INTRODUCTION
In the last few decades, we have seen a drastic change in implant dentistry. The global dental implant market is expected to reach US$13 billion in 2023 [1]. Dental implants have become essential in modern dental practice, with significant advancements such as single-stage flapless (FL) implant placement procedures. These techniques have revolutionized implant placement surgery, improved outcomes, and streamlined treatment approaches [2]. Traditionally, dental implants were placed by elevating the flap for better visualization of the implant site and a better view of anatomical landmarks like the mental foramen, the incisive canal, and local buccal and lingual concavities. It has always helped reduce the risk of bone perforations and penetrating anatomical landmarks [3,4]. However, it had a disadvantage at the time when bones were in limited amounts. The flap elevation often leads to the loss of crestal bone, exposing the implant thread, which influences stress and strain outcomes in the adjacent bone [5]. Flap elevation often tends to tear the flap, leading to flap necrosis due to decreased blood supply, delayed healing, and potential soft tissue scarring.
In recent years, clinicians developed the concept of FL implant surgery to prevent these complications. Patients with sufficient bone volume and gingival tissue that has undergone keratinization have been advised to use this technique [6]. With this method, there is no flap elevation, and the implant is placed straight through the alveolar mucosa. The FL technique’s primary advantage is that it minimizes the risk of postoperative tissue loss [7] and to get relief from soft tissue management after implant placement [8,9]. The other benefit includes less traumatic surgery [10], decreased duration of surgery [2], rapid healing, very few complications, and improved patient acceptance [11]. The FL technique has the advantage of maintaining the blood supply at the bone-periosteum interface, preserving the three-dimensional architecture of both hard and soft tissues surrounding the initial defect. A significant disadvantage of this technique is that the underlying topography of the bone is not visible, leading to an increased risk of perforations (Fig. 1).
The thickness of the gingiva tends to vary across individuals and within the same individual in different parts of the mouth [12]. While a biotype is a unique genetic feature, a phenotype is a complex confluence of genetic and environmental determinants and is site-specific [13]. Implant success depends on many factors but grossly depends on surgical intervention, prosthetic rehabilitation, and hygiene maintenance of dental implants [14]. Numerous research have been conducted to determine the difference between a natural tooth and an implant regarding the gingival biotype [15,16].
Clinicians have been trying hard to determine if the placement of implants by the FL technique influences the longevity of oral implants. The choice between flap and FL is difficult due to a lack of literary evidence. Therefore, the purpose of the present study was to evaluate the impact of the FL implant placement technique compared to traditional flap elevation on gingival biotype, tissue preservation, and overall surgical outcomes.
MATERIALS AND METHODS
Participant selection
The current split-mouth study was conceptualized as a prospective study with forty implants placed by both FL and flap implant surgical techniques. Before this study, a pilot study was conducted with a sample size of five patients, which aimed to recognize the limitations in generalizability and statistical power due to the smaller number size. The earlier study focused on assessing the feasibility of FL dental implants and using it as an exploratory study approach driven by the need to generate hypotheses and gain insights into this novel procedure [7]. This study was carried out after receiving ethical approval from the implant specialty center’s institutional review board at Sewa Charitable Hospital in Udaipur, India (Reference No.: SCHU/IEC/2020/04, Dated August 11, 2020). This study was carried out as per the declaration of Helsinki. Informed consent was obtained from the participants. The primary research was preceded by a pilot where five participants per group were recruited by an independent investigator unaware of the treatment procedure and outcome. Randomization was undertaken by the coin toss method. The sample size calculation using G*Power Version 3.1, keeping the power of study minimum of 80%, was done. Based on the analysis, 20 patients were required to be included per group. Patients were followed at baseline, 3 months, 6 months, and 1 year.
The enrolled patients were aged between 18 and 40 years, with bilateral absence of mandibular first molar. The minimum crestal bone width of 5 mm and vertical bone height from bone crest to the top of mandibular canal 10–12 mm or greater, with adequately healed and remodeled ridge with at least 1.5 mm apical-coronal width of attached nonmobile, preferably keratinized soft tissue. The absence of supra-eruption of opposing teeth and periodontal problems in adjacent teeth were included in the study.
