INTRODUCTION
Over 2 million cases of breast cancer are diagnosed worldwide annually, making it the most common tumor. Nearly one-third of cancer cases in females are breast cancer. Six percent of breast cancer cases undergo metastasis, while de novo metastatic breast cancer has a 5-year survival rate of just 29.0% (Rugo et al., 2022). Breast cancer accounts for 19.2% of all cancer cases in Indonesia, making it the most prevalent cancer. A previous study suggested that most breast cancer patients had already had advanced stages of the disease when they sought treatment (Gautama, 2022). Breast cancer is a diverse illness with various biological characteristics and prognoses (Bertucci et al., 2012). A multidisciplinary approach to treating breast cancer that incorporates elements of surgical oncology, radiation oncology, and medical oncology has been linked to a decrease in the death rates of breast cancer (Rugo et al., 2022). It is very crucial to appropriately identify the earliest stage of breast cancer once it has been diagnosed because this information will influence therapy suggestions (Pilewskie and Morrow, 2017). The degree of expression of the three cellular receptors, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2), that are targets for treatment is used to classify the tumor (Burstein et al., 2015).
Historically, the term triple-negative breast cancer (TNBC) has been used to refer to tumors that do not express ER, PR, or HER2. Compared to ER-positive tumors, TNBCs exhibit rapid development and are more likely to be identified clinically rather than by mammography (Collett et al., 2005) or as interval cancers between mammograms (Phipps et al., 2011). However, inherent variations in breast tissue density among individuals with TNBC may account for these presentation variations (Collett et al., 2005).
TNBC frequently acts in a more combative manner. Contrary to other breast cancer subtypes (such as ER-positive or HER2-positive subtypes), TNBC patients with high expression of programmed cell death ligand 1 (PD-L1) are usually treated with immunotherapy combined with chemotherapy. Nevertheless, no approved targeted therapies are currently available for this subtype of breast cancer (D’Angelo et al., 2020). Approximately 15% of all breast cancer diagnoses globally are considered as this form of malignancy (Palmer et al., 2014), which translates to about 200,000 instances annually (Iwamoto et al., 2012). TNBC is more frequently found in women under the age of 40 as compared with hormone receptor-positive breast cancer (Palmer et al., 2014). According to a study, women under 40 had a twofold higher risk of TNBC than those over 50 (Burstein et al., 2015). In the US, TNBC is also more prevalent among African American women than Caucasian women (Bertucci et al., 2012; Gonzalez-Angulo et al., 2011).
TNBC patients experienced a twofold higher risk for recurrence within 5 years after diagnosis than those with other types of breast cancer, with a mortality rate above 40%. This situation is associated with unclear molecular characteristics of TNBC and the unavailability of treatment for TNBC patients. Therefore, the development of novel targeted treatments and diagnostic tools based on molecular characteristics has become necessary (Burstein et al., 2015; Lehmann et al., 2016). TNBC is characterized by high heterogeneity and only has standard chemotherapy as the treatment option (Zolota et al., 2021). Although it has been proposed that classifying TNBC patients based on pathway-specific molecular abnormalities may help predict which therapy drugs will be most effective, this idea has not been proven useful in routine clinical practice (Korsching et al., 2002).
Some risk factors have been linked to the diagnosis of TNBC (Phipps et al., 2011). Mutation in tumor suppressor BRCA, especially in BRCA1, is present in around 20% of TNBC patients (Bertucci et al., 2012; Gonzalez-Angulo et al., 2011). Contrarily, not more than 6% of all breast cancers have BRCA mutation as a contributing factor (Zolota et al., 2021). Considering this phenomenon, TNBC patients need to be referred for genetic testing and counseling to assess the status of BRCA mutation (Livasy et al., 2006). Furthermore, BRCA germline testing is very important for TNBC patients who are 60 years old or younger (Bertucci et al., 2012). Some studies on the population have discovered that African American women are more likely to develop TNBC than Caucasian women (Palmer et al., 2014). Moreover, compared to postmenopausal status, premenopausal status has been linked to an increased risk of TNBC occurrence (Regan et al., 2006). Obesity and young age at first pregnancy have also been considered risk factors for TNBC, whereas the number of parity and breastfeeding may lower the risks. However, these elements are less consistently reported and are rarely taken into account in therapeutic decisions (Palmer et al., 2014).
Noncoding RNAs, posttranslational histone modifications, and DNA methylation are epigenetic alterations that are very crucial in regulating gene expression in TNBC development (Zolota et al., 2021). The extracellular matrix (ECM) is a complex arrangement of proteins and other molecules with cellular regulatory and structural functions. The ability of the ECM to control crucial cancer cell processes is compromised when the expression of the matrix components is altered (Piperigkou et al., 2018; Zolota et al., 2021). According to recent molecular studies, the tumor microenvironment and ECM changes play a significant role in TNBC, and altering the tumor microenvironment has lately been identified as a promising treatment approach (D’Angelo et al., 2020; Zolota et al., 2021). One way epigenetics play a role in TNBC pathogenesis is by driving the ECM modifications (Piperigkou et al., 2018).
