INTRODUCTION
Cervical cancer is the fourth most common female cancer in terms of morbidity after breast, colorectal, and lung cancer, with a global annual prevalence of 600,000 new cases and 340,000 deaths (Cohen et al., 2019; Sung et al., 2021). One of the major causes recognized as an important etiological agent of cervical cancer is the human papillomavirus (HPV) infection, which is responsible for 99.7% of cervical cancer incidence (Johnson et al., 2019). HPV16 and HPV18 are found to be highly prevalent in most cervical cancer cases, which are transmitted through sexual contact and lead to a squamous intraepithelial lesion (Zhang et al., 2020).
Numerous cervical cancer therapies, including chemotherapy, radiotherapy, and surgery, are available and widely used in the community. Despite evidence of their promising results in treating cervical cancer therapy, several drawbacks such as low efficacy, nonspecificity, and high cost have been pointed out as the main problems for patients receiving these therapies (Shafabakhsh et al., 2019). Additionally, other cancer chemotherapy-related problems are the resistance to anticancer drugs and highly toxic effects leading to the emergence of various health problems such as hepatotoxicity (dos Santos et al., 2007), cardiotoxicity (Al-Majed et al., 2006), and nephrotoxicity (Arany and Safirstein, 2003; Miller et al., 2010). This high toxicity of cancer chemotherapy encourages scientists to combat the toxicological problem by discovering new drug candidates that provide high efficacy with low toxicity and minor side effects.
One of the wild plants with the potential to be developed as an anticancer agent is Cyperus rotundus L. Several studies regarding the anticancer and cytotoxic activity of Cyperus rotundas L. have been reported (Mannarreddy et al., 2017; Simorangkir et al., 2019). Susianti et al. (2018) showed that C. rotundus L. has cytotoxic activity on HeLa cells. Another recent study showed that C. rotundus L. provides a cytotoxic effect on the HeLa human cervical carcinoma cell by regulating apoptosis-associated gene expression (Lin et al., 2019). This anticancer and cytotoxic potential of C. rotundus L. is attributed to the presence of important chemical components that play a role in its biological activity (Hu et al., 2017). A number of articles demonstrated that C. rotundus L. possesses numerous active compounds such as sesquiterpenes (i.e., cyperotundone, isocyperotundone, and cyperusol A) (Wang et al., 2021), flavonoids (i.e., quercetin, myricetin, and kaemferol) (Samariya and Sarin, 2013), and alkaloids (i.e., rotundine A, rotundine B, and rotundine C) (Jeong et al., 2000). However, the presence of those components in C. rotundus L. is diverse depending on the ecological conditions (Lawal and Oyedeji, 2009), which can influence the pharmacological activity of C. rotundus L. Nonetheless, no research has been conducted on the effects of the ecological zone on the cytotoxic activity of C. rotundus L.
Therefore, this study aims to compare the cytotoxic activities of C. rotundus L. collected from three different ecological zones against HeLa human cervical cancer cells in vitro.
MATERIALS AND METHODS
Materials
Fresh C. rotundus L. rhizomes were collected from Lampung province, Indonesia. The HeLa cells were obtained from the Medical Parasitology Laboratory, Faculty of Medicine and Public Health, Universitas Gadjah Mada. The chemicals and reagents were as follows: Roswell Park Memorial Institute (RPMI) 1640 media (GIBCO), fetal bovine serum (FBS) (Sigma-Aldrich), penicillin-streptomycin (Sigma-Aldrich), fungison (GIBCO), Sodium bicarbonate (Sigma-Aldrich), 4(2-hydroxyethyl)-1-piperazine-ethane-sulphonic acid (Sigma-Aldrich), dimethyl sulfoxide (Sigma-Aldrih), aquabidest (Bratachem), ethanol 70% (Bratachem), ethanol 96% (Bratachem), yellow 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich), trypan blue (Sigma-Aldrich), and sodium dodecyl sulfate (SDS) (Bio-Rad).
