INTRODUCTION
In present-day drug discovery, 15% of drugs are derived from natural products-based biopharmaceuticals. Studies focused on disease indications include oncology, metabolic, and musculoskeletal system disorders. Thus, investments in sustainable biotechnology are of vital importance in drug discovery. Pharmaceutical industries rely on natural products-based biotechnology to discover and develop new drug products, processes, and methods and improve existing ones [1,2].
Relevant to this study, metabolic disorders type 2 diabetes (T2D) and obesity remain among the world’s top maladies prompting the World Health Organization to declare both as epidemics. Approximately 422 million people globally are affected by diabetes, while 650 million suffer from obesity [3,4]. In the Philippines, diabetes and obesity are recognized as emerging lifestyle diseases, with 6 million and 5.6 million affected Filipinos, respectively [5]. In addition, COVID-19 outcomes are worse in individuals with metabolic disorders [6]. Patients with obesity and diabetes have been reported to suffer from threefold increased susceptibility to critical COVID-19 [7]. In addition, most Filipinos rely on medicinal plants in the form of supplements as preventive and therapeutic regimens. Thus, the discovery of new therapeutic natural product classes with dual-targeting enzyme activities focused on the metabolic disorders of diabetes and obesity is a logical strategy.
The search for natural products with inhibitory activities against several disease-implicated proteins is an efficient approach to developing new molecular drug archetypes. Multi-targeting therapeutics are effective and may mitigate resistant diseases, especially metabolic disorders [8]. With recent advances in bioinformatics and computational biotechnology, several proteins may be targeted based on their structural and functional information from databases which may pave the way for the discovery of multitargeting natural products against infectious [9,10], cancer [11,12], and metabolic disorders [9–13].
Natural products are known for their myriad of pharmaceutical utilities, including their antidiabetic and antiobesity properties [13,14]. Plant-derived molecular archetypes with multidisease-acting properties are advantageous and elicit good responses against metabolic diseases with multifactorial origins, such as diabetes and obesity. In the Philippines, medicinal plants have been extensively tapped in drug discovery for their multi-disease-targeting secondary metabolites [15,16]. Among these natural products are alkaloids, nitrogen-containing compounds usually in a heterocyclic ring. Alkaloids are undoubtedly among the most prolific classes of plant-derived secondary metabolites with reported activities against cancer, asthma, obesity, diabetes, and multidrug-resistant pathogens [17,18].
In line with our ongoing efforts to investigate pharmaceutically important phytoconstituents from Philippine endemic, native, and medicinal plants, we explored the tetrahydrobisbenzylisoquinoline alkaloids from Phaeanthus ophthalmicus (Roxb. Ex G.Don) J.Sinclair, a lowland woody shrub which mostly inhabits forests in the island of Luzon in the Philippines. This plant is traditionally used for treating conjunctivitis, ulcer, and minor wounds. Recent studies reported its antibacterial, anticancer, and immunomodulatory properties [11, 19]. Herein, we describe the antagonistic activities of P. ophthalmicus tetrahydrobisbenzylisoquinoline alkaloidal phytoconstituents tetrandrine (1) and limacusine (2) (Fig. 1) against enzymes implicated in the pathophysiology of T2D and obesity α-glucosidase, dipeptidyl peptidase-IV (DPP-IV), porcine pancreatic lipase (PPL), and human recombinant monoacylglycerol lipase (MAGL) using colorimetric inhibition assays and molecular docking studies.
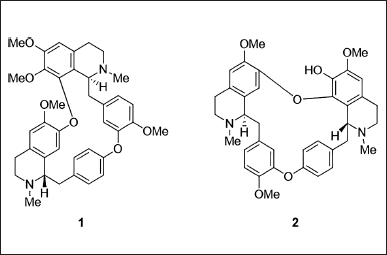 | Figure 1. Tetrahydrobisbenzylisoquinoline alkaloids from P. ophthalmicus. [Click here to view] |
MATERIALS AND METHODS
Compounds
Tetrahydrobisbenzylisoquinoline alkaloids 1 and 2 were obtained from the alkaloid (leaf) extracts of P. ophthalmicus using purification procedures previously reported by Magpantay and co-workers [19]. Briefly, an aliquot of the crude DCM-methanol extract was subjected to acid-base extraction by gradient change in pH (5 and 9) to obtain two alkaloid extracts. As previously described, the alkaloid extract obtained at pH 9 was subjected to iterative column chromatography to afford the major alkaloids 1 and 2.