According to recent research, epigenetic changes contribute to the early and subsequent development of breast cancer (Zolota et al., 2021). The ECM experiences major structural changes as cancer progresses, while epithelial to mesenchymal transition (EMT) is also brought on during this process (Moustakas and Heldin, 2014). These actions are strongly connected to epigenetic modifiers and other processes. According to recent findings, matrix changes in cancer cells can be regulated by epigenetics, and these changes may further improve cell survival and therapy resistance (Bertucci et al., 2012; Lehmann et al., 2011). In line with this, methods for focusing on the tumor’s microenvironment and its epigenetic pathways represent potential therapeutic approaches (Robertson, 2016).
This review discusses the significance of the molecular characteristics of TNBC, including epigenetic modifications in relation to diagnosis and treatment. Articles from available databases were collected and reviewed to elucidate the nature of TNBC, including its clinical and molecular characteristics. We also review the epigenetic modifications associated with breast cancer, particularly TNBC, and its potential influence on diagnosis and treatment. Finally, we summarize hypotheses related to the potential use of epigenetic alteration in clinical practice as an object for further investigation.
DIAGNOSIS OF TNBC
Although TNBC is not similar to basal breast cancers, and there is significant genetic variety in TNBC, the clinical phenotype of TNBC consists primarily of basal-like subtypes (Bertucci et al., 2008, 2012). For instance, a study using genetic and molecular profiling discovered four subtypes of TNBCs: luminal androgen receptor, mesenchymal, basal-like immunosuppressed, and basal-like immune-activated (Sher et al., 2022).
In a different study, gene expression profiles that identified the basal subtype were correlated with the classification of 172 TNBC tumors determined by immunohistochemistry (IHC) staining. The basal subtype was only assigned to 71% of TNBCs. In a converse examination using 160 tumors, 77% of basal tumors were classified as TNBC based on IHC (Iwamoto et al., 2012). Molecular and genetic studies, including gene expression and mutational analysis, have also implied that the clonal spectra in TNBC are highly variable and broad (Bertucci et al., 2012; Gonzalez-Angulo et al., 2011). The overexpression of epidermal growth factor receptor and cytokeratins 5/6 and the lack of HER2 and hormone receptors-related genes are distinctive gene clusters expressed genomically in basal-like breast cancer (Bertucci et al., 2008; Livasy et al., 2006). Gene expression has been used to distinguish different subtypes of TNBC (Prat et al., 2010; Zolota et al., 2021).
Other gene expression analyses also indicated that tumor suppressor gene p53 and BRCA genes have been found to be altered or expressed abnormally in TNBC (Teschendorff et al., 2007). These molecular characteristics may affect the treatment response to platinum and other substances that directly damage DNA (Mathe et al., 2016; Stirzaker et al., 2015). These studies have yielded conflicting findings, with different investigators’ conclusions (Carey et al., 2006; Sørlie et al., 2001). In addition, no study has yet offered any clinical considerations (Lehmann et al., 2011; Phipps et al., 2011).
Women diagnosed with localized TNBC at age 60 or younger or with metastatic TNBC at any age are strongly recommended to undergo BRCA1 mutation testing, regardless of family history, due to its significant correlation with TNBC (Carey et al., 2006). Moreover, several studies also unraveled that BRCA mutational analysis has major therapeutic advantages for those with metastatic TNBC (Sher et al., 2022).
Patients with metastatic TNBC should be confirmed by biopsy followed by a comprehensive evaluation of ER, PR, and HER2 since primary TNBC might have different features compared to the metastatic one (Avery-Kiejda et al., 2017). For instance, further analysis of two studies revealed a 28%, 13%, and 5% difference in PR, ER, and HER2, respectively, between primary and recurrent TNBC (Avery-Kiejda et al., 2017; Zolota et al., 2021). The 22C3 pharmDX analysis is a powerful tool for evaluating patients who get pembrolizumab. The companion immunohistochemical test for SP142, immune cells stably expressing PD-L1, has already been utilized to select patients receiving atezolizumab (Cortes et al., 2020; Khaled and Bidet, 2019). The US Food and Drug Administration has already approved both tests as companion diagnostics. If the amount of tissue is enough, it is also advisable to perform some analysis for tumor mutational burden, mismatch repair deficit, and microsatellite instability (Djahansouzi et al., 2022; Phipps et al., 2011).
TNBC THERAPY APPROACH
Nonmetastatic disease
Patients with TNBC have similar options for neoadjuvant or adjuvant treatment as those with other breast cancer characteristics. With regard to all breast cancer subtypes, the same general concepts for surgical management and radiation therapy alternatives are used. Similar principles govern the surgical management of breast cancer, radiation therapy application, and systemic treatment (Bertucci et al., 2012; Lehmann et al., 2011, 2016). Chemotherapy, to be given in either the adjuvant or neoadjuvant setting, is advised for patients with TNBC who have lymph nodes that are pathologically implicated or a tumor that is larger than 0.5 cm in size (independent of tumor size). Chemotherapy is more likely to be beneficial for larger tumors than smaller ones since the risk of recurrence rises on a continuum. Patients with tumors between 1 and 5 mm typically do not require chemotherapy, but we carefully discuss the matter with them to optimize the benefit for the patients (Liedtke et al., 2008).