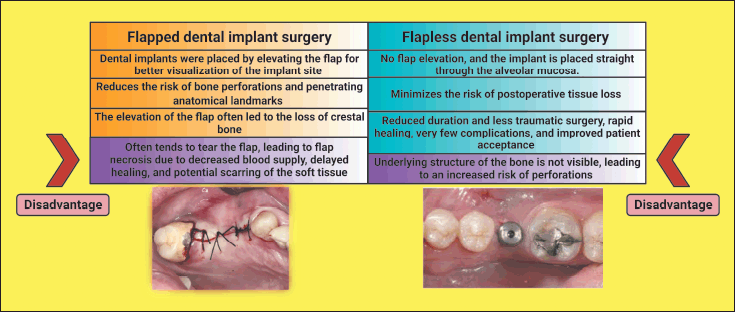 | Figure 1. Chart showing the comparison between flapped and flapless implant surgery. This figure has been drawn utilizing the premium version of BioRender with the License number KA256LFY6Y. Image Credit: Susmita Sinha. [Click here to view] |
Participants with insufficient bone volume, type 4 bone, surgical sites requiring bone augmentation, participants with poor oral hygiene, and smokers were excluded from the study. The study also excluded medically compromised patients suffering from systemic disorders.
Surgical technique
Before surgery, all patients were requested to rinse their mouths with 0.2% chlorhexidine. The face of the patient was disinfected with 7.5% povidone-iodine. The oral cavity was prepped with 5% povidone-iodine, and the patient was draped according to conventional surgical guidelines. Local anesthetic with epinephrine (Xylocaine, Astra Zeneca, London, UK) was used to block the regional nerve supply and aid in hemostasis. To expose the mandibular bone, a full-thickness envelope flap was lifted both buccally and lingually with a no. 9 periosteal elevator, and necessary precautions were taken to avoid damaging the flap. The surgical stent was put on after adequate exposure to the crestal bone (Fig. 2). A crestal mini-incision, approximately 5 mm horizontally with the alveolar crest, was made in the center of the implant site for the FL procedure. The local gingiva was exposed to a depth of around 6 mm, within the range of a big-diameter implant [17–21].
Procedure for implant placement
A no. 6 round bur was used to create a pilot hole. The pilot drill was used to prepare the center of the implant site for the initial depth of bone preparation for the implant length. The osteotomy was designed in the prescribed drill sequence (Osstem™, Seoul, South Korea). Following drilling, the crestal heights of the face and lingual plates were evaluated using osteotomy depth and mucosal thickness surrounding the crest. The probe was then gently introduced into the osteotomy walls to determine if the cortical plate had been perforated and if any soft tissue debris remained in the prepared location. The implants were then placed 2 mm below the bone’s crest. Following the installation of the cover screws, the incised wounds were sutured with a single Silk 4-0 suture. Dental implants were manually put in both groups using a wrench, and postoperative radiographs were collected.
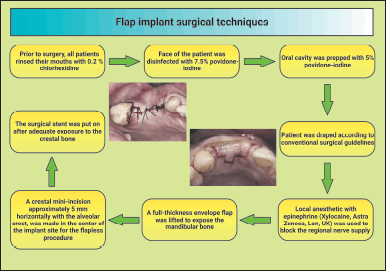 | Figure 2. Shematic diagram showing the flap implant surgical techniques. This figure has been drawn utilizing the premium version of BioRender with the License number TD25NYVSSI. Image Credit: Susmita Sinha. [Click here to view] |
Postoperative care
The patient has been prescribed an antibiotic regimen of amoxicillin 500 mg thrice daily and an analgesic of 400 mg. Additionally, the patient was instructed to rinse twice daily with 0.2 percent chlorhexidine for 2 weeks and to resume regular brushing 1 week after surgery. The patient was encouraged to practice good dental hygiene during the healing process. Three days after the implantation surgery, patients were examined for a check-up to assess postoperative pain and swelling and to monitor painkiller use. After 7 days, patients were reviewed for a second check-up, during which sutures were removed, and oral hygiene instructions were given. A 1-month and 3-month follow-up was conducted.