Biological assays
?-Glucosidase inhibitory assay
The enzyme α-glucosidase and its substrate p-nitrophenyl α-glucopyranoside (p-NPG) were acquired from Sigma, USA. Acarbose served as the drug control (positive). The α-glucosidase inhibitory assay followed a standard protocol previously described with minor modifications [13]. Compounds 1 and 2 were dissolved in dimethyl sulfoxide (DMSO) and yielded 1, 10, 100, 250, 500, and 1,000 μg/ml concentrations. To test for the α-glucosidase inhibition, 8 μl test alkaloid was mixed with 112 μl NaH2PO4-Na2HPO4 buffer (pH 6.8) and 20 μl α-glucosidase solution (0.2 U/ml) before incubation for 15 minutes at 37ºC. To measure the initial absorbance, 405 nm was set as the wavelength of analysis in the Glomax multimode microplate reader (Promega). A 20 μl 2.5 mM p-NPG was then included in the mixture, and additional incubation for 15 minutes at 37ºC was performed. Halting of the reaction was done by adding 80 μl 0.2 M Na2CO3 solution. At 405 nm, the final absorbance was recorded. The formula below was utilized to compute the % glucosidase inhibition:
The inhibitory activity versus α-glucosidase expressed in half-maximal inhibitory concentration (IC50) values were determined based on the equation of the line given by the plot of % inhibition versus concentration.
DPP-IV inhibitory assay
DPP-IV activity was measured using a DPP-IV inhibitor screening test kit (Cayman Chemical, Ann Arbor, MI), which provided a fluorescence-based technique for screening DPP-IV inhibitors. The fluorogenic substrate Gly-Pro-aminomethylcoumarine (AMC) was used in the test to quantify DPP-IV activity. The fluorescence of the free AMC group was investigated using an excitation wavelength of 350–360 nm and an emission wavelength of 450–465 nm as a result of DPP cleavage of the peptide linkage.
The test alkaloids 1 and 2 were first dissolved in DMSO to provide a 50 mM stock solution, which was then diluted in DMSO to achieve varied concentrations. Finally, the test chemicals were added to a 96-well plate in a total volume of 10 μl at a concentration of 50 μM. According to the manufacturer’s instructions, the assay was carried out by adding 30 μl of diluted assay buffer and 10 μl of diluted enzyme solution to a 96-well plate with 10 μl of solvent (blank) or solvent-dissolved test chemicals. To begin the reaction, a diluted substrate solution was added, and the plate was incubated at 37°C for 30 minutes. Following incubation, fluorescence was measured using a plate reader by Promega GloMaxExplorer, Germany, with an excitation wavelength of 350 nm and an emission wavelength of 450 nm. The sigmoidal dose-response fitting tool in GraphPad Prism was used to determine IC50 values from experimental data [9]. The mean values were obtained from three replicates.
Pancreatic lipase inhibitory assay
Alkaloids 1 and 2 were tested for lipase inhibitory action using a colorimetric assay that measures the release of p-nitrophenol butyrate (p-NPB) [8]. Using a stock solution of 1 mg/ml test alkaloids in DMSO, four separate working solutions with the following concentrations were prepared: 0.1, 1.0, 10, and 100 μg/ml. To generate the lipase enzyme solution, 1 mg of PPL was dissolved in 1 ml Tris-HCl buffer (25 mmol Tris, 25 mmol NaCl, pH=7.4). Lipase inhibition assays were carried out in triplicate by pre-incubating a 96-well microtiter plate at 250°C for 15 minutes with 20 μl of each compound, 70 μl Tris-HCl buffer, and 10 μl of pancreatic lipase enzyme. The addition of 15 μl of p-NPB initiated the enzymatic hydrolysis, and the resulting mixture was incubated at 370°C (30 minutes). To measure the amount of produced p-nitrophenolate ions following the reaction, we used a Glomax Promega microplate multi-modal reader at 405 nm. Orlistat was used as a positive control, while DMSO was used as a negative control. The formula shown below measured the lipase inhibition (%):
where A represents the activity induced in the absence of an inhibitor, a is the negative control with no inhibitor, B is the lipase activity in the presence of an inhibitor, and b is the negative control counterpart. Alkaloids 1 and 2 lipase inhibitory activity as expressed in IC50 were calculated using the least-squares regression line of the logarithm function of sample concentration (log) versus pancreatic lipase inhibitory activity (%) plots.