Methods
Sample collection and extraction
The C. rotundus L. rhizome was collected from three different locations based on the altitude of the area: the highland, lowland, and coastal areas located in Lampung province, Indonesia. Then, 500 g of fresh samples was grounded and extracted by maceration technique using 1 l ethanol (technical grade, 96%) for 24 hours at room temperature. The liquid extract was then evaporated using a rotary evaporator to obtain the solid extract. The following equation was used to calculate the yield of the extract:
In vitro cytotoxic assay
The entire process for in vitro cytotoxic assay was undertaken following the methods of Susianti et al. (2018) with some minor modifications as mentioned below:
Preparation of RPMI 1640 media
100 ml of RPMI 1640 was mixed with 2% penicillin-streptomycin and 0.5% fungison. FBS 10% was added to half of the media for the treatment (media B), where the other half was only mixed with FBS 0.5% for starvation purposes (media A).
HeLa cells harvesting
HeLa cells were extracted from a tissue culture flask by trypsinization using 0.5% trypsin. The cells were then incubated with 5% CO2 at 37°C for 10 minutes, followed by adding 1 ml media for cell resuspension. The cell density was measured by observing 10 μl cells mixed with 40 μl 0.4% trypan blue on the microscope assisted with a hemocytometer. The cell concentration per ml was the average of cells in each grid on the hemocytometer (four grids with dimensions 1 × 1 × 0.1 mm) multiplied by 104. The cytotoxic assay needs 2 × 104/100 μl of media in each well. Then, the cell suspension was diluted according to the number of cells to be filled in each well.
Cytotoxic test
The cytotoxic test was conducted by MTT assay using a 96-well microplate. About 2 × 104 HeLa cells suspended in media B were inserted into the well plate and incubated with 5% CO2 for 24 hours at 37°C. After the incubation, media B in the well plate was removed, replaced with fresh media A, and treated according to Table 1. Microculture was further incubated with 5% CO2 for 24 hours at 37°C. After the second incubation, media A was removed and replaced with fresh 100 μl of media A and 10 μl of MTT reagent. The microculture was then incubated with 5% CO2 for 4 hours, and 100 μl of 10% SDS was added in 0.01% HCl. The microplate was shaken for 5 minutes at room temperature, wrapped with aluminum foil, and incubated at room temperature overnight. The microplate was then measured using an ELISA reader at λ 595 nm. The percentage of cell viability was evaluated using the following equation:
where A is the mean control cell absorbance; B is the mean control media absorbance; C is the mean sample absorbance.
Data from the cytotoxic MTT assay were then analyzed using the percentage of the live cells from each sample to the response-dose curve to obtain the IC50.
Cytotoxic data analysis
The cell viability counting from the cytotoxic assay was statistically analyzed using Minitab 19.0 software. The non-normally distributed data were analyzed using Kruskal–Wallis and post hoc Mann–Whitney tests, whereas the normally distributed data were analyzed using one-way ANOVA and Posthoc Dunnett’s and Fisher’s tests.
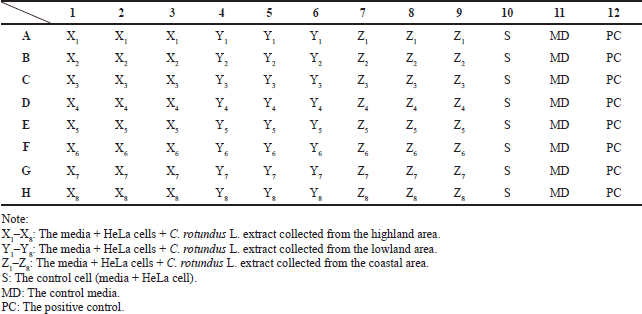 | Table 1. Microculture insertion scheme for cytotoxic assay. [Click here to view] |
Chemical composition analysis
The chemical components of the samples were analyzed using gas chromatography-mass spectrometry (GC-MS) analysis. The peaks observed in the chromatogram were then assessed by comparing their MS data with the library reference.
