INTRODUCTION
Insulin was the first peptide hormone developed as a therapeutic agent due to the critical role played in glucose regulation. Besides the function performed in blood glucose regulation, insulin possesses neuroprotective and neurotrophic effects, including learning and memory enhancement (Akintola and Van Heemst, 2015). Kullmann et al. (2016) reported that a decrease in insulin concentration and receptors-binding ability causes reduced memory function and symptoms of Alzheimer’s disease (AD). The early study on insulin therapy for AD was through intravenous infusion, which improved memory performance (Kern et al., 2001), but this showed a higher risk of hypoglycemia (Watson et al., 2003). Therefore, there is a strong interest in the direct delivery of insulin to the brain through the intranasal route.
Previous insulin studies for AD therapy using insulin liquid by intranasal route showed positive results in patients with AD and cognitive impairment. Administration of insulin at a strength of 20 IU for 12 months decreased the progression of white matter hyperintensity volume, which was associated with improved biomarker profiles for cognitive function (Kellar et al., 2021). Otherwise, insulin is now available in a liquid preparation stored at 2°C–8°C, referred to as a cold chain product (Heinemann et al., 2021). This is related to the instability of insulin in water and at temperatures above 32°C (Bahendeka et al., 2019).
Insulin undergoes chemical and physical degradation due to exposure to high temperatures, sunlight, and agitated stress, which impairs its stability and biological potency. The chemical degradation commonly occurs through deamidation reactions and the formation of high molecular weight (MW) polymers, while physically, insulin can aggregate into fibrils (Heinemann et al., 2021). Therefore, insulin handling poses a significant challenge, particularly in tropical countries, due to the need for high expenses and adequate knowledge among healthcare professionals.
Food and Drug Administration reported that insulin in liquid preparations possesses a 1-month shelf-life because of stability problems (FDA, 2019). This is a limitation of liquid insulin compared to the dry formulation, which is superior in terms of stability during storage and distribution. The higher stability is attributed to the degradation rate reduction orchestrated by limited molecular mobility. Most degradation processes require the mobility of a protein’s molecule. Once a protein is in a dry state, the molecular mobility is greatly limited, leading to an improvement in stability (Mensink et al., 2017). On the other hand, it suggests that the insulin dry powder (IDP), which was formulated with sugar stabilizers, was more stable compared to the formulations without stabilizers.
The accelerated stability testing at 60°C showed that insulin formulated with trehalose or a combination of trehalose and dextran in a weight ratio of 1:1 exhibited an increase in stability of 68%–75% (Tonnis et al., 2015). Trehalose, a nonreducing sugar with a high Tg value (Mensink et al., 2017), and inulin, which also possesses a high Tg value and flexibility in its protein coating, were both chosen as the materials. A high Tg value is related to the conformational stability of a protein; hence, the higher it is, the more stable a system will be due to the significant resistance in the aggregation-prone conformation. However, several studies reported lower stability in polysaccharides with higher Tg values than those with lesser Tg. This Tg can be attributed to the stabilization properties due to the smaller flexible molecules. Therefore, these aspects ensure the combination provides good physical stability, which is also related to chemical stability (Furlan et al., 2011; Hinrichs et al., 2005).
Due to insulin sensitivity to temperature, IDP should be prepared at cold temperatures. One method that can be applied is spray freeze-drying (SFD), which produces spherical particles that fulfill the intranasal dry powder requirements (Eggerstedt et al., 2012; Ishwarya et al., 2015). In the previous study, IDP was produced using the SFD method and resulted in a spherical, rough surface, and microsize insulin particle powder. In this preliminary study, IDP can be obtained without delay or interruptions during the SFD process. IDP with the best characteristics was demonstrated by a formulation with a combination of trehalose and inulin at a weight ratio of 1:1 (Muntu et al., 2021). However, stability testing has not yet been performed.
In this study, the stability study of insulin SFD powder has been conducted to examine the effect of adding a sugar stabilizer on the stability parameters of IDP products. First, seven formulations of IDP were prepared using the SFD method with a variation in the composition of trehalose to inulin. Physicochemical characterization was carried out to ensure the fulfillment of IDP specification for the nasal route and stability criteria, including determination of the powder’s morphology, glass transition temperature (Tg) and thermal energy, crystallinity, moisture content, and insulin content. Moreover, the stability study was performed at room temperature and 40°C for the long-term stability study and elevated temperatures for the accelerated stability study to obtain the shelf-life of IDP. Therefore, this study aimed to provide the most stable IDP for AD therapy with nose-to-brain delivery.
The novelty of this work lies in the use of the SFD method to develop IDPs for AD therapy via the intranasal route, which has not been explored previously. While previous research has focused on liquid formulations of insulin for nasal delivery to the brain (Kamei and Takeda-morishita, 2015) or powder formulations for diabetes therapy via oral or inhalation routes (Goldberd and Wong, 2015) this study addresses the gap in knowledge by investigating the stability and efficacy of IDP formulations for AD therapy via the intranasal route. This study further adds novelty by using a combination of trehalose and inulin as protective agents for insulin to ensure stability and efficient delivery to the brain. Additionally, this study investigates the effects of varying the weight ratio of trehalose and inulin on the properties and stability of IDP formulations.
MATERIALS AND METHODS
Materials
Recombinant human insulin, trehalose, inulin, hydroxypropyl methylcellulose (HPMC) E5, and Poloxamer 188 of pharmaceutical grade were purchased from Sigma Aldrich (St. Louis, MO). The water for injection was purchased from Ikapharmindo Putramas (Jakarta, Indonesia). Whatman nylon membrane filters 0.2 μm was obtained from GF Healthcare Life Sciences (Germany). Moreover, KH2PO4, NaOH, HCl (proanalysis), acetonitrile, and methanol of gradient grade for liquid chromatography, and trifluoroacetic acid for spectroscopy were purchased from Merck (Darmstadt, Germany).
Preparation of SFD IDP for nose-to-brain delivery
A solution of recombinant human insulin (50 IU) and sugar at a 1:1,000 w/w ratio was prepared in demineralized water with the pH adjusted to 5.8 until a 20 ml volume was achieved. Various sugar combinations were formulated in seven different compositions of trehalose and inulin. Sugar used were trehalose-inulin in the ratio of 5:1 (F1), 2:1 (F2), 1:1 (F3), 1:2 (F4), 1:5 (F5), 0:1 (F6), and 1:0 (F7). The next step involved spray-freezing the solutions using liquid nitrogen. A metal container was filled with approximately 5 l of liquid nitrogen before the process begins. The solutions were sprayed using a fluid nozzle (Model B-191 Buchi mini spray dryer; Buchi Co., Ltd., Flawil, Switzerland) and rapidly frozen into droplets using liquid nitrogen. During the spray-freezing process, it is important to refill the liquid nitrogen when it has evaporated to approximately 2 l. The spray-freezing process should be continued until the entire solution is formed into frozen droplets. The frozen droplets were then subjected to sublimation through the freeze-drying (FD) process in ScanVac CoolSafe FDs, DVS-1000 (Surface Measurement Systems Limited, London, UK) at a vacuum pressure of 0.04 MPa and a temperature of −45°C for 50 hours. The primary drying of frozen droplets was ensured to be conducted below the Tg’ for all formulations. Subsequently, secondary drying was performed for 36 hours at −20°C and the same pressure as in the primary drying to produce minimal residual moisture. Finally, the resulting powder was collected and stored in tightly sealed glass vials over silica gel in a vacuum desiccator at 25°C. This entire process was conducted based on a preliminary study that optimized the IDP manufacturing process parameters (Muntu et al., 2021).