Human MAGL inhibitory assay
The MAGL inhibition was assessed using the MAGL screening assay kit (Cayman Chemical, Ann Arbor, MI, USA), an absorbance-based screening test. 10 μl of MAGL enzyme, 10 μl of DMSO, and 150 μl 1X Assay Buffer were combined in three separate wells for 100% initial activity wells. A mixture composed of 160 μl of 1X Assay Buffer and 10 μl of DMSO was added for the three background wells. For the inhibitor wells, the initial activity wells had the same composition, but instead of 10 μl of DMSO, it was replaced with 10 μl of inhibitor or positive control JZL195. The resulting mixtures in each well were gently mixed and incubated for 15 minutes, RT. To initiate the reaction in each well, we added 10 μl substrate. The contents of the 96-well plate were mixed by gentle shaking for 10 seconds to mix before being incubated at room temperature for 10 minutes. We used the plate reader to measure absorbance at 405–415 nm (Promega GloMaxExplorer, Germany). The calculation of IC50 values was based on the experimental data processed in GraphPad Prism software sigmoidal dose-response fitting [9]. Three replicate experiments yielded the mean values.
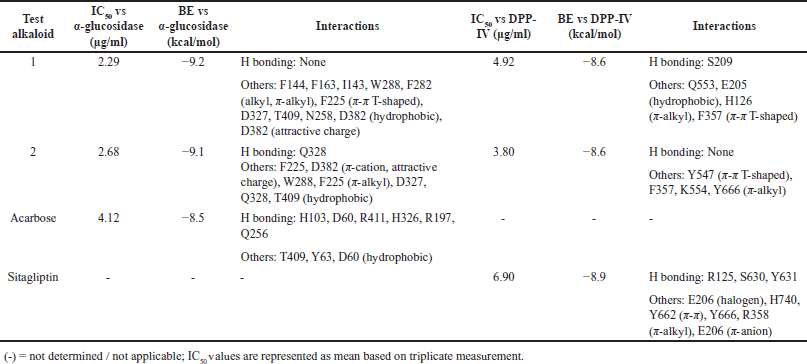 | Table 1. In vitro and in silico α-glucosidase and DPP-IV inhibitory activities of alkaloids 1 and 2. [Click here to view] |
Molecular docking studies
Ligand preparation
In silico simulations particularly molecular docking of alkaloids 1 and 2 was performed. The SMILES notations of the ligands (alkaloids 1 and 2) were processed in Avogadro (version 1.20) to yield the mol2 files, which were then opened in University of California San Francisco (UCSF) Chimera (version. 1.16) [11].
Protein preparation
Before docking, PDB IDs were first fetched from the Protein Data Bank: 5ZCC for α-glucosidase, 2RIP for DPP-IV, 1ETH for PPL, and 3PE6 for MAGL. All non-standard residues and existing co-crystallized structures were removed [20].
Protein minimization and molecular docking simulations
Protein minimization was performed by the “structure editing” feature in UCSF Chimera. The specific parameters included were: 100 steps with 0.02 Å step size for the steepest descent method and 10 steps with 0.02 Å step size for the conjugate gradient method. The Gasteiger charge method was selected [21]. Molecular docking simulations were performed after the addition of the ligands in the UCSF Chimera. The active site protocol “flexible ligand into the flexible active site” with generated grid was utilized [22]. Interacting amino acids and ligand moieties were visually interpreted in BIOVIA Discovery Studio (version 4.1).
Prediction of ADME and other pharmacokinetic properties
Analysis of the druggability of ligands was performed based on Lipinski’s rule of five (LRo5). Absorption, distribution, metabolism, and excretion (ADME) properties and LRo5 profile of alkaloids 1 and 2 were predicted in silico using SWISSADME [23]. SMILES-formatted ligands were uploaded to http://www.swissadme.ch/index.php to obtain their molecular weight, number of H-bond acceptors and donors, lipophilicity (MlogP) and number of violations in LRo5, and determine drug-likeness [24].