Molecular docking prediction
The molecular docking study was conducted on cyclin-dependent kinase 2 (CDK2), Murin Double Minute 2 (MDM2)-p53, and caspase-9. The crystallographic structure of the macromolecules was retrieved from Protein Data Bank (PDB) with the PDB IDs for CDK2 (2UZE), MDM2-p53 (4IPF), and caspase-9 (3D9T). The BIOVIA Discovery Studio Visualizer was used to remove water, ligands, and other unnecessary components to prepare the macromolecule for molecular docking. The 3D structure of the chemical compounds of C. rotundus L. was obtained from the PubChem database. The molecular docking study was undertaken using AutoDock 4.2, assisted by AutoDock Tools. The chemical compounds were docked to the active site of the macromolecule following the binding site of the native ligand of each macromolecule. The docking parameters use the default value with 100 runs of the Genetic Algorithm (GA) and Lamarckian GA as the output. Compounds with the smallest binding free energy and inhibition constant (Ki) were declared the best compounds.
RESULTS AND DISCUSSION
Sample collection and extraction
Cyperus rotundus L. rhizomes were collected in January 2021 from three different locations in Lampung province, Indonesia, with the specific locations as follows: Talang Sepuh (highland coordinate -5.377263, 104.792544), Sukarame (lowland coordinate −5.3790486, 105.3041975), and Pasir Sakti (coastal coordinate −5.5383300, 105.7876040). Plant samples were identified by Dr. Sri Wahyuningsih, and a voucher was kept in our department with identification number 001CYPBL. According to the botanical identification, C. rotundus belonged to the Plantae kingdom, the Poales order, the Cyperaceae family, the Cyperus L. genus, and the C. rotundus L species. The extracts obtained from the three locations differed slightly in terms of yield, as shown in Table 2.
 | Table 2. The yield of C. rotundus L. collected from three different ecological zones. [Click here to view] |
As shown in Table 2, the altitude greatly influences extract yield. The higher the altitude, the higher the yield of extracts. Indeed, the main compound in C. rotundus L. is essential oils with high volatility characteristics. In the highland areas, the content of essential oils increased, as it increases proportionally with the elevation of the altitude (Lallo et al., 2022). The production of essential oil is affected by several factors, including plant species-related biotic factors and environment-related abiotic factors (Boaro et al., 2019). In this study, environment-related abiotic factors are recognized as the main factors influencing the production of essential oils. The ecological zones where the samples were collected have different temperatures, with the annual average temperature being 24°C–30°C, 24°C–32°C, and 24°C–34°C for Talang Sepuh, Sukarame, and Pasir Sakti, respectively.
Chemical composition analysis
The gas chromatography-mass spectrometry results revealed that the extracts of C. rotundus L. collected from three locations have slightly different chemical compositions. The extracts collected from the highland, lowland, and coastal areas have 118, 42, and 87 chemical constituents, respectively. The GC chromatograms of extracts are illustrated in Figure 1.
As illustrated in Figure 1, extracts collected from the highland area provide 118 peaks indicating that GC-MS detects 118 chemical compounds. Among these constituents, six major components were observed: δ-selinene, caryophyllene oxide, longiverbenone, cyperotundone, methyl trisporate B, and 2,5-octadecadienoic acid-methyl ester. In the lowland ecological zone, 42 chemical compounds were observed in an extract with four main constituents: cyperotundone, n-hexadecanoic acid, chlorfenapyr, and diisooctyl phthalate. On the other hand, 87 chemical compounds were observed with seven major constituents, namely, cyperotundone, α-neoclovene, caryophyllene oxide, panaxjapyne A, methyl trisporate B, n-hexadecanoic acid, and oleic acid. The comparison of the major chemical constituents of three different extracts is summarized in Table 3.