SFD IDP characterization
IDP was characterized by its morphology, thermal properties, crystallinity, and moisture content. The various techniques used in the characterization of IDP for each specific parameter will be explained in the following section.
Shape and surface morphology characterization of IDP
The IDP particle’s shape and surface morphology were observed using scanning electron microscopy (SEM) APOLLO X (AMETEK Inc., USA) with an excitation voltage of 10.00 kV and a working distance of 7–10 mm. The samples were coated with gold-palladium for 90 seconds using a sputter coater (Bio-Rad SC 502, VG Microtech, Uckfield, UK) with an electric potential of 2.0 kV at 10 mA for 10 minutes under air pressure of 1.3–13.0 mPa. Observations were performed at a magnification of 250×.
Thermal properties characterization of IDP
Differential scanning calorimetry (DSC) 1/500 (Mettler-Toledo, Greifensee, Switzerland) was used to analyze the thermal properties of IDP. A certain amount of IDP samples and all tested materials were weighed at approximately 4 mg, placed in an aluminum DSC crucible plate, and then pressed. The samples were placed in the sample plate of the instrument, then heated from 30°C to 300°C. The heating rate used was 10°C/minute.
Crystallinity characterization of IDP
X-ray powder diffraction (XRPD) by X’pert PRO PANalytical, The Netherlands, was used to determine the crystallinity. All IDP samples were filled into a horizontally shaped sample holder and excess powder was cleaned. The sample surface should be smooth and parallel to the edge of the holder. Scanning was conducted at 2.00°/minute above 2θ in the range of 3.0°–40.0°.
Moisture content characterization of IDP
The moisture content was measured using the moisture content analyzer HB43 (Mettler Toledo, Switzerland). The IDP samples were weighed at approximately 1 g and dried at 105°C until a constant weight was achieved. The moisture content of the IDP was then determined by calculating the difference between the initial and final weights, expressed as a percentage of the final weight.
Insulin assay
Insulin content in IDP was assayed by a reversed-phase high-performance liquid chromatography (HPLC) method following the previous study with a slight modification. Samples were prepared by dissolving the IDP in 500 μl of 0.05 N HCl. The internal standard used in this method is 2.0 ml of 10 μg/ml methylparaben. The chromatographic assay was performed on an HPLC system (Water Alliance E2695, Milford, MA) equipped with an ultraviolet detector at a wavelength of 276 nm using HPLC column Osaka Soda Capcell Pak C18 UG120 5 um 4,6 × 150 mm (Osaka Soda Co., Ltd., Osaka, Japan) as a stationary phase. The mobile phase used in this method was acetonitrile and 0.1% trifluoroacetic acid solution in the composition of 30:70 at a flow rate maintained at 1.0 ml/minute. The injection volume of the sample was 20 μl (Surini et al., 2003).
The stability study of the SFD IDP
The long-term stability of IDP was evaluated in seven different formulations through triplicate studies. IDPs were placed in tightly sealed glass vials and stored in a desiccator at room temperature (25°C ± 2°C) and in an oven at 40°C ± 2°C. In addition, the humidity was monitored periodically between 58.70% ± 4.76%. The samples of all formulations were subjected to physicochemical characterization. Insulin content of IDP was observed by measuring the content of insulin that remained in the samples at sampling points of 0, 1, 2, 3, 4, 12, and 24 weeks of storage. Moreover, the physical stability of IDP from each formulation was observed by examining the powder’s morphology, thermal properties, crystallinity, and moisture content at 0, 1, 3, and 6 months of storage.
In addition, the accelerated stability study was carried out to examine the chemical characteristic of IDP from each formulation during storage in various accelerated temperatures. The insulin content that remained in IDP after storage in an oven (Memmert, Germany) at 40°C ± 2°C, 50°C ± 2°C, and 60°C ± 2°C was determined at 0, 1, 2, 3, and 4 weeks of storage using an HPLC assay. According to the results, the degradation rate constant of insulin at 25°C (k25) can be determined to further analyze its activation energy (Ea), half-life, and shelf life.
RESULTS AND DISCUSSION
Physicochemical characteristics of SFD IDP
The study aimed to develop a stable dry powder form of insulin to be used in nose-to-brain delivery for AD treatment. The insulin was physically engineered with sugar stabilizers trehalose and inulin in seven different weight ratios and prepared using the SFD method. The physical and chemical properties of the resulting IDP were characterized to ensure that IDP fulfills the intranasal route specification required for nose-to-brain delivery in AD therapy. In addition, long-term and accelerated stability studies were conducted to determine the most stable formulation and to provide information on the degradation rate constant, half-life, and shelf life of the insulin in IDP.
Morphology of SFD IDP
SEM images provided various structural and morphological information on the SFD IDP in 250× magnification, as presented in Figure 1. All formulations were discovered to have spherical shapes and rough surface properties. These results indicate that the variation in trehalose and inulin ratio does not affect the shape and morphology of the particles produced. Particle shape and surface are typically influenced by the drying method and the formulation components of the powder. The spherical shapes were developed during the SFD process involving solution atomization. The process involves the spray of a liquid feedstock into a cryogenic liquid, which instantaneously freezes the droplets into spherical particles (Ishwarya et al., 2015). Similar particles’ morphology was also demonstrated in previous studies that developed mucoadhesive microspheres. This research also employed a spray-drying process, which contributed to the formation of spherical microparticles with rough surfaces (Surini et al., 2009). Previous studies that used high MW sugars also demonstrated spherical particle shapes. Materials comprising sugars with high MW have a low capacity as plasticizers and are, therefore, crucial in forming spherical microparticles (Loksuwan, 2006). As in other studies, the FD process is known to result in rough and porous surfaces (Tian et al., 2018), which is consistent with our findings.
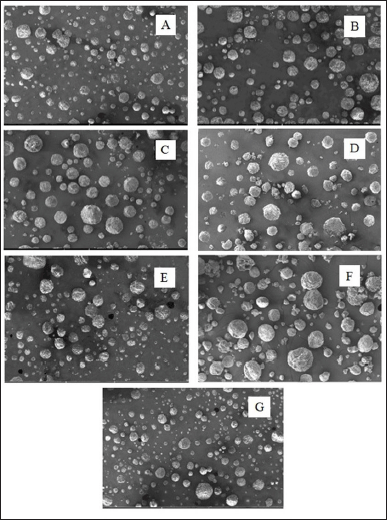 | Figure 1. SEM images of F1 (A), F2 (B), F3 (C), F4 (D), F5 (E), F6 (F), and F7 (G) IDP morphology in 250× magnification. [Click here to view] |
The rough surface characteristics of the resulting IDP powder particles may be attributed to the SFD’s process parameters. During the formulation of IDPs, a solid material concentration of 88 mg/ml was utilized based on the solubility of each constituent (Muntu et al., 2021). The solution’s concentration of solutes may impact the resulting particles’ surface characteristics. High concentrations of solutes can lead to the formation of particles with rougher surfaces, whereas lower concentrations can result in smoother surfaces (Li, 2013). The rapid dehydration, as reported by Al-Muhtaseb et al. (2004), that occurs during the FD is attributed to sublimation and can induce structural stresses in the cellular components of the materials. Moreover, this process leads to shrinkage and the formation of a rougher surface (Al-Muhtaseb et al., 2004).