Clinical parametric evaluation
Soft tissue thickness (STT)
A modified caliper was used to record the STT. The examiners were calibrated, so the gingival tissue thickness was directly measured without undue pressure to the gingiva at approximately 2 mm apical to the free gingival margin on the mid-facial aspect. This location was chosen because it is usually still in the keratinized zone, and the measurement is unlikely to be obstructed by the facial bone level. One of the two examiners held the modified caliper during the measurement, and the gingival thickness was recorded to the nearest 0.1 mm. The measurements were made until two duplicate values were registered and recorded. The gingival biotype was considered thin if the measurement was ≤ 1.0 mm and thick if it measured >1.0 mm [22]. This parameter was recorded at baseline, 3 months, 6 months, and 1 year.
Buccolingual width (BLW)
Measuring the BLW of the alveolar ridge is critical for presurgical implant placement diagnosis. The precise buccolingual dimension will ensure that the diameter of the implant will not exceed the dimensions of the alveolar bone. The ridge-mapping technique involves a series of measurements with a specially designed caliper. The sharp points of the caliper penetrate the anesthetized mucosa until the surface of the bone is reached. A millimeter scale near the handle end of the caliper will give an accurate reading of ridge thickness.
Ridge mapping necessitates three measurements taken at each implant site: one at the level of the ridge crest, near where the implant’s center and apex would be positioned. This parameter was recorded at baseline, 3 months, 6 months, and 1 year.
Statistical analysis
The present study was conducted by placing forty dental implants in twenty patients (n = 20) in the first molar area of the mandible using both flapped and FL implant surgical procedures prepared. STATA (Version 15) tabulated and statistically analyzed the results. The mean and standard deviation of the data were provided. Repeated measure analysis of variance was conducted for inter-group comparisons at baseline, 3 months, 6 months, and 1 year, with 95% confidence intervals. We also used a multivariate regression model to evaluate the between-group differences. A p-value was considered significant when it was less than 0.05.
RESULTS
Demography data
The study collected the data of twenty patients from the population, 65% males and 35% females, with a mean age of 32.3 ± 6.04 years (18–40 years).
Quantitative analysis
Soft tissue thickness
The STT showed a time-dependent increase from baseline to 6 months (p < 0.011) and then to 1 year in both FL and flapped groups (p < 0.002) (Fig. 3). The baseline to 3 months (p < 0.042), 6 months (p < 0.018), and 1-year (p < 0.002) comparisons significantly increased in either group (Fig. 1). However, the mean value of STT at 1 year was significantly (p < 0.001) higher in the flapped group than in the FL group (Table 1).
Intergroup comparative analysis
When comparisons were made for STT between FL and flapped groups, it was observed that there was a time-dependent increase in the thickness in both groups. A significant increase was seen in the flapped group compared to the FL group at baseline, 6 months, and 1 year (Fig. 3 and Table 1).
Data were presented as mean ± SD. The multivariate regression model was used to estimate the p-value. Age and sex were adjusted in the regression model.
Buccolingual width
There was a time-dependent decrease in the BLW of the ridge. The BLW significantly reduced between baseline and 3 months (p < 0.042), with 6 months (p < 0.001), and with one year (p < 0.001) within flapless and flapped groups. However, the reduction was more pronounced in the flapped than in the FL group (Fig. 4).
Data were presented as mean ± SD. A multivariate regression model was used to estimate the p-value. Age and sex were adjusted in the regression model.
Further, a time-dependent decrease was observed in BLW in both flapped and FL groups. A significant reduction was observed between the two groups at 6 months (p = 0.040) and 1 year (p = 0.050) (Table 2 and Fig. 3). However, intergroup quantitative analysis for baseline and 3 months showed a non-significant (p > 0.10) decrease in BLW.
DISCUSSION
Advances in oral implantology have led to more conservative and less traumatic surgical techniques, particularly in flap design [23]. The mucogingival flap design has revolutionized implant surgery, improving patient comfort, minimizing trauma, and preserving bone loss.
Although flap-based dental implantation provides better visualization during surgery, it has certain drawbacks [2,8,9]. One significant disadvantage is the potential for bone resorption, particularly in poorly vascularized crestal bone. When the soft tissue is elevated, the blood supply to the crestal bone decreases, increasing resorption [2,24–27]. Some studies have suggested that flap elevation can stimulate healing and bone resorption [23,28,29].
On the other hand, the FL technique has gained recognition as a minimally traumatic approach with minimal crestal bone resorption and positive esthetic outcomes. This technique offers several advantages over traditional methods, including reduced complications, pain, swelling, bleeding, surgical time, and preservation of tissues and blood supply. These benefits improve patient satisfaction, comfort, and faster recovery [2].