Metastatic disease
TNBC falls under many of the general guidelines that also apply to other phenotypes of advanced breast cancer. Chemotherapy has been considered the main systemic therapy for TNBC because endocrine-based therapy and HER2-directed treatments are less successful. However, several studies have implied that targeted treatments, such as immune checkpoint inhibitors and poly [adenosine diphosphate (ADP)-ribose] polymerase inhibitors, may yield good outcomes in TNBC. Combination chemotherapy may be appropriate in the metastatic setting for patients with extensive or rapidly progressing visceral disease, in whom it is believed that the higher the chance of response, the higher the risk of toxicity. However, no cohort studies are comparing single-agent sequential cytotoxic chemotherapy to combination chemotherapy, demonstrating an improvement in overall survival (OS) (Cortes et al., 2020; Khaled and Bidet, 2019).
In patients with advanced TNBC, a randomized trial (KEYNOTE-355) found that adding pembrolizumab to chemotherapy marginally increased progression-free survival (PFS). Pembrolizumab also increased OS in the subset of tumors that expressed PD-L1 and had a combined positive score (CPS) of at least 10 (23 vs. 16 months). OS and PFS advantages were seen in the CPS 1 subgroup of patients who were PD-L1 positive, though neither trend was statistically significant. In light of these findings, studies combine immunotherapy with immune checkpoint inhibitors for patients with PD-L1-positive TNBC (Bertucci et al., 2012; Khaled and Bidet, 2019; Phipps et al., 2011).
RECENT INSIGHTS INTO EPIGENETICS
DNA methylation is a heritable epigenetic mark in which DNA methyltransferases covalently transfer a methyl group to the C-5 of the cytosine ring in the DNA. Anywhere along the genome in mammals, cytosines are methylated. Specific noninvasive biomarkers that can provide an early diagnosis of the disease are still not utilized in treating breast cancer at this moment (Khaled and Bidet, 2019; Sher et al., 2022; Zolota et al., 2021). Environmental exposures have an impact on epigenetic-sensitive signatures, which are mediated by direct molecular mechanisms, primarily controlled by DNA methylation, that control the interaction of genetic and nongenetic risk factors during cancerogenesis (Djahansouzi et al., 2022). An early stage of the development of cancer is marked by the inactivation of tumor suppressor genes caused by promoter hypermethylation (Stirzaker et al., 2015). Therefore, it seems to be a practical clinical strategy for early detection, more precise risk stratification, and personalized treatment for patients with breast cancer to use liquid-based assays to detect both targeted and genome-wide DNA methylation changes. By separating the circulating tumor DNA from plasma, a more accessible biospecimen, it is possible to map the DNA methylation profile (Bertucci et al., 2012; Lehmann et al., 2011; Stirzaker et al., 2015; Teschendorff et al., 2007). Additionally, gene-specific DNA methylation may affect how sensitive a patient is to chemotherapy, hormone therapy, and immunotherapy, suggesting new potential drug targets (Mathe et al., 2016; Stirzaker et al., 2015). Most notably, in patients who have resistance to anticancer treatment, the use of epigenetic drugs administered alone or in combination with anticancer therapies has recently produced remarkable results (Mathe et al., 2016; Zolota et al., 2021). This review aims to inform readers about DNA methylation changes that may be related to the onset of breast cancer and their potential clinical application in the areas of diagnosis, prognosis, and treatment (Koboldt et al., 2012; Zolota et al., 2021).
Covalent modifications known as posttranslational modifications (PTM) include methylation, phosphorylation, acetylation, ubiquitylation, SUMOylation, glycosylation, and ADP-ribosylation on histone proteins. PTMs are essential for the structure and operation of chromatin. The term “epigenetic markers” has also been applied to histone changes (Regan et al., 2006). By changing the structure of chromatin, the PTMs that occur in histone proteins can have an impact on gene expression (Bertucci et al., 2012; Lehmann et al., 2011, 2016). Histone modifications play a pivotal role in various cellular activities, including DNA damage and repair, mitosis, meiosis, chromosome packaging, and transcriptional activation and inactivation (Lin et al., 2010). A number of clinical disorders, including cancer, have been linked to defects in the PTM pathway. In human breast cancer cells, loss of H3K4me2 caused by the histone lysine demethylase KDM1A was linked to EMT (Avery-Kiejda et al., 2017; Zolota et al., 2021). The DNA damage marker γH2AX has recently demonstrated its prognostic value. High levels of γH2AX in TNBC were linked to poor OS and shorter telomere length (Lin et al., 2010).