As described in Table 3, one compound was found in all extracts, and several are present in only one or two. Cyperotundone is the only compound found in all extracts with different amounts in each extract. This compound was most abundant in C. rotundus L. extract collected from the highlands compared to the other ecological zones. Caryophyllene oxide and methyl trisporate B were found in the highland and coastal areas and were not detected in the lowland areas. In contrast, longiverbenone, δ-selinene, and 2,5-ocatdecadienoic acid-methyl ester were only found in the highland extract; n-hexadecanoic acid and chlorfenapyr only in the lowland extract; α-neoclovene, panaxjapyne A, and oleic acid were only in the coastal extract. This difference might be attributed to the influence of the nutrients in each ecological zone, which play a vital role in the secondary metabolites biosynthesis pathway by acting as cofactors of enzymes and electron transfer (Bhat et al., 2020). However, soil elements related to the environmental conditions of the ecological zone of the samples were not observed in this work. We assumed that individual changes in environmental factors such as light, temperature, soil fertility, and salinity may alter the chemical content of C. rotundus L., which is also explained by Yang et al. (2018), who found that production of secondary metabolite in plant strongly depends on those environmental factors even when other parameters remained stable.
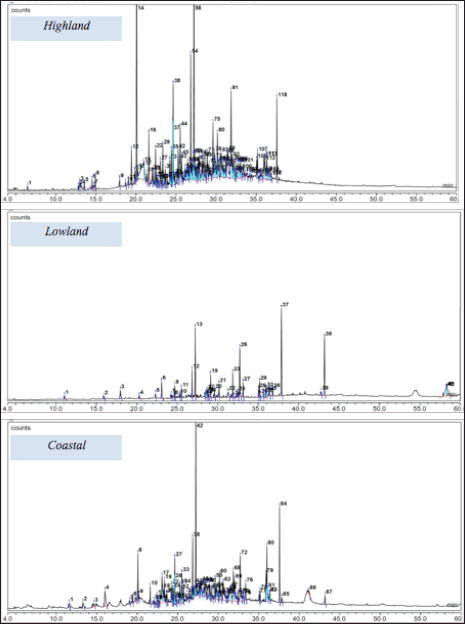 | Figure 1. Chromatogram of C. rotundus L. extracts collected from three ecological zones. [Click here to view] |
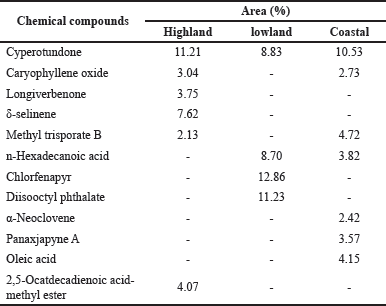 | Table 3. The main chemical composition of C. rotundus L. extracts collected from three different ecological zones. [Click here to view |
Cytotoxic activity
The cytotoxic activity of C. rotundus L. extracts collected from three ecological zones was tested on HeLa cervical cancer cells. The cytotoxic activity of the extracts was determined by observing the cell density and viability. The result revealed that the cell density of the HeLa cells treated with C. rotundus L. extracts collected from the highland areas decreased, which was slightly lower than that of the positive control. However, the cell density of HeLa cells treated with C. rotundus L. extracts collected from the lowland and coastal areas remained constant with no significant change. The cell death was determined by observing the cell viability response to each treatment’s concentration. The higher the cell viability, the lower the cytotoxic effect of C. rotundus L. extracts.
As illustrated in Figure 2, C. rotundus L. extracts collected from three ecological zones possess different cytotoxic activity on HeLa cervical cancer cells in a concentration-dependent manner. Cyperus rotundus L. extract collected from highlands decreased more than 50% of the viability of the HeLa cells in the concentration of 62.5 μg/ml, whereas the extracts from the lowland and coastal areas need 500 μg/ml and >500 μg/ml, respectively. Accordingly, the IC50 value of extract from the highland, lowland and coastal areas were 69.26 μg/ml, 43.49 ± 1.78 g/ml, and 108.51 mg/ml, respectively. It indicates that C. rotundus L. collected from the highland area was more potent than those from the lowland and coastal areas.
The difference in the cytotoxic activity of C. rotundus L. extracts collected from three different ecological zones was attributed to the variation in the chemical compositions in each extract. δ-Selinene and longiverbenone were the main constituents of C. rotundus L. extract collected from highlands, which were not found in the lowland and coastal areas. Studies regarding the cytotoxic activity of those compounds have revealed that δ-selinene and longiverbenone have cytotoxic activity (Keawsa-ard et al., 2012; Rahman and Anwar, 2010). In doing so, δ-selinene and longiverbenone were presumed to play a significant role in the cytotoxic activity of C. rotundus L. extract.