According to consensus, peri-implant STT is crucial for implant health and aesthetic, masticatory, and hygiene outcomes. Increasing and/or maintaining gingival thickness around implants improves the final implant-supported prosthesis. This is frequently taken to minimize or nullify the influence of the shades of the abutment (such as titanium alloy, gold, or zirconia) on the buccal aspect of the mucosa [30–35]. In addition, the research illustrates how the gingival biotype compensates for any underlying bone deficits caused by negative osseous remodeling patterns before or after functional loading.
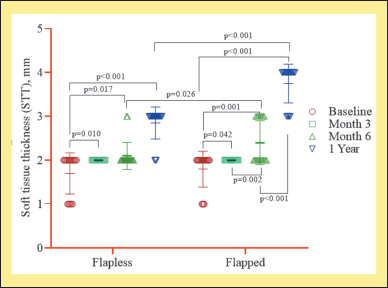 | Figure 3. Intragroup quantitative evaluation of STT between FL and flapped groups (n = 20). This figure has been drawn utilizing the premium version of BioRender with the License number KA256LFY6Y. Image Credit: Susmita Sinha. [Click here to view] |
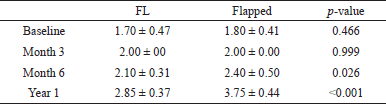 | Table 1. Intergroup (STT) difference between FL (Group 1) and flapped groups (Group 2). [Click here to view] |
Although most studies in this area have focused on the effect of mucosal thickness augmentation for aesthetic purposes, multiple studies found that performing soft tissue grafting procedures for mucosal thickness gain resulted in significantly less interproximal marginal bone loss over time [36,37]. This study also reiterates the concept of preserving STT. There is no consensus regarding the minimum acceptable mucosal thickness needed to minimize marginal bone loss and mucosal recession, create predictable long-term functional and aesthetic outcomes, and achieve these objectives. Preserving STT is essential to achieve optimal functioning and aesthetic outcomes in dental surgery, especially in cases where implants are used. When the soft tissue surrounding an implant is thin, there is a higher risk of marginal bone loss and mucosal recession, leading to complications such as implant failure or an unattractive smile. Therefore, preserving STT is critical for the long-term success of dental implant procedures. However, a significant number of scientific researches on this topic found that the mucosal effect of the abutment shadow was reduced in areas with a minimum mucosal thickness of around 2 mm [22,30,36].
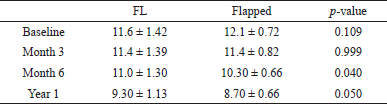 | Table 2. Intergroup evaluation of BLW for FL (Group 1) and flapped groups (Group 2). [Click here to view] |
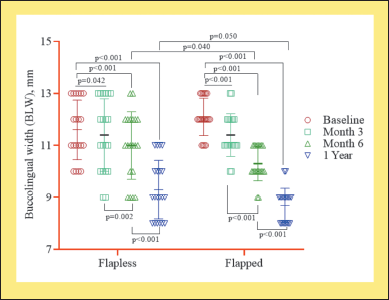 | Figure 4. Quantitative evaluation of BLW between FL and flapped group (n = 20). [Click here to view] |
The present study showed a time-dependent increase in the thickness of the soft tissue in both groups, but the consistency was more prominent in the flapped group. This finding indicates the undergoing healing process and could imply that using a flap during the surgical procedure may have provided some additional benefits to the healing process. This result aligns with the findings of several other studies [24,25,38]. This increase in thickness in flapped surgery can be attributed to the laceration caused by the underlying mucoperiosteal flap. Once the laceration occurs, the polymorphonuclear neutrophils and macrophages rush to the damaged site. Fibroblasts and fibroblast-like cells are the most predominant reparative cells that migrate to the surgical site. Fibroblast migration in the extracellular matrix depends on precise recognition and interaction with specific components of the matrix. Collagen synthesis takes place, which leads to the formation of granulation tissue. As time passes by, these granulation tissues mature and form fibrous tissue. This hyperinflammatory reaction is thought to be the reason for the increased STT in flapped surgery [39].