Additionally, the flow rate of the material solution in the IDP formulation was set at 1 ml/minute based on the results of preliminary testing (Muntu et al., 2021), which required a slow flow rate during the solution spraying process. This rate could potentially influence the freezing rate in the SFD process and the surface characteristics of the resulting powder particles. The freezing conditions during the spray-freeze process may also affect the surface roughness of the particles. Rapid freezing conditions may lead to the formation of particles with smoother surfaces, while slower freezing may result in rougher surfaces (Li, 2013). The resulting rough surface of IDP is consistent with the outcomes of insulin SFD nanosuspension (Schiffter et al., 2010), which also generated a highly porous powder structure with a large, irregular, and rough surface in the SEM photographs.
Thermal properties of SFD IDP
The particles obtained from the SFD process using sugar stabilizers appeared as a fine white powder. As shown in Figure 2 and Table 1, the DSC analysis of IDP revealed endothermic peaks with Tg values ranging from 97.4°C to 117.98°C, requiring thermal energy between −180.70 and −12.42 J/g when heated from 25°C–200°C at a rate of 10°C/minute. The statistical analysis indicated a significant increase in Tg value with an increase in the inulin ratio (p-value = 0.000). The DSC results suggested that formulations combining trehalose and inulin required more thermal energy than trehalose alone (F7). Inulin has a higher Tg value than trehalose, and combining both resulted in higher Tg values and thermal energy (Hinrichs et al., 2001; Mensink et al., 2017; Pouya et al., 2018; Teekamp et al., 2017).
The individual Tg values of insulin and all excipients were measured. As presented in Figure 2, insulin had the lowest Tg value, followed by poloxamer, HPMC, and trehalose, whereas inulin had the highest Tg value. These findings are consistent with previous studies that have also compared the use of polysaccharides and disaccharides. The use of polysaccharides, such as dextran, inulin, and pullulan, resulted in higher Tg values than disaccharides, such as trehalose and sucrose (Hinrichs et al., 2001; Mensink et al., 2017; Teekamp et al., 2017).
The Tg refers to the temperature at which a solid material undergoes a transition from a rigid, glassy state to a more flexible, rubbery state, which can lead to changes in the physical properties of the drug product. The Gordan-Taylor equation is a mathematical equation that elucidates the effect of Tg on the stability of a drug product. This equation is frequently employed to predict the stability of a drug product under different storage conditions. The use of mathematical models, such as the Gordan-Taylor equation, aids in predicting the stability of a drug product under different storage conditions, allowing for the optimization of storage conditions to guarantee long-term stability (Schugmann and Foerst, 2022).
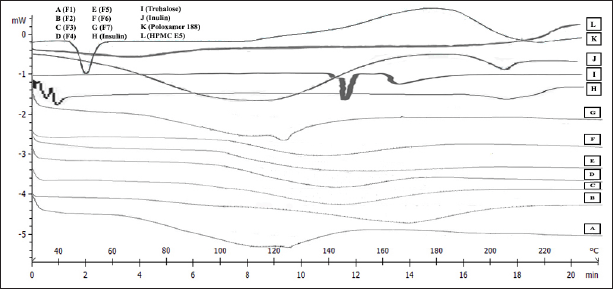 | Figure 2. Endothermic curves of F1 (A), F2 (B), F3 (C), F4 (D), F5 (E), F6 (F), F7 (G) IDP, insulin (H), trehalose (I), inulin (J), poloxamer 188 (K), and HPMC E5 (L). [Click here to view] |
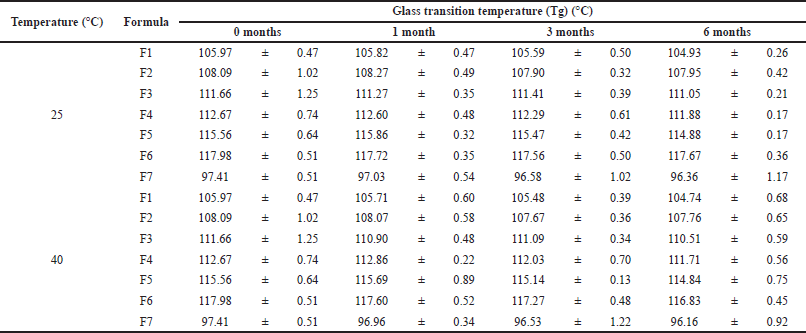 | Table 1. The glass transition temperatures (Tg) (°C) of IDP during stability study for 0, 1, 3, and 6 months at room temperature and 40°C. [Click here to view] |
Based on the above Tg results of the IDPs, IDP F7 obtained the lowest Tg due to the use of single trehalose. According to the Gordon-Taylor equation, the storage of the product should consider the Tg value of the product. All IDPs should be stored at a temperature below the Tg value of the IDPs. The storage of IDPs is planned at room temperature, which is considered suitable because it is significantly lower than the Tg of all IDP formulations.
This is consistent with previous studies using trehalose, dextran, or pullulan in insulin powder formulations. The highest Tg value was obtained with the combination of trehalose and dextran with the highest MW. The MW of a substance can influence its Tg value. The use of dextran with a higher MW resulted in an increased Tg value of the formulated powder. The increase in intermolecular forces between molecules resulting from an increment in a substance’s MW leads to a higher Tg. This phenomenon elevates the degree of rigidity in the amorphous structure of the substance, impeding the mobility of molecules and causing an increase in Tg.
Moreover, higher MW substances tend to form more physical cross-links, further augmenting the degree of rigidity and Tg. Therefore, the relationship between MW and Tg can be explained by the influence of molecular interactions on the physical properties of the substance (Wei and Torkelson, 2020). Conversely, the Tg value showed a decreasing trend with decreasing MW of dextran or in the presence of trehalose in combination. Similar results were found using pullulan, where the highest Tg value was observed in the formulation without the addition of trehalose. The addition of trehalose into pullulan formulations resulted in a decrease in Tg value (Tian et al., 2018). Trehalose, classified as a disaccharide, exhibits a lower Tg value than polysaccharides, such as dextran, inulin, and pullulan. The incorporation of trehalose into the formulation has been shown to contribute to a reduction in the Tg value of the powder (Tonnis et al., 2015).
Crystallinity of SFD IDP
The XRPD diffractogram analysis was conducted on seven different IDP formulations containing trehalose and inulin as stabilizers. The analysis aimed to identify the physical state and crystallinity of IDPs.