Most primary transcripts produced by genomic transcription but not translated into proteins are noncoding RNAs (ncRNA) (Piperigkou et al., 2018). Many studies have already reported the potential uses of these previously thought of as “junk” molecules, and new understandings have developed (Shah et al., 2012). In breast cancer, ncRNAs control intracellular and intercellular signaling (Shah et al., 2012) and adjust ER levels and activity, cell growth, migration, apoptosis, and stemness to regulate various cellular activities (Koboldt et al., 2012; Zolota et al., 2021). Additionally, ncRNAs can be packaged into exosomes to enable local or systemic delivery of microRNAs (miRNAs) and long noncoding RNAs (lncRNAs) to cells, enabling intercellular communication. Although lncRNAs and miRNAs are thought to be therapeutic targets for breast cancer, more research is required before the potential for controlling their activities can be put to clinical use (Lehmann et al., 2016; Shah et al., 2012).
THE ROLES OF EPIGENETICS IN TNBC
Recent research has shown that genetic and epigenetic variables are crucial in the onset and spread of breast cancer (Zolota et al., 2021). According to earlier studies, epigenetic alterations play an important role in the development and spread of breast cancer (Sher et al., 2022). Epigenetic changes like DNA methylation and histone modification, which result in the silencing of particular genes, make up one of the first steps in the cancer-inducing pathway (Sher et al., 2022; Zolota et al., 2021).
DNA methylation
Numerous studies have examined the genetic and epigenetic markers that make up the molecular components of TNBC (Khaled and Bidet, 2019; Zolota et al., 2021). A genetic BRCA1 gene mutation has been connected to TNBC (Moustakas and Heldin, 2014). The oncogenic characteristics of cancer may be affected by epigenetic alteration (Moustakas and Heldin, 2014). Breast cancer’s molecular characteristics and histological subtypes were connected to alterations in DNA acetylation and methylation (Piperigkou et al., 2018). Moderate-to-low levels of histone lysine acetylation, lysine methylation, and arginine methylation were observed in breast cancer subtypes with poor prognoses (Mathe et al., 2016). However, the investigations could not establish how the molecular profiles can help the TNBC point of care. The use of these molecular markers in TNBC and the implications for clinical management as a result have not been fully investigated (Khaled and Bidet, 2019; Mathe et al., 2016; Zolota et al., 2021).
In recent studies, differentially methylated regions have been identified as potential biomarkers (Stirzaker et al., 2015). They refer to genomic regions, typically CpGs, where breast cancer cells and healthy breast tissue exhibit markedly different methylation patterns. Using a cutting-edge method for early diagnosis, previous study showed that the detection of circulating DNA in blood plasma is useful in identifying patients with early-stage breast cancer (Mathe et al., 2016). Like epigenetics, miRNAs can alter gene expression in a heritable manner without changing the DNA sequence (Mathe et al., 2016). Expression dysregulation has been linked to several illnesses, including cancer. When the expression levels of miRNAs were analyzed in breast cancer cell lines after chemotherapy application, it was found that chemotherapy-induced changes in breast cancer-related miRNAs (Zolota et al., 2021). The results of a pilot study with a small number of TNBC patients demonstrated the presence of previously identified miRNAs in urine and serum samples (Khaled and Bidet, 2019; Zolota et al., 2021). Identification of miRNA in urine and plasma may be used for early breast cancer diagnosis, according to a recent study that tried to examine a breast cancer-specific miRNA panel (Lehmann et al., 2016; Prat et al., 2010).
DNA methylation inhibitors (DNMTi) are compounds that can activate tumor suppressor genes deactivated by DNA methylation. 5-Azacytidine (5-aza-CR; azacitidine) is the first DNMTi, a nucleoside analog that binds to RNA and DNA. Only DNA can be integrated by the 5-aza-CR deoxy derivative, 5-aza-2′deoxycytidine (5-aza-CdR, decitabine). Its incorporation into DNA suppresses DNA methylation by trapping DNMTi during tumor cell replication (D’Angelo et al., 2020; Zolota et al., 2021).
Histone PTM is related to cancer’s abnormal gene silencing (Nagelkerke et al., 2011). A common alteration is a decrease in histone acetylation levels brought on by increased histone deacetylases (HDAC) expression (Zolota et al., 2021). Unfortunately, HDAC inhibitors (HDACi), which are used to return histone acetylation levels to normal, reduce the acetylation of both histone and nonhistone proteins without being selective, leading to unfavorable side effects (Zolota et al., 2021).
DNA methylation patterns in TNBCs resemble other breast cancer subtypes (Mathe et al., 2016). For instance, CpG islands and coastlines exhibit hypermethylation, whereas intragenic areas exhibit hypomethylation (Mathe et al., 2016). The methylated genes differ between tumor subtypes even if the quantity of methylation in CpG islands of TNBC and non-TNBC tumors is the same (Koboldt et al., 2012). DNA methylation revealed widespread epigenetic changes in the methylome of breast cancer, which can be used to categorize various subtypes, stages, and outcomes (Koboldt et al., 2012).