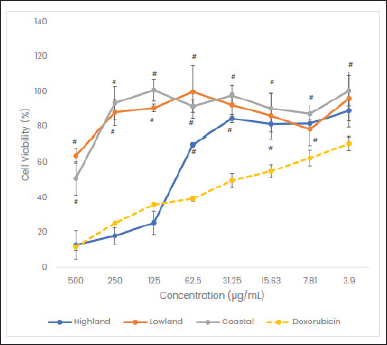 | Figure 2. Cytotoxic activity of C. rotundus L. extracts collected from three different ecological zones on HeLa cells. #p < 0.05 indicates a significant difference compared with doxorubicin. All concentrations from all groups were analyzed using Kruskal–Wallis and post hoc Mann–Whitney tests, while each concentration from each group was tested using one-way ANOVA and post hoc Dunnett’s tests. [Click here to view] |
Molecular docking prediction of bioactive compounds
The bioactive compound with cytotoxic activity from C. rotundus L. samples collected from Lampung, Indonesia, was determined by observing their ligand-protein complex affinity on CDK2, MDM2-p53, and caspase-9 using molecular docking. However, the validation of docking methods was initially undertaken to identify the parameters that will be used for the docking process of the test compounds. In the validation process, the root means score deviation (RMSD) obtained from the re-docking of the native ligand is critical to confirm the validation result. The RMSD value less than 2 ? is a criterion that indicates the docking methods are valid because as the RMSD value gets closer to zero, the pose of the re-docking ligand and the original native ligand becomes more similar (Santos et al., 2020). The validation result is illustrated in Figure 3.
As shown in Figure 3, the re-docking of the native ligand of each target macromolecule obtained an RMSD value <2 ?, indicating that the docking method was valid. Therefore, the docking parameters can be applied for further molecular docking of the test compounds. The complex ligand-protein affinity was assessed by observing the binding free energy, inhibition constant (Ki), and molecular interactions. Ligands with the lowest (more negative) binding free energy and inhibition constant (Ki) are considered well-bound, indicating that they have a better affinity with the macromolecule. The docking results of C. rotundus L. bioactive are shown in Table 4.
Chemical compounds derived from C. rotundus L. have different affinities to each target macromolecule. Every compound tends to be well-bound and interacts with one or more macromolecules. As described in Table 4, in caspase-9, the two best compounds, methyl trisporate B and δ-selinene, possess more negative binding free affinity and smaller Ki compared to any other C. rotundus L. compounds with a binding free energy of −6.84 and −6.32 Kcal/mol and Ki of 9.68 and 23.33 μM, respectively. The compounds fit in the caspase-9 pocket and interact with several important amino acid residues, as illustrated in Figure 4. Methyl trisporate B interacts with Arg-308 and Gly-306 via hydrogen interaction, whereas δ-selinene interacts with Trp-310, Leu-307, Arg-307, and Cys-309 via pi-sigma, alkyl, and pi-alkyl interactions. Additionally, diisooctyl phthalate and longiverbenone also showed a potential activity on caspase-9, but their affinity was less than that of methyl trisporate B.
In CDK2, methyl trisporate B and diisooctyl phthalate are the best compounds with the lowest binding free energy and Ki than other C. rotundus L. compounds. They possess an excellent affinity with CDK2 by interacting with various amino acid residues in the CDK2 binding pocket. As illustrated in Figure 5, methyl trisporate B interacts with Lys-89, Asp-86, and Leu-83 via hydrogen bond, while diisooctyl phthalate interacts with Lys-89, Ile-10, Val-18, Phe-80, Val-64, Ala-31, Leu-83, Leu-134, Ala-144, and Asp-86 via hydrogen bond, pi-sigma, and alkyl interactions. Other potential compounds from C. rotundus L. for inhibitor CDK2 were δ-selinene and longiverbenone. Their complex ligand-protein affinity with CDK2 was close to that of methyl trisporate B and diisooctyl phthalate.