The mucosal thickness also depends on the BLW or peri-implant bone thickness, which is the horizontal dimension of the anatomical bone. Not just the soft tissue over the bone but the underlying bone is a vital parameter ?for correctly evaluating surgical, esthetic, and prosthetic outcomes. Coronal bone remodeling consisted entirely of a buccolingual constriction of the crestal ridge. New bone apposition to fill the peri-implant defect and buccal and lingual bone resorption could be responsible for the pattern of bone rearrangement. This remodeling decreased the width of the alveolar ridge and existed around all examined implants. However, the delayed implants had smaller buccolingual bone widths when the initial measurements were recorded [40]. There has been sparse literature evidence in which the dimension of the buccolingual aspect of the bone pre- and post-implant has been evaluated. There are some studies indicating the changes in the buccolingual dimension of bone following the extraction of a tooth. The subsequent healing of the socket and the bone growth within the tooth socket would eventually become synchronized with the resorption of the alveolar ridge. The buccal side would show more obvious bone loss in horizontal and vertical directions, making the ridge shorter and narrower [21,40]. Multiple studies suggested that socket remodeling had a more enormous impact in the horizontal direction than in the vertical [30,41,42]. In their investigation, extraction sockets lost significantly more breadth than height during the healing process. Consistent with earlier studies, the current data support that the essential components necessary to induce bone healing are primary implant stability, bony walls capable of sustaining a solid blood clot, and primary flap closure [43]. This study also stated a time-dependent decrease in the BLW of the ridge in both groups. The 6-month intergroup comparison showed a significant reduction, whereas it was less critical at the end of a year. This suggests that there was some level of bone resorption or loss occurring in both groups over time. The difference between the groups became less significant at the end of a year, which may indicate some natural bone remodeling or regeneration occurring over time.
Another study suggested criteria to evaluate the success of implants. As per this criterion, an annual bone loss should be less than 0.2 mm after the implant becomes functional. This criterion is necessary for long-term implant success [44,45]. Since the crestal bone present has become the primary criterion for evaluating implants’ success, it has become essential to save every bit of it. Nevertheless, the buccolingual dimension of the bone is paramount; hence, any changes recorded after the placement of implants will give clinicians logical prognostic attributes. This parametric evaluation of the BLW in the initial years of implants has shown a positive correlation between flap elevation and gingival recession. Hence, moving to the FL technique was needed to preserve crestal bone.
CONCLUSION
In conclusion, the evolution of oral implantology has brought about advancements in flap design and surgical techniques to improve patient comfort, reduce trauma, and preserve bone loss. Although it provides better surgical field visualization, the mucogingival flap technique has disadvantages, such as bone resorption due to the poorly vascularized crestal bone. On the other hand, the FL technique has emerged as a minimal traumatic approach, resulting in reduced bone resorption and improved esthetic outcomes.
Preserving STT around implants has been recognized as crucial for implant health and aesthetic outcomes. Studies have shown that increasing or maintaining gingival thickness can minimize the influence of abutment shadows on the buccal aspect of the mucosa and reduce interproximal marginal bone loss. The FL technique has been found to contribute to the increase in STT during the healing process, possibly due to the hyperinflammatory reaction and granulation tissue formation.
Furthermore, evaluating the buccolingual dimension of the bone is essential for assessing surgical, esthetic, and prosthetic outcomes. Changes in the BLW of the ridge over time have been observed, emphasizing the need for bone preservation techniques. The long-term success of dental implant procedures relies on preserving crestal bone, as indicated by the implant successcriteria, including minimal annual bone loss.
Overall, advancements in flap design, soft tissue preservation, and bone maintenance techniques have significantly improved dental implant procedures’ functional and esthetic outcomes.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This study obtain no finnacial support.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study was carried out after receiving ethical approval from the implant specialty center’s institutional review board at Sewa Charitable Hospital in Udaipur, India (Reference No.: SCHU/IEC/2020/04, dated August 11, 2020).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral about jurisdictional claims in published institutional affiliation.