A high risk of developing metastatic disease and dying has been correlated with hormone-negative breast cancers that lack CpG island methylation (Koboldt et al., 2012). Hypomethylated TNBC cells were recently revealed to have a lower OS rate (Mathe et al., 2016; Stirzaker et al., 2015). Despite being one of the first gene-specific DNA methylation abnormalities to be identified in cancer, hypomethylation’s causes have only recently been found (Koboldt et al., 2012). A number of studies have been carried out recently to learn more about the mechanisms of hypomethylation in cancer, particularly in hematological cancers (Khaled and Bidet, 2019; Zolota et al., 2021). TNBCs provide a chance to learn more about the molecular causes of DNA hypomethylation and their connection to prognosis (Mathe et al., 2016).
Since a previous study reported that several genes were not methylated in TNBC methylation signatures, it was determined that TNBC heterogeneity is most associated with other pathways. A multiplatform dataset describing genome-wide analyses of gene expression, DNA methylation, and miRNA expression in primary TNBCs, surrounding healthy tissues, and metastases-related organs is required to identify novel indicators implicated in TNBC management (Mathe et al., 2016).
A group of genes, including ANKRD30B, IL6ST, and MEG3, had differentially methylated probes, indicating that they were specific to TNBC (Mathe et al., 2016). An additional group of differentially methylated genes was discovered in lymph node metastases (Branham et al., 2012; Mathe et al., 2016). Better survival was linked to increased methylation levels in several genes, such as GREB1, EGR1, and ITIH5, and decreased methylation of AMIGO2. The same study found an inverse relationship between EGR1 downregulation and its methylation (Zolota et al., 2021).
Posttranslational histone modifications
Numerous PTM, including the addition of acetyl, methyl, ubiquitin, and phosphor groups, can occur in histones (Lin et al., 2010). A lysine residue in the N-terminal tail of histones modified by histone acetylation produces a more open chromatin structure, while histone deacetylation produces a more closed chromatin structure (Lin et al., 2010). These modifications are carried out by the enzymes histone acetyltransferase and histone deacetyltransferase (Livasy et al., 2006). Gene silencing can be associated with DNA methylation along with histone deacetylation, whereas gene transcription can be induced by the demethylation of DNA and acetylation of histone tail (Nagelkerke et al., 2011).
Figure 1 describes several histone changes connected to breast cancer. Researchers have also investigated potential histone modification-related molecules linked to EMT in TNBC (Khaled and Bidet, 2019; Zolota et al., 2021). Interestingly, previous studies revealed an increased level of H3K4me3 and H3K4ac level in the MDA-MB-231 TNBC cell line as compared with MCF7 luminal cell line, suggesting the importance of those marks in breast cancer subtype characterization (Troester et al., 2006; Yahfoufi et al., 2018).
DNMT1, which can be recruited by EZH2, catalyzes the methylation of gene promoter regions that will inhibit this gene expression. According to one theory, in basal breast cancer cells, EZH2 activates H3K27me3, which is linked to the CDH1 promoter, to silence E-cadherin, hence maintaining the mesenchymal cells (Khaled and Bidet, 2019; Sher et al., 2022).
Cancer stem cells (CSCs) have been shown to differ from non-CSCs in their DNA and histone methylation patterns (Koboldt et al., 2012; Prat et al., 2010). H3K4me2 and H3K27me3 marks play an important role by modulating Wnt and GnRH signaling in the MDA-MB-231 TNBC cell line, increasing their capacity for invasion and tumorigenesis both in vivo and in vitro. Although the precise mechanism is unclear, breast CSCs are thought to influence TNBC aggression (Koboldt et al., 2012; Prat et al., 2010).
The anticancer effect of DNMTis and HDACis was recently investigated in TNBC cell line using several assays such as viability, apoptosis, migration and invasion, 3D culture, and clonogenic assays (Koboldt et al., 2012; Prat et al., 2010). The most efficient DNMTi and HDACi were used to treat several types of breast cancer cell lines (Khaled and Bidet, 2019; Lehmann et al., 2016). DNMTis and HDACis have been reported to have the ability to decrease the aggressivity of TNBC by reprogramming EMT (Koboldt et al., 2012; Lehmann et al., 2016). Another study also unraveled that by modulating EMT, epigenetic-based therapies showed some promise for TNBC treatment (Stirzaker et al., 2015).
Noncoding RNA–mediated gene regulation
ncRNAs can be classified into small and long ncRNAs. Short ncRNAs, or those with fewer than 200 nucleotides, include miRNA, piwi-interacting RNA, tiny nuclear RNA, and small-interfering RNA. Because they cleave messenger RNA (mRNA) or stop translation, miRNAs are the most extensively researched short ncRNAs in cancer (Sher et al., 2022). Unlike mRNAs, which are involved in gene transcription, miRNAs are RNA sequences that control the expression of DNA primarily posttranscriptionally (Avery-Kiejda et al., 2017; Zolota et al., 2021). They are pivotal in oncogenesis, including TNBC (Zolota et al., 2021).