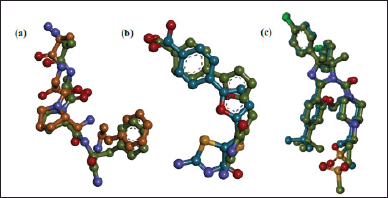 | Figure 3. The molecular docking validation result. (a) Caspase-9 (RMSD 1.59 ?), (b) CDK2 (RMSD 1.67 ?), and (c) MDM2-p53 (RMSD 1.77 ?). Note: green color for re-docking ligand. [Click here to view] |
In MDM2-p53, methyl trisporate B and δ-selinene possess more negative binding free energy and lower inhibition constant (Ki) than other C. rotundus L. compounds. They fit in the MDM2-p53 binding pocket and interact with several important amino acid residues in the MDM2-p53 active site. Methyl trisporate B has a binding free energy of −7.03 Kcal/mol and Ki of 7.03 μM and interacts directly with Gln-55, Gly-54, and His-92 via hydrogen bond and pi-sigma interaction. In contrast, δ-selinene provides binding free energy −6.65 Kcal/mol and Ki 13.37 μM and directly interacted with Tyr-96, Ile-95, Leu-53, Leu-50, and His-92 via alkyl and pi-alkyl interactions, as illustrated in Figure 6. Other compounds such as longiverbenone and cyperotundone, even though their affinity with MDM2-p53was not better than those of methyl trisporate B and δ-selinene, they also have the potential to be the MDM2-p53 inhibitor indicated by their binding free energy and Ki close to that of methyl trisporate B and δ-selinene.
Therefore, methyl trisporate B and δ-selinene were the compounds to contribute the most to C. rotundus L. cytotoxic activity. Methyl trisporate B was found in extracts collected from the highland and coastal areas, whereas δ-selinene was only found in extracts collected from the highlands. Obviously because highland extracts have δ-selinene, they have high cytotoxic activity on HeLa cervical cancer cells compared to the extracts from the coastal and lowland areas. Additionally, extracts from the highland also possess longiverbenone contributing to the cytotoxic activity of C. rotundus L., which was not found in extracts from the lowland and coastal areas. Indeed, the presence of these compounds makes C. rotundus L. extracts collected from the highland more potent than those from the lowland and coastal. This result is reinforced by several findings that revealed longiverbenone and d-selinene possess cytotoxic activities by inhibiting various human cancer cell lines (Keawsa-ard et al., 2012; Rahman and Anwar, 2010). However, the cytotoxicity of methyl trisporate B has not been reported. Our finding provides insight into the potential of methyl trisporate B as a novel anticancer agent. Further research, including molecular dynamic simulation and in vitro assay, must be undertaken to justify methyl trisporate B potential as an anticancer candidate.
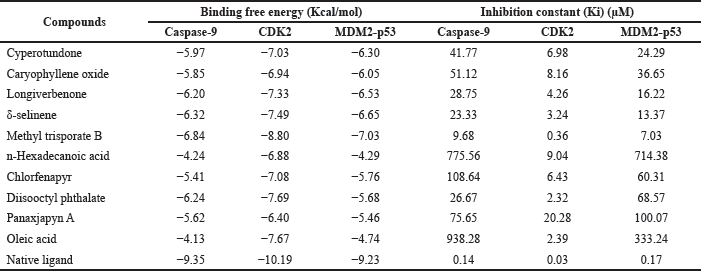 | Table 4. The docking scores of the bioactives from C. rotundus L. extracts. [Click here to view] |
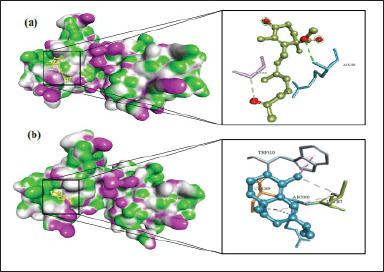 | Figure 4. 3D molecular interactions of (a) methyl trisporate B and (b) δ-selinene with caspase-9. [Click here to view] |