As presented in Figure 3, IDP F1-F6 consisting of trehalose and inulin produced an amorphous solid form of the powder. In contrast, IDP F7, solely composed of trehalose, showed a significant increase in intensity for several peaks, indicating the presence of crystal formation. Moreover, a new high-intensity peak was observed in F7, indicating semicrystalline dry powder formation from the use of trehalose as the sole sugar component. The 2θ values of the formulations were analyzed to determine the crystal structure of the samples. The characteristic peaks of powder crystallinity were also examined to identify the degree of crystallinity and the crystal structure of the powders. The 2θ values of the formulations were found to be within the typical range of 3.0°–40.0° for IDP powders. The XRPD patterns of the samples exhibited characteristic peaks indicative of the presence of crystalline structures (Fawcett et al., 2019). The results suggest that the powders had a high degree of crystallinity, with a peak around 20° 2θ, which is a characteristic of insulin.
The identification of the crystalline property of IDP F7, as indicated by the presence of a high-intensity peak, is consistent with a previous study conducted by Hinrichs et al. (2001), who used trehalose as a positive control in a study on cryoprotectants. The crystal growth in the trehalose-stabilized dry powder is believed to be caused by high environmental relative humidity and trehalose hygroscopic characteristics (Wu et al., 2019). Additionally, FD powder preparations with trehalose only as a stabilizer are hygroscopic, resulting in increased water adsorption on the surface, inducing crystal formation because of the amorphous phase separation. The use of crystalline trehalose in this study produces a semicrystalline sugar glass insulin system, while the application of an amorphous inulin and trehalose combination produces an amorphous system, as reported by Xie and Taylor (2016). We used trehalose as a single sugar stabilizer in IDP F7. Trehalose is known to have hygroscopic properties similar to polyvinylpyrrolidone (PVP) used by Xie and Taylor (2016). Both trehalose and PVP exhibit hygroscopic properties, which enable them to adsorb moisture from the surrounding environment. This moisture absorption can result in the formation of crystalline structures within the powder’s system (Xie and Taylor, 2016) or sugar glass systems of dry powder formulations (Teekamp et al., 2017). These findings may have implications for the stability and efficacy of IDP formulations, particularly those intended for nose-to-brain delivery in AD therapy.
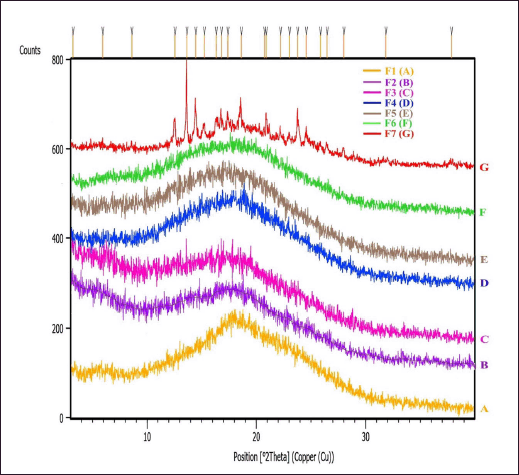 | Figure 3. XRPD diffractogram of F1 (A), F2 (B), F3 (C), F4 (D), F5 (E), F6 (F), and F7 (G) IDP. [Click here to view] |
Moisture content of SFD IDP
The analysis of the moisture content of IDP showed that the moisture content percentages ranged from 2.19% to 3.44%, as shown in Table 2. The percentage decreased to less than 3% in F3, F4, F5, and F6 when higher inulin concentrations were used.
IDP formulation with a high inulin ratio in the powder formula resulted in a significant decrease in moisture content. IDP F6 with the highest weight ratio of inulin exhibited the lowest moisture content. Although all the percentages were different due to trehalose hygroscopic characteristics, all IDP formulations contained a moisture content of less than 3.5%. Dry powder with low moisture content can enhance product stability and quality. These results can improve the flow characteristics of the powder and decrease the risk of aggregation during product storage and distribution, as well as prolong the shelf life of the product. The high inulin concentration in the formulation caused a decrease in moisture content, which is consistent with previous studies on trehalose alone as a positive control in cryoprotectant studies. The Tg value from DSC also supported this finding, as it indicated that the Tg value and hygroscopicity of sugar are inversely proportional (Mensink et al., 2017; Pouya et al., 2018). The dry powder formulations were crystalline in nature due to the high environmental relative humidity and trehalose hygroscopic characteristics. During the formulation process, exposure to high humidity can induce amorphous-amorphous phase separation followed by crystallization in hygroscopic materials, such as trehalose and PVP. These results subsequently trigger the formation of crystalline structures in the powder (Xie and Taylor, 2016).
Based on the physical characterization, the IDP prepared by SFD using trehalose and inulin as stabilizers met the intranasal route specification required for nose-to-brain delivery in AD therapy (Cheng et al., 2001; Kundoor and Dalby, 2011; Xi et al., 2016). First, the IDPs showed spherical shape particles, typically in the microsize range. These results can support efficient deposition in the olfactory region and rapid absorption across the olfactory epithelium (Jeong et al., 2023). Second, the IDP formulation also exhibited stable formulation with high Tg values that can prevent aggregation. This is particularly significant in the development of dry powder formulations for inhalation, as a lower Tg value can enhance the flowability and dispersibility of the powder (Trenkenschuh, 2021). Therefore, the use of a combination of disaccharides with low Tg and polysaccharides with high Tg is expected to enhance delivery efficacy. In this study, the formulation also includes appropriate excipients, such as poloxamer as absorption enhancers and HPMC as mucoadhesive agents. These materials can improve the bioavailability of insulin and enhance its residence time in the nasal cavity (Jeong et al., 2023).
In line with previous research conducted by Friesen et al. (2008) on hydroxypropyl methylcellulose acetate succinate-based spray-dried dispersions, a dry powder formulation was obtained that maintained its amorphous state and did not undergo crystallization during storage at various temperatures. The dry powder obtained in their study exhibited a single amorphous phase, which allowed the drug to remain amorphous and dispersed over practical storage times. Similarly, the IDPs in formulations F1–F6 demonstrated amorphous characteristics, with Tg values significantly higher than the planned storage temperature at room temperature (Friesen et al., 2008).
Insulin content of SFD IDP
The insulin contents in IDP formulations were determined by the HPLC method with a retention time (Rt) of 2.64 minutes for insulin and 5.02 minutes for methylparaben. The insulin contents were calculated using the insulin standard curve with the regression equation of y = 0.0115×–0.6111 (r = 0.999) and the overlay chromatogram of eight insulin contents were shown in Figure 4. The HPLC method used in this study not only determines insulin content but can also detect any degradants that may cause peak broadening due to slight differences in Rt. A relatively stable insulin peak at a consistent Rt indicates it is not interfered with by insulin degradants. Desamido insulin is a common insulin degradant that may appear (Ambarsari et al., 2022). However, during the insulin content testing, we ensured that stability testing was carried out and no desamido insulin or other impurities and degradants were detected. This was confirmed by insulin peaks that shared the same Rt and peak shape (data not presented) as the chromatogram observed in the system suitability testing and insulin standard curve (Fig. 4). All insulin peaks showed consistent Rt, tailing factor, and resolution values, indicating the specificity of the analysis method.