Compared to normal tissue, TNBC microarray profiling identified several lncRNAs with distinctive expression patterns (Bertucci et al., 2012; Teschendorff et al., 2007). However, their roles, connections to other pathways, and significance have not yet been determined (Livasy et al., 2006; Prat et al., 2010). A microarray analysis study identified that ER dysregulation in TNBC is related to lncRNA LINC00993 (Zolota et al., 2021). A different lncRNA, MALAT1, has recently been found to contribute to TNBC metastasis and has been suggested as a promising marker for HER2+ prognosis determination (Djahansouzi et al., 2022). Figure 2 depicts the part that epigenetic regulation plays in TNBC carcinogenesis and potential treatment options.
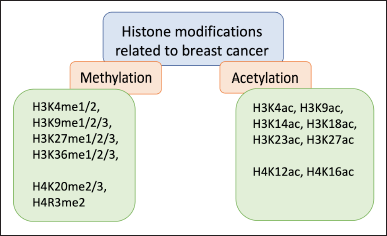 | Figure 1. Histone methylations and acetylations associated with breast cancer. [Click here to view] |
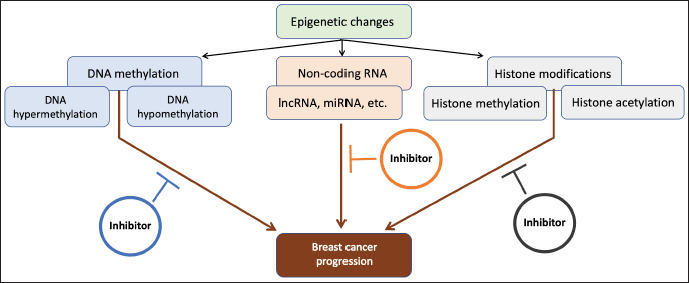 | Figure 2. Potential therapeutic strategies based on epigenetic regulation on carcinogenesis in TNBC. [Click here to view] |
CONCLUSION
Epigenomics can signal breast cancer progression, according to a growing body of research. In signaling pathways linked to tumor metastasis, abnormal protein expression may result from the epigenomic profiles, which include DNA methylation and histone modification, and may result in the progression of the tumor. Detecting epigenetic information, such as DNA methylation and histone modification, is now possible using various new techniques, including next-generation sequencing and microfluidics. However, based on the pace of advancement, single-cell epigenome analysis is currently less advanced than single-cell transcriptomics and genome analysis.
TNBC is a malignancy that exhibits a variety of histologic and molecular traits. Clinical diagnostics are insufficient, especially during the early stages, to identify TNBC. The use of these genetic markers is restricted to higher-grade tumors, although the available polygenic risk model can aid in disease recurrence prediction. Additionally, the genetic mutation of BRCA1/2, the primary genetic contributor to breast cancer, is insufficient for biomarker application in TNBC diagnosis. To better diagnose and treat TNBC patients, more research on potential epigenetic variables is important.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This study was funded by the Universitas Padjadjaran grant for EFA.
CONFLICTS OF INTEREST
The authors do not have any conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral in terms of jurisdictional claims in published institutional affiliation.
REFERENCES
Avery-Kiejda KA, Mathe A, Scott RJ. Genome-wide MiRNA, gene and methylation analysis of triple negative breast cancer to identify changes associated with lymph node metastases. Genom Data, 2017; 14(December):1–4; https://doi.org/10.1016/J.GDATA.2017.07.004.
Bertucci F, Finetti P, Birnbaum D. Basal breast cancer: a complex and deadly molecular subtype. Curr Mol Med, 2012; 12(1):96; https://doi.org/10.2174/156652412798376134.
Bertucci F, Finetti P, Cervera N, Esterni B, Hermitte F, Viens P, Birnbaum D. How basal are triple-negative breast cancers? Int J Cancer, 2008; 123(1):236–40; https://doi.org/10.1002/IJC.23518.
Branham MT, Marzese DM, Laurito SR, Gago FE, Orozco JI, Tello OM, Vargas-Roig LM, Roqué M. Methylation profile of triple-negative breast carcinomas. Oncogenesis, 2012; 1(7):e17; https://doi.org/10.1038/ONCSIS.2012.17.
Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SAW, Savage MI, Osborne CK, Hilsenbeck SG, Chang JC, Mills JB, Lau CC, Brown PH. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res, 2015; 21(7):1688–98; https://doi.org/10.1158/1078-0432.CCR-14-0432.
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MCU, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the carolina breast cancer study. JAMA, 2006; 295(21):2492–502; https://doi.org/10.1001/JAMA.295.21.2492.