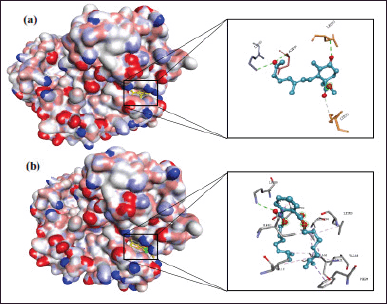 | Figure 5. 3D molecular interactions of (a) methyl trisporate B and (b) diisooctyl phthalate with CDK2. [Click here to view] |
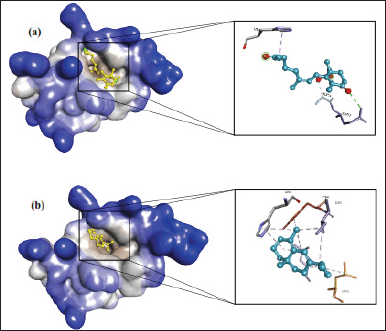 | Figure 6. 3D molecular interactions of (a) methyl trisporate B and (b) δ-selinene with MDM2-p53. [Click here to view] |
CONCLUSION
Ecological zones influence the chemical compositions and cytotoxic activity of C. rotundus L. The chemical compositions of C. rotundus L. collected from three ecological zones were slightly different. The cytotoxic activities of three C. rotundus L. extracts were also diverse. Cyperus rotundus L. extracts collected from the highland were more potent on HeLa cells with moderate cytotoxic activity than the lowland and coastal ones. This potential cytotoxic activity of C. rotundus L. extracts collected from the highland areas was attributed to the presence of methyl trisporate B, δ-selinene, and longiverbenone.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research receives no external funding.
CONFLICTS OF INTEREST
The authors declare there are no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Al-Majed AA, Sayed-Ahmed MM, Al-Yahya AA, Aleisa AM, Al-Rejaie SS, Al-Shabanah OA. Propionyl-L-carnitine prevents the progression of cisplatin-induced cardiomyopathy in a carnitine-depleted rat model. Pharmacol Res, 2006; 53:278–86; https://doi.org/10.1016/j.phrs.2005.12.005.
Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol, 2003; 23:460–4; https://doi.org/10.1016/s0270-9295(03)00089-5.
Bhat BA, Islam ST, Ali A, Sheikh BA, Tariq L, Islam SU, Dar TUH. The role of micronutrients insecondary metabilsm of plants. Plant Micronutr, 2020; https://doi.org/10.1007/978-3-030-49856-6.
Boaro CSF, Vieira MAR, Campos FG, Ferreira G, De-la-Cruz-Chacón I, Marques MOM. Factors influencing the production and chemical composition of essential oils in aromatic plants from Brazil. In: Malik S (Eds.). Essential oil research, Springer International Publishing, Cham, Switzerland, pp. 19–47, 2019.
Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet (London, England), 2019; 393:169–82; https://doi.org/10.1016/S0140-6736(18)32470-X.
dos Santos NAG, Martins NM, Curti C, Bianchi MLP, dos Santos AC. Dimethylthiourea protects against mitochondrial oxidative damage induced by cisplatin in liver of rats. Chem Biol Interact, 2007; 170:177–86; https://doi.org/10.1016/j.cbi.2007.07.014.
Hu QP, Cao XM, Hao DL, Zhang LL. Chemical composition, antioxidant, DNA damage protective, cytotoxic and antibacterial activities of Cyperus rotundus rhizomes essential oil against foodborne pathogens. Sci Rep, 2017; 7:1–9; https://doi.org/10.1038/srep45231.
Jeong SJ, Miyamoto T, Inagaki M, Kim YC, Higuchi R. Rotundines A−C, three novel sesquiterpene alkaloids from Cyperus rotundus. J Nat Prod, 2000; 63:673–5; https://doi.org/10.1021/np990588r.
Johnson CA, James D, Marzan A, Armaos M. Cervical cancer: an overview of pathophysiology and management. Semin Oncol Nurs, 2019; 35:166–74; https://doi.org/10.1016/j.soncn.2019.02.003.