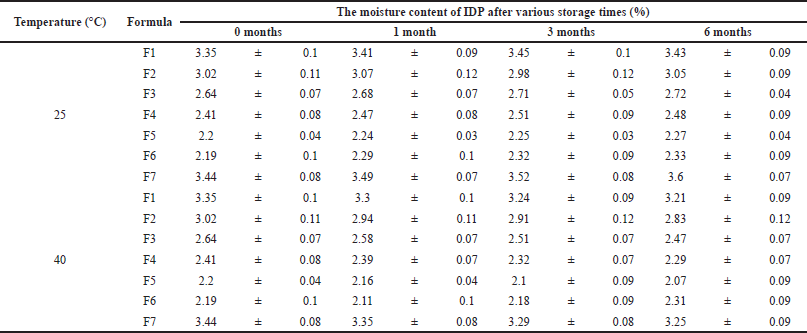 | Table 2. The moisture content of IDP during stability study for 0, 1, 3, and 6 months both at room temperature and 40°C. [Click here to view] |
The results show the differences in the insulin content in IDPs, are within the range of 92.38% ± 0.17% to 101.28% ± 0.75%, as presented in Table 3. The insulin content in IDPs of F1, F2, F3, F4, and F5 was significantly higher than that of F6 and F7. It might be caused by the sugar stabilizer composition in IDP formulations, where F1, F2, F3, F4, and F5 contained trehalose and inulin. Applying these two materials jointly at a specific ratio increases insulin protection during the formulation process due to the environment (Mensink et al., 2017; Pouya et al., 2018; Teekamp et al., 2017). The use of a combination of trehalose and inulin in the IDP formulation resulted in higher Tg values compared to using trehalose alone, as indicated in Table 1. These higher Tg values can protect the conformation of insulin and maintain insulin content during the manufacturing process. This finding is consistent with previous studies that used a combination of trehalose and dextran to formulate reconstituted insulin powder. The use of this combination at a 1:1 weight ratio resulted in higher Tg values and insulin contents compared to using a single sugar (Tonnis et al., 2015).
Stability study of SFD IDP
This report presents a stability study of SFD IDP formulations. The study evaluates the chemical and physical parameters of all IDP formulations, focusing on the insulin content parameter, which was tested on five IDP formulations (F1, F2, F3, F4, and F5). IDP formulations in F6 and F7 were excluded from this testing due to the low insulin content, as the formula contained either only trehalose or inulin. The study employed long-term and accelerated stability testing conditions to assess the stability of IDP formulations over time. In addition to the chemical stability, the physical stability of SFD IDP was also observed at long-term stability storage. The parameters observed in this study included the powder’s morphology, thermal properties, crystallinity, and moisture content. The results of this study are presented and discussed in the following sections.
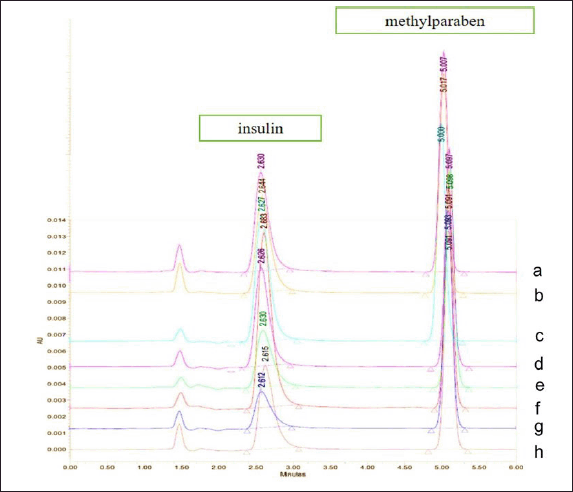 | Figure 4. The overlay of the HPLC chromatogram of insulin in eight different contents using acetonitrile and 0.1% trifluoroacetic acid solution (30:70) as a mobile phase and the C18 column as a stationary phase. [Click here to view] |
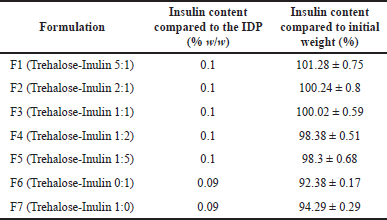 | Table 3. The insulin content of IDP. [Click here to view] |
Long-term stability of SFD IDP
Figure 5a presents the profile of insulin content recovered in IDP F1–F5 at room temperature (25°C). The stability testing conducted during 1, 2, 3, 4, 12, and 24 weeks showed a significant decrease in insulin content in all five IDP formulations compared to the 0 month. However, during 6 months of storage, all IDP formulations maintained a high remaining insulin content ranging from 83.08% ± 0.44% to 97.43% ± 0.07%. Notably, IDP F2 and F3 exhibited the highest remaining insulin content, exceeding 95%. These findings are consistent with the insulin content testing in the IDPs. The higher insulin contents observed in IDPs F2 and F3 are likely attributed to the specific composition of a combination of trehalose and inulin sugars. Inulin contributes to higher Tg values, resulting in improved physical stability and higher insulin content of the IDPs. Additionally, trehalose contributes to sufficiently high Tg values and offers flexibility in encapsulating insulin, leading to improved insulin stability (Tonnis et al., 2015).
A long-term stability study at an elevated temperature of 40°C showed a significant decrease in insulin content for all IDP formulations. After 1 month of storage, the remaining insulin content in the IDPs ranged from 78.5% ± 0.24% to 87.18% ± 0.56%. Moreover, IDP F5 exhibited the lowest remaining insulin content of 71.68% ± 0.11% after 6 months of storage. Conversely, IDPs of F2, F3, and F4 maintained insulin content above 80%, as depicted in Figure 5b. These formulations utilized a specific weight ratio of trehalose and inulin combinations, which protected the conformation of insulin during the storage and formulation process.
The protection provided by the combination of trehalose and inulin in the IDP formulation may impact the remaining insulin content. It could be attributed to the sugar composition and Tg of the formulated IDPs. Trehalose, a disaccharide with a high Tg value, imparted flexibility to coat insulin. Meanwhile, inulin, a polysaccharide with a higher Tg value than trehalose, increased the Tg value of the formulated IDPs and provided adequate physical stability. In addition, inulin has smaller flexible molecules than the other polysaccharides (Furlan et al., 2011; Hinrichs et al., 2005). These results are in line with previous studies, including Tonnis et al. (2015) who used dextran, inulin, and a combination of trehalose and dextran for insulin, HBsAg, lactate dehydrogenase (LDH), and β-galactosidase formulation. In addition, the best storage stability was shown in the formulation using a combination of dextran and trehalose with a weight ratio of 1:1 (Tonnis et al., 2015).
The lower stability of insulin content in IDP F5 was attributed to its low weight ratio of trehalose. Furthermore, it may affect the reduction in insulin content and the flexibility of the sugar glass used to coat insulin. Nevertheless, the stability testing results suggest that the optimal combination of trehalose and inulin, as seen in IDP F2 and F3, can provide high stability and maintain remaining insulin content during long-term storage. Moreover, storing the IDPs in a desiccator and the use of silica gel can protect IDPs from moisture exposure, which can affect the crystallization and stability of protein content.