REFERENCES
1. Alghamdi HS, Jansen JA. The development and future of dental implants. Dent Mater J. 2020;39(2):167–72. doi: https://doi.org/10.4012/dmj.2019-140
2. Divakar TK, Gidean Arularasan S, Baskaran M, Packiaraj I, Dhineksh Kumar N. Clinical evaluation of placement of implant by flapless technique over conventional flap technique. J Maxillofac Oral Surg. 2020;19(1):74–84. doi: https://doi.org/10.1007/s12663-019-01218-9
3. Gnigou M, Goutzanis L, Sarivalasis S, Petsinis V. Retrieval of displaced implants inside the maxillary sinus: two case reports and a short review. Int J Implant Dent. 2019;5(1):24. doi: https://doi.org/10.1186/s40729-019-0173-7
4. Kageyama I, Maeda S, Takezawa K. Importance of anatomy in dental implant surgery. J Oral Biosci. 2021;63(2):142–52. doi: https://doi.org/10.1016/j.job.2021.01.002
5. Hudieb MI, Wakabayashi N, Abu-Hammad OA, Kasugai S. Biomechanical effect of an exposed dental implant’s first thread: a three-dimensional finite element analysis study. Med Sci Monit. 2019;25:3933–40. doi: https://doi.org/10.12659/MSM.913186
6. Diana M, Dallemagne B, Chung H, Nagao Y, Halvax P, Agnus V, et al. Probe-based confocal laser endomicroscopy and fluorescence-based enhanced reality for real-time assessment of intestinal microcirculation in a porcine model of sigmoid ischemia. Surg Endosc. 2014;28(11):3224–33. doi: https://doi.org/10.1007/s00464-014-3595-6
7. Jain P, Jain M, Alharbi AAM, Gaikwad RN, Porwal A, Rai N. Clinical evaluation of flap and flapless implant surgical procedures on gingival biotype: a prospective study. Eur Chem Bull. 2023;12(Special Issue 1):631–40. doi: https://doi.org/10.31838/ecb/2023.12.s1
8. Yadav S, Sachs E, Vishwanath M, Knecht K, Upadhyay M, Nanda R, et al. Gender and growth variation in palatal bone thickness and density for mini-implant placement. Prog Orthod. 2018;19(1):43. doi: https://doi.org/10.1186/s40510-018-0241-1
9. Yadav MK, Verma UP, Parikh H, Dixit M. Minimally invasive transgingival implant therapy: a literature review. Natl J Maxillofac Surg. 2018;9(2):117–22. doi: https://doi.org/10.4103/njms.NJMS_52_17
10. Cai H, Liang X, Sun DY, Chen JY. Long-term clinical performance of flapless implant surgery compared to the conventional approach with flap elevation: a systematic review and meta-analysis. World J Clin Cases. 2020;8(6):1087–103. doi: https://doi.org/10.12998/wjcc.v8.i6.1087
11. Ionescu A, Dodi A, Petcu LC, Nicolescu MI. Open healing: a minimally invasive protocol with flapless ridge preservation in implant patients. Biology (Basel). 2022;11(1):142. doi: https://doi.org/10.3390/biology11010142
12. Kim DM, Bassir SH, Nguyen TT. Effect of gingival phenotype on the maintenance of periodontal health: an American Academy of Periodontology best evidence review. J Periodontol. 2020;91(3):311–38. doi: https://doi.org/10.1002/JPER.19-0337
13. Vlachodimou E, Fragkioudakis I, Vouros I. Is there an association between the gingival phenotype and the width of keratinized gingiva? A systematic review. Dent J (Basel). 2021;9(3):34. doi: https://doi.org/10.3390/dj9030034
14. Montero J. A review of the major prosthetic factors influencing the prognosis of implant prosthodontics. J Clin Med. 2021;10(4):816. doi: https://doi.org/10.3390/jcm10040816
15. Aguilar-Duran L, Mir-Mari J, Figueiredo R, Valmaseda-Castellón E. Is measurement of the gingival biotype reliable? Agreement among different assessment methods. Med Oral Patol Oral Cir Bucal. 2020;25(1):e144–9. doi: https://doi.org/10.4317/medoral.23280
16. Nettemu SK, Nettem S, Singh VP, William SS, Gunasekaran SS, Krisnan M, et al. Multilevel analysis of site, implant, and patient-level factors with peri-implant bleeding on probing: a cross-sectional study. Int J Implant Dent. 2021;7(1):77. doi: https://doi.org/10.1186/s40729-021-00315-0