Keawsa-ard S, Liawruangrath B, Liawruangrath S, Teerawutgulrag A, Pyne SG. Chemical constituents and antioxidant and biological activities of the essential oil from leaves of Solanum spirale. Nat Prod Commun, 2012; 7:955–8.
Lallo S, Lewerissa AC, Rafi’i A, Usmar U, Ismail I, Tayeb R. Pengaruh ketinggian tempat tumbuh terhadap aktivitas antioksidan dan sitotoksik ekstrak rimpang lengkuas (Alpinia galanga L.). Maj Farm Dan Farmakol, 2022; 23:118–23; https://doi.org/10.20956/mff.v23i3.9406.
Lawal OA, Oyedeji AO. Chemical composition of the essential oils of Cyperus rotundus L. from South Africa. Molecules, 2009; 14:2909–17; https://doi.org/10.3390/molecules14082909.
Lin CH, Peng SF, Chueh FS, Cheng ZY, Kuo CL, Chung JG. The ethanol crude extraction of Cyperus rotundus regulates apoptosis-associated gene expression in HeLa human cervical carcinoma cells in vitro. Anticancer Res, 2019; 39:3697–709; https://doi.org/10.21873/anticanres.13518.
Mannarreddy P, Denis M, Munireddy D, Pandurangan R, Thangavelu KP, Venkatesan K. Cytotoxic effect of Cyperus rotundus rhizome extract on human cancer cell lines. Biomed Pharmacother, 2017; 95:1375–87; https://doi.org/10.1016/j.biopha.2017.09.051.
Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel), 2010; 2:2490–518; https://doi.org/10.3390/toxins2112490.
Rahman MS, Anwar M. Antibacterial and cytotoxic activity of longiverbenone isolated from the rhizome of Cyperus scariosu. Bangladesh J Microbiol, 2010; 25:82–4.
Samariya K, Sarin R. Isolation and identification of flavonoids from Cyperus rotundus Linn. in vivo and in vitro. J Drug Deliv Ther, 2013; 3:109–13; https://doi.org/10.22270/jddt.v3i2.460.
Santos KB, Guedes IA, Karl ALM, Dardenne LE. Highly flexible ligand docking: benchmarking of the dockthor program on the LEADS-PEP protein-peptide data set. J Chem Inf Model, 2020; 60:667–83; https://doi.org/10.1021/acs.jcim.9b00905.
Shafabakhsh R, Reiter RJ, Mirzaei H, Teymoordash SN, Asemi Z. Melatonin: a new inhibitor agent for cervical cancer treatment. J Cell Physiol, 2019; 234:21670–82; https://doi.org/https://doi.org/10.1002/jcp.28865.
Simorangkir D, Masfria M, Harahap U, Satria D. Activity anticancer n-hexane fraction of Cyperus rotundus L. rhizome to breast cancer MCF-7 cell line. Open Access Maced J Med Sci, 2019; 7:3904–6; https://doi.org/10.3889/oamjms.2019.530.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2021; 71:209–49; https://doi.org/https://doi.org/10.3322/caac.21660.
Susianti Susianti S, Yanwirasti Y, Darwin E, Jamsari J. The cytotoxic effects of purpel nutsedge (Cyperus rotundus L.) tuber essential oil on the HeLa cervical cancer cell line. Pak J Biotechnol, 2018; 15:1–23.
Wang Q, Yi C, Duan W, Duan Y, Lou J, Zeng G, Yin J. Two new sesquiterpenoids isolated from Cyperus rotundus L. Nat Prod Commun, 2021; 16; https://doi.org/10.1177/1934578X21991687.
Yang L, Wen KS, Ruan X, Zhao YX, Wei F, Wang Q. Response of plant secondary metabolites to environmental factors. Molecules, 2018; 23:1–26; https://doi.org/10.3390/molecules23040762.
Zhang S, Xu H, Zhang L, Qiao Y. Cervical cancer: epidemiology, risk factors and screening. Chin J Cancer Res, 2020; 32:720–8; https://doi.org/10.21147/j.issn.1000-9604.2020.06.05.