In addition to insulin content stability, the physical characteristics of IDP were also observed. All SFD IDPs experienced no significant changes in particle morphology after 6 months of storage. They remained stable in this parameter under stability testing storage conditions, including room temperature (25°C ± 2°C) and an elevated temperature of 40°C ± 2°C (data not shown). The SEM analysis showed that the particles maintained spherical shapes with rough surfaces. These align with previous studies using trehalose and raffinose in powder formulations for protein delivery, which led to the stability of the spherical nanoparticles’ shape and surface morphology during 12 weeks of storage at 25°C (Ógáin et al., 2011). The combination of disaccharide and polysaccharide was found to provide good particle morphology properties and stability to IDP formulation.
The thermal stability properties of all IDP formulations were also evaluated under two storage temperatures. The Tg values were measured over a 6-month period and the results showed no significant difference between the Tg values of each formulation, as presented in Table 1. These results suggested that the Tg values of IDP formulations remained stable even when stored at an elevated temperature of 40°C. The combination of trehalose and inulin was found to provide good thermal stability to IDP formulations. These findings are consistent with previous studies that investigated the stability of other proteins in various sugar-based formulations (Mensink et al., 2017; Pouya et al., 2018; Teekamp et al., 2017). Tonnis et al. (2015) also reported that combining dextran and trehalose provided the best storage stability for insulin, HBsAg, LDH, and β-galactosidase formulations.
In contrast, formulations that used a single sugar showed lower stability results. Another previous study has reported on the use of trehalose and raffinose in powder formulations for protein delivery, which resulted in stable Tg values for up to 12 weeks of storage at 25°C (Ógáin et al., 2011). The use of desiccators and silica gel has been shown to enhance the stability of IDP formulations, particularly in high-humidity environments.
Furthermore, a protein’s conformational stability is linked to its Tg value. A higher Tg value implies a more stable system because of the larger resistance in the aggregation-prone conformation. Nevertheless, some studies have reported that polysaccharides with higher Tg values may have lower stability than those with lesser Tg, which could be due to the stabilization properties of smaller flexible molecules. Therefore, the combination of trehalose and inulin provides good physical stability, which is also related to chemical stability (Furlan et al., 2011; Hinrichs et al., 2005).
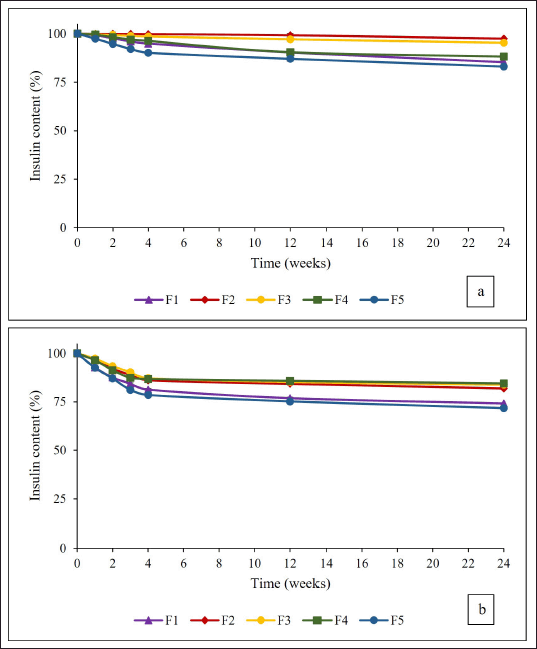 | Figure 5. The stability profile of IDP formulations F1–F5 during 24 weeks of storage at room temperature (a) and elevated temperature of 40°C (b). [Click here to view] |
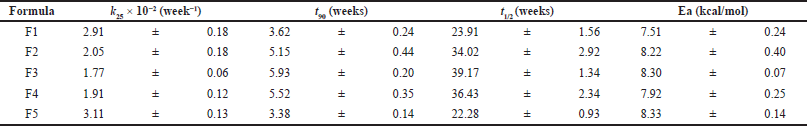 | Table 4. Values of the stability parameters (k25, t90, t1/2 and Ea) for IDP F1–F5 obtained from the accelerated stability study. [Click here to view] |
Consistent with the thermal properties stability, the crystallinity of all IDP formulations exhibited insignificant changes during the 6-month storage period, both at room temperature and elevated temperature of 40°C (data not shown). The XRPD patterns were obtained for the six amorphous insulin formulated with inulin alone or in combination with trehalose (F1–F6), which remained stable over the storage period. The XRPD patterns of these formulations exhibited characteristic broad peaks, indicating their amorphous nature. On the other hand, the XRPD pattern of the F7 formulation, which did not contain inulin, showed sharp peaks in its diffraction pattern, indicating a semicrystalline structure.
These results align with previous studies that have demonstrated the ability of inulin and trehalose to stabilize amorphous forms of insulin and other proteins (Mensink et al., 2017; Pouya et al., 2018; Teekamp et al., 2017). These results also agree with previous research that used trehalose and raffinose in powder formulations for protein delivery, which maintained stability in crystallinity for up to 12 weeks of storage at 25°C (Ógáin et al., 2011). The stability of the amorphous forms of insulin prepared using inulin and trehalose (Muntu et al., 2021) is crucial for developing effective protein delivery systems. The XRPD pattern provides additional evidence for stability.
Moisture content analysis was carried out on IDP formulations after 1, 3, and 6 months of storage, and the results are shown in Table 2. There were no significant differences in moisture content between each formulation during 6 months of storage, indicating that all formulations were stable at room temperature and elevated temperature of 40°C. This stability may be attributed to the use of a desiccator and silica gel to protect against moisture exposure. The moisture content parameter is related to the crystallinity of the powder, which was found to be stable during the stability testing due to the controlled environmental relative humidity (Hinrichs et al., 2001). These proper stability findings are consistent with other studies that have used trehalose and mannitol in a recombinant spray-dried powder formulation. An anti-IgE monoclonal antibody demonstrated protein stability, and aggregation was minimized in trehalose formulations with excipient-to-protein ratios ranging from 300:1 to 500:1. This ratio aligns with the protein-to-stabilizer ratio of 1:1,000, which was utilized to reduce aggregation and crystallization (Andya et al., 2003). Previous studies have indicated that incorporating trehalose and raffinose in powder formulations for protein delivery can result in stable products. These formulations demonstrated no increase in moisture content for up to 12 weeks of storage at 25°C (Ógáin et al., 2011).