Collett K, Stefansson IM, Eide J, Braaten A, Wang H, Eide GE, Thoresen S, Foulkes WD, Akslen LA. A basal epithelial phenotype is more frequent in interval breast cancers compared with screen detected tumors. Cancer Epidemiol Biomarkers Prev, 2005; 14(5):1108–12; https://doi.org/10.1158/1055-9965.EPI-04-0394.
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SH, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, Iwata H, Masuda N, Otero MT, Gokmen E, Loi S, Guo Z, Zhao J, Aktan G, Karantza V, Schmid P. KEYNOTE-355: randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol, 38(15_suppl):1000; https://doi.org/10.1200/JCO.2020.38.15_SUPPL.1000.
D’Angelo E, Lindoso RS, Sensi F, Pucciarelli S, Bussolati B, Agostini M, Collino F. Intrinsic and extrinsic modulators of the epithelial to mesenchymal transition: driving the fate of tumor microenvironment. Front Oncol, 2020; 10(July); https://doi.org/10.3389/FONC.2020.01122.
Djahansouzi S, Hanstein B, Rein D, Clees M, Rath W. The rate of estrogen receptor-conversion associated with tumor progression in estrogen receptor-positive breast cancer patients following adjuvant tamoxifen administration. Cancer Rep, 2022; 5(1); https://doi.org/10.1002/cnr2.1431.
Gautama W. Breast cancer in Indonesia in 2022: 30 years of marching in place. Indones J Cancer, 2022; 16(1):1–2; https://doi.org/10.33371/IJOC.V16I1.920.
Gonzalez-Angulo AM, Timms KM, Liu S, Chen H, Litton JK, Potter J, Lanchbury JS, Stemke-Hale K, Hennessy BT, Arun BK, Hortobagyi GN, Do KN, Mills GB, Meric-Bernstam F. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res, 2011; 17(5):1082–89; https://doi.org/10.1158/1078-0432.CCR-10-2560.
Iwamoto T, Booser D, Valero V, Murray JL, Koenig K, Esteva FJ, Ueno NT, Zhang J, Shi W, Qi Y, Matsuoka J, Yang EJ, Hortobagyi GN, Hatzis C, Symmans WF, Pusztai L. Estrogen receptor (ER) MRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J Clin Oncol, 2012; 30(7):729–34; https://doi.org/10.1200/JCO.2011.36.2574.
Khaled N, Bidet Y. New insights into the implication of epigenetic alterations in the EMT of triple negative breast cancer. Cancers, 2019; 11(4); https://doi.org/10.3390/CANCERS11040559.
Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF, Fulton LL, et al. Comprehensive molecular portraits of human breast tumours. Nature, 2012; 490(7418):61–70; https://doi.org/10.1038/NATURE11412.
Korsching E, Packeisen J, Agelopoulos K, Eisenacher M, Voss R, Isola J, Van Diest PJ, Brandt B, Boecker W, Buerger H. Cytogenetic alterations and cytokeratin expression patterns in breast cancer: integrating a new model of breast differentiation into cytogenetic pathways of breast carcinogenesis. Lab Invest, 2002; 82(11):1525–33; https://doi.org/10.1097/01.LAB.0000038508.86221.B3.
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest, 2011; 121(7):2750–67; https://doi.org/10.1172/JCI45014.
Lehmann BD, Jovanovi? B, Chen X, Estrada MV, Johnson KN, Shyr Y, Moses HL, Sanders ME, Pietenpol JA. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS ONE, 2016; 11(6); https://doi.org/10.1371/JOURNAL.PONE.0157368.
Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol, 2008; 26(8):1275–81; https://doi.org/10.1200/JCO.2007.14.4147.
Lin T, Ponn A, Hu X, Law BK, Lu J. Requirement of the histone demethylase LSD1 in snai1-mediated transcriptional repression during epithelial-mesenchymal transition. Oncogene, 2010; 29(35):4896–904; https://doi.org/10.1038/ONC.2010.234.
Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, Moore DT, Perou CM. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol, 2006; 19(2):264–71; https://doi.org/10.1038/MODPATHOL.3800528.
Mathe A, Wong-Brown M, Locke WJ, Stirzaker C, Braye SG, Forbes JF, Clark SJ, Avery-Kiejda KA, Scott RJ. DNA methylation profile of triple negative breast cancer-specific genes comparing lymph node positive patients to lymph node negative patients. Sci Rep, 2016; 6(September); https://doi.org/10.1038/SREP33435.
Moustakas A, Heldin P. TGFβ and matrix-regulated epithelial to mesenchymal transition. Biochim Biophys Acta Gen Subj, 2014; 1840(8):2621–34; https://doi.org/10.1016/J.BBAGEN.2014.02.004.
Nagelkerke A, Van Kuijk SJA, Sweep FCGJ, Nagtegaal ID, Hoogerbrugge N, Martens JWM, Timmermans MA, Van Laarhoven HWM, Bussink J, Span PN. Constitutive expression of γ-H2AX has prognostic relevance in triple negative breast cancer. Radiother Oncol, 2011; 101(1):39–45; https://doi.org/10.1016/J.RADONC.2011.07.009.