RESULTS
In vitro and in silico inhibitory activities of 1 and 2 versus diabetes-associated enzymes α-glucosidase and DPP-IV
Both alkaloids 1 and 2 from the Philippine medicinal plant P. ophthalmicus were tested against T2D-implicated enzymes α-glucosidase and DPP-IV in vitro and using molecular docking simulations. Alkaloids 1 (IC50 = 2.29 μg/ml) and 2 (IC50 = 2.68 μg/ml) showed stronger inhibitions against α-glucosidase as opposed to the positive drug control acarbose (IC50 = 4.12 μg/ml) (Table 1). To probe its putative binding mechanism, both alkaloids were docked onto the α-glucosidase active site (PDB ID: 5ZCC) (Fig. 2a and b). Alkaloid 1 [binding energy (BE)= −9.2 kcal/mol) interacted with the active site through alkyl and π-alkyl bonding with F144, F163, I143, W288, and F282. It also formed π-π T-shaped bond with F225 and hydrophobic bonds with D327, T409, N258, and D382. An attractive charge with D382 was also noted (Fig. 2a). Meanwhile, alkaloid 2 (BE = −9.1 kcal/mol] established a hydrogen bonding with Q328 and π-cation and an attractive charge with F225 and D382. Other interactions include residues W288, F225 (π −alkyl), D327, Q328, and T409 (hydrophobic) (Fig. 2b). Molecular docking with acarbose, on the other hand, showed corroborative results with the in vitro assay data. Despite the richness of hydrogen bonding interactions, a weaker binding affinity was obtained compared to compounds 1 and 2.
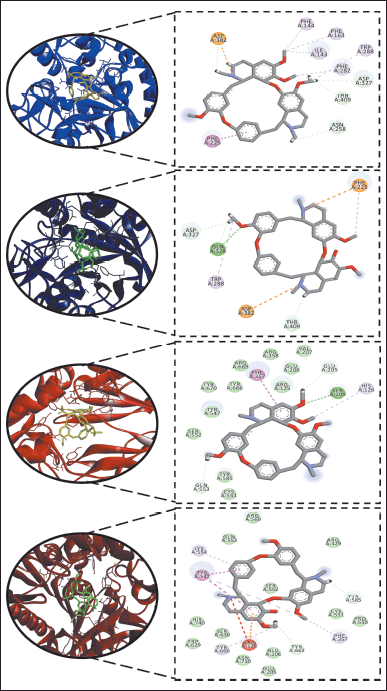 | Figure 2. Dock poses and 2D binding diagrams of alkaloids 1 and 2 versus type 2 diabetes-implicated enzymes: (a) 1 versus ?-glucosidase, (b) 2 versus ?-glucosidase, (c) 1 versus DPP-IV, and (d) 2 versus DPP-IV. [Click here to view] |
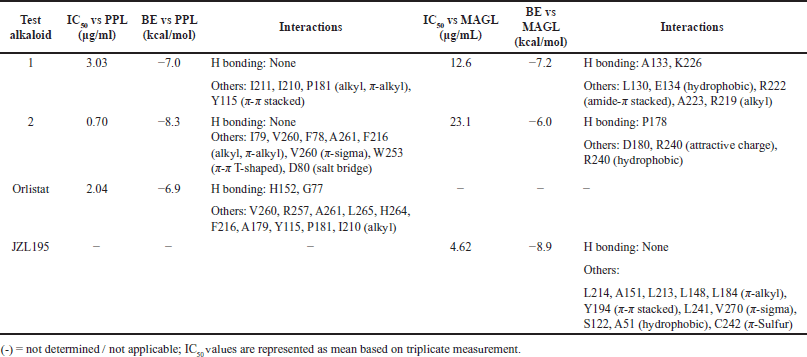 | Table 2. In vitro and in silico PPL and MAGL inhibitory activities of alkaloids 1 and 2. [Click here to view] |
Alkaloids 1 and 2 also showed promising inhibitory activities against DPP-IV (Table 1). Both 1 (IC50 = 4.92 μg/ml) and 2 (IC50 = 3.80 μg/ml) exerted stronger inhibitory activity against DPP-IV compared to the drug control sitagliptin (IC50 = 6.90 μg/ml). Results of molecular docking simulations against DPP-IV (PDB ID: 2RIP) revealed the putative affinity of alkaloid 1 (BE = −8.6 kcal/mol). It interacted with the active site via conventional hydrogen bonding with S209 and hydrophobic interactions with Q553 and E205. It also formed π-alkyl bonding with H126 and π-π T-shaped interaction with F357 (Fig. 2c). Alkaloid 2 (BE = −8.6 kcal/mol) bound to the following residues: Y547 (π-π T-shaped), F357, K554, and Y666 (π-alkyl) (Fig. 2d). Overall, the results of the anti-diabetic biological assays corroborate with the results of the in silico simulations, as evidenced by better IC50 and BE values of alkaloids 1 and 2 compared to acarbose and sitagliptin (positive controls). Molecular docking with sitagliptin showed H-bonding as the principal underlying interaction, with slightly better binding affinity recorded, and different amino acids involved in the H-bonding.