Accelerated stability of SFD IDP
IDP samples were subjected to stress stability testing by placing them in an oven at temperatures of 40°C, 50°C, and 60°C for 0, 1, 2, 3, and 4 weeks. These temperature levels were chosen based on the known temperature at which insulin degrades, leading to a decrease in the insulin content. The rate constant of degradation can be determined. The first-order reaction kinetics of insulin degradation was observed, as evidenced by a higher correlation coefficient value than zero and second-order (r > 0.90). The degradation rate constant was determined at each temperature and used to evaluate the degradation rate constant at 25°C (k25) and other stability parameters, as shown in Table 4, which were calculated using the Arrhenius equation (Sinko, 2016). The k25 values ranged from 1.77 × 10−2 to 3.11 × 10−2 week−1, with the lowest value obtained from IDP F3 followed by F4, F2, and F1, and the highest value from F5. These values are inversely proportional to the half-life and shelf life of the samples, with the shortest values being observed from F5, followed by F1, F2, F4, and F3. The half-life of F1–F5 ranged from 22.28 to 39.17 weeks, while the shelf life ranged from 3.38 to 5.93 weeks. The stability parameters showed that F3 is the best formulation with the lowest k25 value, most prolonged half-life, and shelf life. This finding is consistent with the stability study conducted at room temperature, which showed that F3 maintained a remaining insulin content of over 95% after 6 months of storage. The optimal stability parameters of F3 can be attributed to the maximal protection provided by the trehalose and inulin combination in the sugar glass system.
The results reveal IDP combined with trehalose and inulin stabilizers showing a significant increase in stability. The stability of insulin in liquid form has been reported in previous studies conducted by Okimura et al. (2017). This study reported that the degradation rate constant of liquid insulin at 22°C (k22) was 0.00619/hour. The k25 values of IDP formulations were significantly lower compared to the k22 of liquid insulin. IDP F5 had the highest k25 value among the five formulations, but it was still 33 times lower than the reported k22 value of liquid insulin.
On the other hand, IDP F3 exhibited the lowest k25 value, which was 58 times lower than the k22 value of liquid insulin. These results suggest that the combination of trehalose and inulin in the dry powder sugar glass system offers potential protection for the chemical conformation of insulin (Mensink et al., 2017; Pouya et al., 2018; Teekamp et al., 2017). This is supported by a previous study that investigated dextran, inulin, and a combination of trehalose and dextran in insulin, HBsAg, LDH, and β-galactosidase formulations. The study found that the best storage stability was achieved with the dextran and trehalose combination, with other formulations using a single sugar exhibiting lower stability (Tonnis et al., 2015). The combined protection approach has potential benefits for increasing the stability of insulin in dry powder form.
This study showed that IDP formulations containing trehalose and inulin stabilizers could significantly increase the stability of insulin in dry powder form. Insulin’s chemical stability is affected by various factors, such as temperature, pH, and humidity. Exposure to high temperatures can cause insulin to denature, leading to decreased insulin content. Insulin is more stable in dry states than in solutions because the hydrogen bonds in aqueous solutions promote the formation of dimers or oligomers, making it difficult for insulin to dissolve and reducing its absorption due to the larger molecular size. This denaturation of insulin at high temperatures has been previously documented by Sweetman (2009), while studies by Avanti et al. (2014) and Date et al. (2016) have also highlighted the importance of these factors on insulin stability.
CONCLUSION
A stable IDP prepared using a SFD method has been developed for nose-to-brain delivery in AD treatment. IDP F3, containing trehalose and inulin in a 1:1 weight ratio, was the most stable formulation and had proper stability for 6 months, including insulin content, particle morphology, transition glass temperature (Tg), crystallinity, and moisture content. Additionally, the degradation rate constant (k25), half-life, and shelf life of IDP F3 were determined to be 1.77 × 10−2 week−1, 39.17, and 5.93 weeks, respectively. These findings suggest that IDP F3 may offer prospective benefits for storing and delivering insulin in dry powder form. These results also indicate that IDP F3 is a promising formulation for nose-to-brain delivery in AD treatment. However, further studies are necessary to evaluate the efficacy and safety of IDP F3 in vivo.
AUTHORS’ CONTRIBUTIONS
Cynthia Marisca Muntu conceptualized and designed the study, conducted laboratory experiments, collected and analyzed data, interpreted the results, and drafted and critically revised the manuscript. Christina Avanti and Hayun contributed to the data analysis and interpretation and provided critical feedback on the manuscript. Silvia Surini supervised the study, provided guidance on data interpretation, critically revised the manuscript, and gave final approval for submission.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support provided by the PUTI Grant (contract number NKB-5/UN2.RST/HKP.05.00/2020) from Universitas Indonesia for this study. Additionally, the authors thank Dr. Wouter L.J. Hinrichs from the Groningen Research Institute of Pharmacy at the University of Groningen, the Netherlands, for his valuable comments and insightful discussions on the methodology.
CONFLICTS OF INTEREST
There are no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Akintola AA, Van Heemst D. Insulin, aging, and the brain: mechanisms and implications. Front Endocrinol (Lausanne), 2015; 6:13.
Al-Muhtaseb AH, McMinn WAM, Magee TRA. Shrinkage, density and porosity variations during the convective potato starch gel. In: In: Proceeding of the 14th International Drying Symposium (IDS), São Paulo, Brazil, 2004, pp 1604–11.
Ambarsari C, Suryadi H, Yanuar A. Development of analysis and validation of human insulin-containing matrices using reverse phase-liquid chromatography. J Penelit Pendidik IPA, 2022; 8(5):2195–202.
Andya JD, Hsu CC, Shire SJ. Mechanism of aggregate formation and carbohydrate excipient stabilization of lyophilized humanized monoclonal antibody formulations. AAPS PharmSci, 2003; 5(2):21–35.
Avanti C, Saluja V, Streun ELP, Frijlink HW, Hinrichs WLJ. Stability of lysozyme in aqueous extremolyte solutions during heat shock and accelerated thermal conditions. PLoS One, 2014; 9(1):1–6.
Bahendeka S, Kaushik R, Swai AB, Otieno F, Bajaj S, Kalra S, Bavuma CM, Karigire C. EADSG guidelines?: insulin storage and optimisation of injection technique in diabetes management. Diabetes Ther, 2019; 10(2):341–66; doi: 10.1007/s13300-019-0574-x.
Cheng YS, Holmes TD, Gao J, Guilmette RA, Li S, Surakitbanharn Y. Characterization of nasal spray pumps and deposition pattern in a replica of the human nasal airway. J Aerosol Med, 2001; 14(2):267–80.
Date AA, Hanes J, Ensign LM. Nanoparticles for oral delivery: design, evaluation, and state-of-the-art. J Control Release, 2016; 240:504–26.
Eggerstedt SN, Dietzel M, Sommerfeld M, Süverkrüp R, Lamprecht A. Protein spheres prepared by drop jet freeze-drying. Int J Pharm, 2012; 438(1–2):160–6.
Fawcett T, Gates-Rector S, Gindhart A, Rost M, Kabekkodu S, Blanton J, Blanton T. A practical guide to pharmaceutical analyses using X-ray powder diffraction. Powder Diffr, 2019; 34(2):164–83.
FDA. 2019. Available via https://www.fda.gov/ (Accessed 06 June 2021)
Friesen DT, Shanker R, Crew M, Smithey DT, Curatolo WJ, Nightingale JAS. Hydroxypropyl methylcellulose acetate succinate-based spray-dried dispersions: an overview. Mol Pharm, 2008; 5(6):1003–19.
Furlan LTR, Lecot J, Padilla AP, Campderros ME, Zaritzky N. Effect of saccharides on glass transition temperatures of frozen and freeze-dried bovine plasma protein. J Food Eng, 2011; 106(1):74–9.
