; 53:65–73; https://doi.org/10.1016/S0939-6411(01)00225-9
Varde NK, Pack DW. Microspheres for controlled release drug delivery. Expert Opin Biol Ther, 2004; 4:35–51; https://doi.org/10.1517/14712598.4.1.35
World Health Organization. Oral health surveys: basic methods. World Health Organization, Geneva, Switzerland, n.d.