In vitro and in silico inhibitory activities of 1 and 2 versus obesity-implicated enzymes PPL and MAGL
Against PPL, only limacusine (2) (IC50 = 0.70 μg/ml) showed better inhibitory activity compared to the drug control, orlistat (IC50 = 2.04 μg/ml) (Table 2). Alkaloid 2 also showed a better binding propensity to the active site of PPL (PDB ID: 1ETH) with BE = −8.3 kcal/mol compared to orlistat (BE = −6.9 kcal/mol). Alkaloid 2 interacted with I79, V260, F78, A261, and F216 via alkyl and π-alkyl bonds. It also formed a π-sigma bond with V260, π-π T-shaped bond with W253, and a salt bridge with D80 (Fig. 3). Orlistat with weaker binding affinity was shown to interact with similar amino acid residue interaction targets of 1 and 2, such as V260, A261, F216, P181, and I210.
Alkaloid 1 (IC50 = 12.6 μg/ml) and 2 (IC50 = 23.1 μg/ml) also showed moderate activity against the human MAGL (Table 2). While the positive control JZL195 showed better inhibitory activity (IC50 = 4.62 μg/ml), both alkaloids 1 and 2 showed significant H-bonding with their MAGL (PDB ID: 3PE6) residues (Fig. 3c–d). Compared with alkaloid 2, alkaloid 1 showed better binding affinity to MAGL, forming H-bonding with A133 and K226. It also established hydrophobic interactions with L130 and E134 and alkyl binding with A223 and R219, and amide-π stacked bond with R222. Despite exhibiting better binding activity for positive control JZL195, no H-bonds were observed. However, more amino acid residues were involved during its binding. Results of docking studies corroborated with in vitro data of JZL195 having better IC50 and BE.
Both tetrandrine (1) and limacusine (2) showed drug-likeness in accordance with Lipinski’s rule of five (Table 3). Both alkaloids were also previously reported to confer low toxicity risks in silico [19].
DISCUSSION
In T2D management and therapy, α-glucosidase and DPP-IV are among the pharmaceutical target enzymes. Due to their complementary function, inhibitors of these two enzymes are expected to elicit a synergistic effect in improving glucose fluctuations [25]. α-Glucosidase has long been recognized as a pharmaceutical target in managing postprandial rise in blood glucose. α-Glucosidase inhibitors reduce the rate of hydrolytic cleavage of sugar polymers like oligosaccharides into monosaccharides in the small intestines, thereby delaying the absorptive rate of glucose into the blood [26]. According to Garber et al. [27], α-glucosidase inhibitors serve as the first-line chemotherapeutic agents in T2D. Meanwhile, DPP-IV inhibitors work in type-2 diabetes patients by downregulating DPP-IV, thus increasing hormone incretin levels. Increasing incretin leads to more insulin production and less glucose production in the liver [28]. Although naturally occurring DPP-IV inhibitory alkaloids have been reported from natural sources such as ephedrine and its derivative pseudoephedrine, their bioactivities were not promising. These compounds were also reported to confer toxicity and low drug-likeness [29,30]. Thus, the discovery of alkaloids as promising anti-diabetic pharmacophores with inhibitory activities against these two enzymes is warranted. In our study, both alkaloids tetrandrine (1) and limacusine (2) exhibited promising inhibitory activities against α-glucosidase and DPP-IV, which are even better than the control drugs. This suggests the potential of alkaloids derived from P. ophthalmicus as potential alkaloid-based drug prototypes in developing a new generation of dual-targeting antidiabetic drugs with α-glucosidase and DPP-IV as the main therapeutic targets.