Palmer JR, Viscidi E, Troester MA, Hong CC, Schedin P, Bethea TN, Bandera EV, Borges V, McKinnon C, Haiman CA, Lunetta K, Kolonel LN, Rosenberg L, Olshan AF, Ambrosone CB. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER consortium. J Nat Cancer Inst, 2014; 106(10); https://doi.org/10.1093/JNCI/DJU237.
Phipps AI, Chlebowski RT, Prentice R, McTiernan A, Wactawski-Wende J, Kuller LH, Adams-Campbell LL, Lane D, Stefanick ML, Vitolins M, Kabat GC, Rohan TE, Li CI. Reproductive history and oral contraceptive use in relation to risk of triple-negative breast cancer. J Nat Cancer Inst, 2011; 103(6):470–77; https://doi.org/10.1093/JNCI/DJR030.
Pilewskie M, Morrow M. Axillary nodal management following neoadjuvant chemotherapy: a review. JAMA Oncol, 2017; 3(4):549–55; https://doi.org/10.1001/JAMAONCOL.2016.4163.
Piperigkou Z, Götte M, Theocharis AD, Karamanos NK. Insights into the key roles of epigenetics in matrix macromolecules-associated wound healing. Adv Drug Deliv Rev, 2018; 129(April):16–36; https://doi.org/10.1016/J.ADDR.2017.10.008.
Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res BCR, 2010; 12(5); https://doi.org/10.1186/BCR2635.
Regan MM, Viale G, Mastropasqua MG, Maiorano E, Golouh R, Carbone A, Brown B, Suurküla M, Langman G, Mazzucchelli L, Braye S, Grigolato P, Gelber RD, Castiglione-Gertsch M, Price KN, Coates AS, Goldhirsch A, Gusterson B. Re-evaluating adjuvant breast cancer trials: assessing hormone receptor status by immunohistochemical versus extraction assays. J Nat Cancer Inst, 2006; 98(21):1571–81; https://doi.org/10.1093/jnci/djj415.
Robertson C. The extracellular matrix in breast cancer predicts prognosis through composition, splicing, and crosslinking. Exp Cell Res, 2016; 343(1):73–81; https://doi.org/10.1016/J.YEXCR.2015.11.009.
Rugo HS, Brufsky A, Liu X, Li B, McRoy L, Chen C, Layman RM, Cristofanilli M, Torres MA, Curigliano G, Finn RS, Demichele A. 169P overall survival with first-line palbociclib plus an aromatase inhibitor (AI) versus AI in metastatic breast cancer: a large real-world database analysis. Ann Oncol, 2022; 33:S202; https://doi.org/10.1016/j.annonc.2022.03.188.
Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, Turashvili G, Ding J, Tse K, Haffari G, Bashashati A, Prentice LM, Khattra J, Burleigh A, Yap D, Bernard V, McPherson A, Shumansky K, Crisan A, Giuliany R, Heravi-Moussavi A, Rosner J, Lai D, Birol I, Aparicio S. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature, 2012; 486(7403):395–99; https://doi.org/10.1038/NATURE10933.
Sher G, Salman NA, Khan AQ, Prabhu KS, Raza A, Kulinski M, Dermime S, Haris M, Junejo K, Uddin S. Epigenetic and breast cancer therapy: promising diagnostic and therapeutic applications. Semin Cancer Biol, 2022; 83(August):152–65; https://doi.org/10.1016/J.SEMCANCER.2020.08.009.
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Nat Acad Sci U S A, 2001; 98(19):10869–74; https://doi.org/10.1073/PNAS.191367098.
Stirzaker C, Zotenko E, Song JZ, Qu W, Nair SS, Locke WJ, Stone A, Armstong NJ, Robinson MD, Dobrovic A, Avery-Kiejda KA, Peters KM, French JD, Stein S, Korbie DJ, Trau M, Forbes JF, Scott RJ, Brown MA, Francis GD, Clark SJ. Methylome sequencing in triple-negative breast cancer reveals distinct methylation clusters with prognostic value. Nat Commun, 2015; 6; https://doi.org/10.1038/NCOMMS6899.
Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol, 2007; 8(8); https://doi.org/10.1186/GB-2007-8-8-R157.
Troester MA, Herschkowitz JI, Oh DS, He X, Hoadley KA, Barbier CS, Perou CM. Gene expression patterns associated with P53 status in breast cancer. BMC Cancer, 2006; 6; https://doi.org/10.1186/1471-2407-6-276.
Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients, 2018; 10(11):1–23; https://doi.org/10.3390/nu10111618.
Zolota V, Tzelepi V, Piperigkou Z, Kourea H, Papakonstantinou E, Argentou MI, Karamanos NK. Epigenetic alterations in triple-negative breast cancer—the critical role of extracellular matrix. Cancers, 2021; 13(4):1–17; https://doi.org/10.3390/CANCERS13040713.