Goldberg T, Wong E. Afrezza (insulin human) inhalation powder: a new inhaled insulin for the management of type-1 or type-2 diabetes mellitus. Pharm Ther, 2015; 40(11):735–41.
Heinemann L, Braune K, Carter A, Zayani A, Kramer LA. Insulin storage: a critical reappraisal. J Diabetes Sci Technol, 2021; 15(1):147–59.
Hinrichs WLJ, Prinsen MG, Frijlink HW. Inulin glasses for the stabilization of therapeutic proteins. Int J Pharm, 2001; 215(1–2):163–74.
Hinrichs WLJ, Sanders NN, De Smedt SC, Demeester J, Frijlink HW. Inulin is a promising cryo- and lyoprotectant for PEGylated lipoplexes. J Control Release, 2005; 103(2):465–79.
Ishwarya SP, Anandharamakrishnan C, Stapley AG. Spray freeze-drying: a novel process for the drying of foods and bio-products. Trends Food Sci Technol, 2015; 41(2):161–81.
Jeong SH, Jang JH, Lee YB. Drug delivery to the brain via the nasal route of administration: exploration of key targets and major consideration factors. J Pharm Investig, 2023; 53:119–52.
Kamei N, Takeda-morishita M. Brain delivery of insulin boosted by intranasal coadministration with cell-penetrating peptides. J Control Release, 2015; 197:105–10.
Kellar D, Lockhart SN, Aisen P, Raman R, Rissman RA, Brewer J, Craft S. Intranasal insulin reduces white matter hyperintensity progression in association with improvements in cognition and CSF biomarker profiles in mild cognitive impairment and alzheimer’s disease. J Prev Alzheimer Dis, 2021; 8:240–8.
Kern W, Peters A, Fruehwald-Schultes B. Improving influence of insulin on cognitive functions in humans. Neuroendocrinology, 2001; 74(4):270–80.
Kullmann S, Heni M, Hallschmid M. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev, 2016; 96(4):1169–209.
Kundoor V, Dalby RN. Effect of formulation-and administration-related variables on deposition pattern of nasal spray pumps evaluated using a nasal cast. Pharm Res, 2011; 28(8):1895–904.
Li Z. Morphology of particle produced by spray-freeze drying. Huagong Jinzhan, 2013; 32:270–5.
Loksuwan J. Characteristic of microencapsulated β-carotene formed by spray drying with modified tapioca starch, native tapioca starch and maltodextrin. Food Hydrocoll, 2006; 21:928–35.
Mensink MA, Frijlink HW, Maarschalk KV, Hinrichs WLJ. How sugars protect proteins in the solid state and during drying (review): mechanisms of stabilization in relation to stress conditions. Eur J Pharm Biopharm, 2017; 114:288–95.
Muntu CM, Surini S, Avanti C, Hayun. Preliminary study of insulin dry powder formulation: critical process parameters on spray-freeze-drying and critical material attributes of trehalose and inulin as stabilizers. Int J Appl Pharm, 2021; 13(Special Issue 4):83–8.
Ógáin ON, Li J, Tajber L, Corrigan OI, Healy AM. Particle engineering of materials for oral inhalation by dry powder inhalers I-particles of sugar excipients (trehalose and raffinose) for protein delivery. Int J Pharm, 2011; 405(1–2):23–35.
Okimura K, Yamada J, Miyamoto E. Stability of insulin in saline containing sodium hydrogen sulfite. Jpn J Pharm Health Care Sci, 2017; 43(5):267?72.
Pouya MA, Daneshmand B, Aghababaie S, Faghihi H, Vatanara A. Spray-freeze drying: a suitable method for aerosol delivery of antibodies in the presence of trehalose and cyclodextrins. AAPS PharmSciTech, 2018; 19(5):2247–54.
Schiffter H, Condliffe J, Vonhoff S. Spray-freeze-drying of nanosuspensions: the manufacture of insulin particles for needle-free ballistic powder delivery. J R Soc Interface, 2010; 7(Suppl 4):483–500.
Schugmann M, Foerst P. Systematic investigation on the glass transition temperature of binary and ternary sugar mixtures and the applicability of Gordon–Taylor and Couchman–Karasz equation. Foods, 2022; 11(12):1679.
Sinko PJ. Martin’s physical pharmacy and pharmaceutical sciences. Wolters Kluwer, Philadelphia, PA, 2016.
Surini S, Akiyama H, Morishita M, Nagai T, Takayama K. Release phenomena of insulin from an implantable device composed of a polyion complex of chitosan and sodium hyaluronate. J Control Release, 2003; 90(3):291–301.
Surini S, Anggriani V, Anwar E. Study of mucoadhesive microspheres based on pregelatinized cassava starch succinate as a new carrier for drug delivery. J Med Sci, 2009; 9(6):249–56.
Sweetman SC. Martindale the complete drug reference. 36th edition, Pharmaceutical Press, New York, NY, 2009.
Teekamp N, Tian Y, Visser JC, Olinga P, Frijlink HW, Woerdenbag HJ, Hinrichs WLJ. The addition of pullulan to trehalose glasses improves the stability of β—galactosidase at high moisture conditions. Carbohydr Polym, 2017; 176:374–80.
Tian Y, Visser JC, Klever JS, Woerdenbag HJ, Frijlink HW, Hinrichs WLJ. Orodispersible films based on blends of trehalose and pullulan for protein delivery. Eur J Pharm Biopharm, 2018; 133:104–11.
Tonnis WF, Mensink MA, de Jager A, Maarschalk KV, Frijlink HW, Hinrichs WLJ. Size and molecular flexibility of sugars determine the storage stability of freeze-dried proteins. Mol Pharm, 2015; 12(3):684–94.
Trenkenschuh E. Freeze-drying of nanoparticles: impact of particle properties on formulation and process development. Dissertation, Faculty of Chemistry and Pharmacy, LMU München, München, Germany, 2021.
Watson GS, Peskind ER, Asthana S. Insulin increases CSF Abeta42 levels in normal older adults. Neurology, 2003; 60(12):1899–903.
Wei T, Torkelson JM. Molecular weight dependence of the glass transition temperature (Tg)-confinement effect in well-dispersed poly(2-vinyl pyridine)–silica nanocomposites: comparison of interfacial layer Tg and matrix Tg. Macromolecules, 2020; 53(19):8725–36.
Wu F, Wang N, Pang S, Zhang Y. Hygroscopic behavior and fractional crystallization of mixed (NH4)2SO4/glutaric acid aerosols by vacuum FTIR. Spectrochim Acta A, 2019; 208:255–61.
Xi J, Yuan JE, Alshaiba M, Cheng D, Firlit Z, Johnson A, Nolan A, Su WC. Design and testing of electric-guided delivery of charged particles to the olfactory region: experimental and numerical studies. Curr Drug Deliv, 2016; 13(2):265–74.
Xie T, Taylor LS. Effect of temperature and moisture on the physical stability of binary and ternary amorphous solid dispersions of celecoxib. J Pharm Sci, 2016; 106(1):100–10.