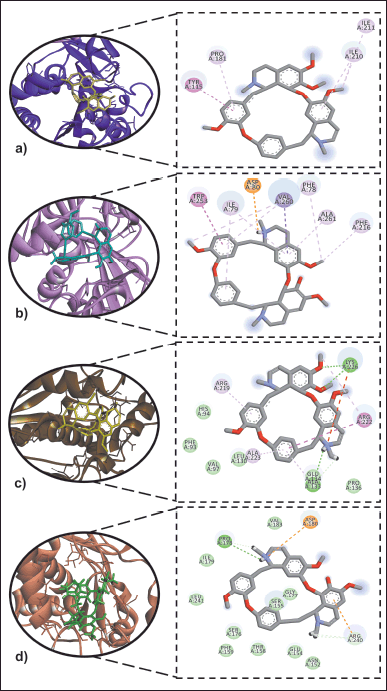 | Figure 3. Dock poses and 2D binding diagrams of alkaloids 1 and 2 versus obesity-implicated enzymes: (a) 1 versus PPL, (b) 2 versus PPL, (c) 1 versus MAGL, and (d) 2 versus MAGL [Click here to view] |
In general, alkaloidal phytoconstituents are known for their pharmaceutical potential, including inhibitory properties against diabetes-implicated enzymes [18,26,31,32]. Studies suggest increasing phenolic moieties while decreasing the degree of methylation may increase the inhibitory potencies of natural products against α-glucosidase [33,34]. In our visualized molecular docking results for alkaloids 1 and 2 against α-glucosidase and DPP-IV (Fig. 2), most interactions with key residues occur at phenolic moieties and nitrogen-bearing ringed structures suggestive of the putative role of polar groups in enhancing antidiabetic potency. Interestingly, results indicated the presence of hydrogen bonds may not be generally associated with better BE. For example, JZL195 exhibited more favorable BE compared to 1 and 2, but no H-bonds were formed between the positive control and MAGL. More H-bonds were established with α-glucosidase residues for acarbose compared to 1 and 2, but both alkaloids showed better binding energies.
Meanwhile, the pancreatic lipase enzyme is a well-known target for anti-obesity drug discovery. This enzyme directly influences dietary fat metabolism and inhibits fat absorption through the small intestine. The excess fat stored tend to accumulate white adipose tissues and cause obesity. Presently, the sole approved pancreatic lipase inhibitor in the market is orlistat. This led researchers to discover and develop new prodrugs against pancreatic lipase based on natural products owing to their structural diversity [35]. Similarly, the endocannabinoid (ECS), a conserved lipid signaling system, is widely recognized as a target for managing obesity. Increased endocannabinoid synthesis is associated with an increased risk of obesity. To modulate ECS activity, the MAGL enzyme is considered a therapeutic target as it inhibits endocannabinoid degradation [36,37]. Interestingly, our results indicated better inhibitory activity of alkaloid 2 against PPL in vitro and in silico compared to orlistat. Thus, further evaluation of alkaloid 2 in vivo is deemed beneficial to developing this compound as a pancreatic lipase-targeting prodrug. On the other hand, both alkaloids 1 and 2 also showed moderate activity against MAGL.
Among plant natural products, alkaloids are among the most extensively studied class in terms of their inhibitory activity against lipases [38]. Studies have indicated that increasing hydroxylation, as with polyphenols, yield better inhibition for better lipase catalytic activity [37]. In our study, structural modification of alkaloids 1 and 2 might enhance their inhibitory properties, especially 2, by increasing their hydroxyl moieties.
 | Table 3. Drug-likeness of alkaloids 1 and 2 according to Lipinski’s rule of five. [Click here to view] |
CONCLUSION
Overall, our findings demonstrated the inhibitory activities of the tetrahydrobisbenzylisoquinoline alkaloidal phytoconstituents tetrandrine (1) and limacusine (2) from the Philippine native and medicinal plant P. ophthalmicus against key enzymes implicated in T2D (α-glucosidase and DPP-IV) and obesity (PPL and human recombinant MAGL). Results of in vitro assays suggest the potential of these alkaloids in discovering new natural product-based drug prototypes against metabolic disorders with known COVID-19 metabolic comorbidities. Molecular docking experiments supported putative binding mechanisms of 1 and 2 in the active pockets of associated target enzymes.