17. Monje A, Insua A, Wang HL. Understanding peri-implantitis as a plaque-associated and site-specific entity: on the local predisposing factors. J Clin Med. 2019;8(2):279. doi: https://doi.org/10.3390/jcm8020279
18. Monje A, Pons R, Insua A, Nart J, Wang HL, Schwarz F. Morphology and severity of peri-implantitis bone defects. Clin Implant Dent Relat Res. 2019;21(4):635–43. doi: https://doi.org/10.1111/cid.12791
19. Monje A, Chappuis V, Monje F, Muñoz F, Wang HL, Urban IA, et al. The critical peri-implant buccal bone wall thickness revisited: an experimental study in the Beagle dog. Int J Oral Maxillofac Implants. 2019;34(6):1328–36. doi: https://doi.org/10.11607/jomi.7657
20. Wadhwa P, Kim SK, Kim HJ, Lim HK, Jia Q, Jiang HB, et al. A Six-year prospective comparative study of wide and standard diameter implants in the maxillary and mandibular posterior area. Medicina (Kaunas). 2021;57(10):1009. doi: https://doi.org/10.3390/medicina57101009
21. Lombardo G, Signoriello A, Marincola M, Nocini PF. Assessment of peri-implant soft tissues conditions around short and ultra-short implant-supported single crowns: a 3-year retrospective study on periodontally healthy patients and patients with a history of periodontal disease. Int J Environ Res Public Health. 2020;17(24):9354. doi: https://doi.org/10.3390/ijerph17249354
22. Kao RT, Curtis DA, Kim DM, Lin GH, Wang CW, Cobb CM, et al. American Academy of Periodontology best evidence consensus statement on modifying periodontal phenotype in preparation for orthodontic and restorative treatment. J Periodontol. 2020;91(3):289–98. doi: https://doi.org/10.1002/JPER.19-0577
23. Sinha S, Kumar S, Sonoo PR, Kumar U, Siddhartha R, Singh SK. Evaluation of bone regeneration around implants with and without flap elevation. J Pharm Bioallied Sci. 2021;13(Suppl 1):S699–705. doi: https://doi.org/10.4103/jpbs.JPBS_691_20
24. Al-Juboori MJ. Rotational flap to enhance buccal gingival thickness and implant emergence profile in the esthetic zone: two cases reports. Open Dent J. 2017 Jun 30;11:284–93. doi: https://doi.org/10.2174/1874210601711010284
25. Al-Juboori MJ, Ab Rahman S, Hassan A, Bin Ismail IH, Tawfiq OF. What is the effect of initial implant position on the crestal bone level in flap and flapless technique during healing period? J Periodontal Implant Sci. 2013;43(4):153–9. doi: https://doi.org/10.5051/jpis.2013.43.4.153
26. Singla N, Kumar S, Jain S, Choudhary S, Dandiwal N, Nandalur KR. Crestal bone changes around immediately loaded single-piece implants using flap and flapless technique: a radiographic study. J Contemp Dent Pract. 2018;19(8):949–54. Available from: https://pubmed.ncbi.nlm.nih.gov/30150495/
27. Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev. 2019;146:97–125. doi: https://doi.org/10.1016/j.addr.2018.09.010
28. Lemos CAA, Verri FR, Bonfante EA, Santiago Júnior JF, Pellizzer EP. Comparison of external and internal implant-abutment connections for implant supported prostheses. A systematic review and meta-analysis. J Dent. 2018;70:14–22. doi: https://doi.org/10.1016/j.jdent.2017.12.001
29. Camps-Font O, Rubianes-Porta L, Valmaseda-Castellón E, Jung RE, Gay-Escoda C, Figueiredo R. Comparison of external, internal flat-to-flat, and conical implant-abutment connections for implant-supported prostheses: a systematic review and network meta-analysis of randomized clinical trials. J Prosthet Dent. 2021:S0022-3913(21)00529-1. doi: https://doi.org/10.1016/j.prosdent.2021.09.029
30. Avila-Ortiz G, Chambrone L, Vignoletti F. Effect of alveolar ridge preservation interventions following tooth extraction: a systematic review and meta-analysis. J Clin Periodontol. 2019;46(Suppl 21):195–223. doi: https://doi.org/10.1111/jcpe.13057