AUTHOR CONTRIBUTIONS
JAHM and LCJL were responsible for conceptualization, investigation, data collection and analysis, manuscript preparation, and manuscript editing. APGM was reponsible for conceptualization, design, manuscript preparation, editing, and review. All authors have read and approved the present version of the manuscript.
FINANCIAL SUPPORT
This work was supported in part by the International Foundation for Science (Grant no. F/5376-1).
CONFLICTS OF INTERESTS
The authors declare that they have no conflicts of interest in the publication.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Berdigaliyev N, Aljofan M. An overview of drug discovery and development. Future Med Chem. 2020;12(10):939–47.
2. Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT. Natural products in drug discovery: Advances and opportunities. Nat Rev Drug Discov. 2021;20(3):200–16.
3. World Health Organization [Internet]. Diabetes [cited 2022 Dec 18] Available from https://www.who.int/health-topics/diabetes#tab=tab_1
4. World Health Organization [Internet]. Obesity [cited 2022 Dec 18]. Available from https://www.who.int/health-topics/obesity#tab=tab_1
5. Duante CA, Canag JL, Patalen CF, Austria RE, Acuin CC. Factors associated with overweight and obesity among adults 20.0 years and over: results from the 2013 national nutrition survey, Philippines. Philipp J Sci. 2019;148(1):7–20.
6. Kalligeros M, Shehadeh F, Mylona EK, Benitez G, Beckwith CG, Chan PA, et al. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity. 2020;28(7):1200–4.
7. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534–43.
8. Quimque MT, Magsipoc RJ, Llames LC, Flores AI, Garcia KY, Ratzenbo?ck A, et al. Polyoxygenated cyclohexenes from Uvaria grandiflora with multi-enzyme targeting properties relevant in type 2 diabetes and obesity. ACS Omega. 2022;7(41):36856–64.
9. Quimque MT, Notarte KI, Adviento XA, Cabunoc MH, de Leon VN, Lugtu EJ, et al. Polyphenolic natural products active in silico against SARS-CoV-2 spike receptor binding domains and non-structural proteins-a review. Comb Chem High Throughput Screen. 2023;26(3):459–88.
10. Liu M, El-Hossary EM, Oelschlaeger TA, Donia MS, Quinn RJ, Abdelmohsen UR. Potential of marine natural products against drug-resistant bacterial infections. Lancet Infect Dis. 2019;19(7):e237–45.
11. Malaluan IN, Manzano JA, Muñoz JE, Bautista TJ, Dahse HM, Quimque MT, et al. Antituberculosis and antiproliferative activities of the extracts and tetrahydrobisbenzylisoquinoline alkaloids from Phaeanthus ophthalmicus: in vitro and in silico investigations. Philipp J Sci. 2022;151(1):371–81..
12. Manzano JA, III CL, Quimque MT, Macabeo AP. In silico potentials of Alpinia galanga constituents against human placental aromatase vital in postmenopausal estrogen-dependent breast cancer pathogenesis. Philipp J Sci. 2022;151(6A):2101–15.
13. Macabeo AP, Pilapil LA, Garcia KY, Quimque MT, Phukhamsakda C, Cruz AJ, et al. Alpha-glucosidase-and lipase-inhibitory phenalenones from a new species of Pseudolophiostoma originating from Thailand. Molecules. 2020;25(4):965.
14. Macabeo AP, Rubio PY, Alejandro GJ, Knorn M. An α-glucosidase inhibitor from Drepananthus philippinensis. Procedia Chem. 2015;14:36–9.
15. de Jesus MS, Macabeo AP, Ramos JD, de Leon VN, Asamitsu K, Okamoto T. Voacanga globosa spirobisindole alkaloids exert antiviral activity in HIV latently infected cell lines by targeting the NF-kB cascade: in vitro and in silico investigations. Molecules. 2022;27(3):1078.