31. Bas BB, Cakan U. Evaluation of the effect of anodization-colored titanium abutments and zirconia substructure thickness on zirconia substructure color: an in vitro study. Niger J Clin Pract. 2022;25(12):2024–9. doi: https://doi.org/10.4103/njcp.njcp_484_22
32. Hutton CG, Johnson GK, Barwacz CA, Allareddy V, Avila-Ortiz G. Comparison of two different surgical approaches to increase peri-implant mucosal thickness: a randomized controlled clinical trial. J Periodontol. 2018;89(7):807–14. doi: https://doi.org/10.1002/JPER.17-0597
33. Kim DG, Kwon HJ, Jeong YH, Kosel E, Lee DJ, Han JS, et al. Mechanical properties of bone tissues surrounding dental implant systems with different treatments and healing periods. Clin Oral Investig. 2016;20(8):2211–20. doi: https://doi.org/10.1007/s00784-016-1734-2
34. Lops D, Stellini E, Sbricoli L, Cea N, Romeo E, Bressan E. Influence of abutment material on peri-implant soft tissues in anterior areas with thin gingival biotype: a multicentric prospective study. Clin Oral Implants Res. 2017;28(10):1263–8. doi: https://doi.org/10.1111/clr.12952
35. Shen XT, Li JY, Luo X, Feng Y, Gai LT, He FM. Peri-implant marginal bone changes with implant-supported metal-ceramic or monolithic zirconia single crowns: a retrospective clinical study of 1 to 5 years. J Prosthet Dent. 2022;128(3):368–74. doi: https://doi.org/10.1016/j.prosdent.2020.12.010
36. Thoma DS, Naenni N, Figuero E, Hämmerle CHF, Schwarz F, Jung RE, et al. Effects of soft tissue augmentation procedures on peri-implant health or disease: a systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(Suppl 15):32–49. doi: https://doi.org/10.1111/clr.13114
37. Thoma DS, Gil A, Hämmerle CHF, Jung RE. Management and prevention of soft tissue complications in implant dentistry. Periodontol 2000. 2022;88(1):116–29. doi: https://doi.org/10.1111/prd.12415
38. Puisys A, Auzbikaviciute V, Vindasiute-Narbute E, Zukauskas S, Razukevicus D, Dard MM. Full versus partial thickness flap to determine differentiation and over keratinization of non-keratinized mucosa. A 3-year split-mouth randomized pilot study. Clin Exp Dent Res. 2021;7(6):1061–8. doi: https://doi.org/10.1002/cre2.468
39. Gonzalez AC, Costa TF, Andrade ZA, Medrado AR. Wound healing—a literature review. An Bras Dermatol. 2016;91(5):614–20. doi: https://doi.org/10.1590/abd1806-4841.20164741
40. Amin V, Kumar S, Joshi S, Hirani T, Shishoo D. A clinical and radiographical comparison of buccolingual crestal bone changes after immediate and delayed implant placement. Med Pharm Rep. 2019;92(4):401–7. doi: https://doi.org/10.15386/mpr-1213
41. Lekovic V, Camargo PM, Klokkevold PR, Weinlaender M, Kenney EB, Dimitrijevic B, et al. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J Periodontol. 1998;69(9):1044–9. doi: https://doi.org/10.1902/jop.1998.69.9.1044
42. Pagni G, Pellegrini G, Giannobile WV, Rasperini G. Postextraction alveolar ridge preservation: biological basis and treatments. Int J Dent. 2012;2012:151030. doi: https://doi.org/10.1155/2012/151030
43. Moghaddas O, Naddafpour N, Farhadi S, Nikookar P, Khandan S. Comparison of healing time and the histopathology of bone formation following tooth extraction using freeze-dried bone allograft: a randomized controlled clinical trial. J Adv Periodontol Implant Dent. 2022;14(2):69–75. doi: https://doi.org/10.34172/japid.2022.020
44. Chen S, Darby I. Inter-implant distance and buccal bone thickness for a novel implant design: a preclinical study. Clin Oral Investig. 2023;27(6):3261–74. doi: https://doi.org/10.1007/s00784-023-04942-2
45. Karthik K, Sivakumar, Sivaraj, Thangaswamy V. Evaluation of implant success: a review of past and present concepts. J Pharm Bioallied Sci. 2013;5(Suppl 1):S117–9. doi: https://doi.org/10.4103/0975-7406.113310