16. Quimque MT, Notarte KI, de Leon VN, Manzano JA, Muñoz JE, Pilapil IV DY, et al. Computationally repurposed natural products targeting SARS-CoV-2 attachment and entry mechanisms. In: Adibi S, Griffin P, Sanicas M, Rashidi M, Lanfranchi F, editors. Frontiers of COVID-19: scientific and clinical aspects of the novel coronavirus 2019. Cham, Switzerland: Springer International Publishing; 2022. pp 505–37.
17. Gupta MK, Sharma PK. Textbook of Pharrmacognosy, 3rd ed. Meerut, India: Pragati Prakashan; 2014.
18. Kumar A, Aswal S, Semwal RB, Chauhan A, Joshi SK, Semwal DK. Role of plant-derived alkaloids against diabetes and diabetes-related complications: a mechanism-based approach. Phytochem Rev. 2019;18:1277–98.
19. Magpantay HD, Malaluan IN, Manzano JA, Quimque MT, Pueblos KR, Moor N, et al. Antibacterial and COX-2 inhibitory tetrahydrobisbenzylisoquinoline alkaloids from the Philippine medicinal plant Phaeanthus ophthalmicus. Plants. 2021;10(3):462.
20. Fernandez RA, Quimque MT, Notarte KI, Manzano JA, Pilapil IV DY, de Leon VN, et al. Myxobacterial depsipeptide chondramides interrupt SARS-CoV-2 entry by targeting its broad, cell tropic spike protein. J Biomol Struct Dyn. 2022;40(22):12209–20.
21. Wang J, Wang W, Kollman PA, Case DA. Automatic atom type and bond type perception in molecular mechanical calculations. J Mol Graph Model. 2006;25(2):247–60.
22. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–12.
23. Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7(1):42717.
24. de Leon VN, Manzano JA, Pilapil DY, Fernandez RA, Ching JK, Quimque MT, et al. Anti-HIV reverse transcriptase plant polyphenolic natural products with in silico inhibitory properties on seven non-structural proteins vital in SARS-CoV-2 pathogenesis. J Genet Eng Biotechnol. 2021;19(1):1–7.
25. Kurozumi A, Okada Y, Mori H, Arao T, Tanaka Y. Efficacy of α–glucosidase inhibitors combined with dipeptidyl–peptidase–4 inhibitor (alogliptin) for glucose fluctuation in patients with type 2 diabetes mellitus by continuous glucose monitoring. J Diabetes Investig. 2013;4(4):393–8.
26. Kumar S, Narwal S, Kumar V, Prakash O. α-Glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacogn Rev. 2011;5(9):19–29.
27. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the American association of clinical endocrinologists and American college of endocrinology on the comprehensive type 2 diabetes management algorithm–2016 executive summary. Endocr Pract. 2016;22(1):84–113.
28. Scheen AJ. DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab. 2012;38(2):89–101.
29. Chen KK. The acute toxicity of ephedrine. J Pharmacol Exp Therapeut. 1926;27(1):61–76.
30. Ojeda-Montes MJ, Ardid-Ruiz A, Tomás-Hernández S, Gimeno A, Cereto-Massagué A, Beltrán-Debón R, et al. Ephedrine as a lead compound for the development of new DPP-IV inhibitors. Future Med Chem. 2017;9(18):2129–46.
31. Macabeo AP, Aguinaldo A. Chemical and phytomedicinal investigations in Lunasia amara. Pharmacogn Rev. 2008;2(4):317–25.
32. Macabeo AP, Alejandro GJ, Hallare A, Vidar W, Villaflores O. Phytochemical survey and pharmacological activities of the indole alkaloids in the genus Voacanga thouars (apocynaceae) --an update. Pharmacogn Rev. 2009;3(5):143 -53.
33. Kurihara H, Mitani T, Kawabata J, Takahashi K. Inhibitory potencies of bromophenols from Rhodomelaceae algae against α-glucosidase activity. Fish Sci. 1999;65(2):300.
34. Gao H, Huang YN, Gao B, Xu PY, Inagaki C, Kawabata J. α-Glucosidase inhibitory effect by the flower buds of Tussilago farfara L. Food Chem. 2008;106(3):1195–201.
35. Kumar A, Chauhan S. Pancreatic lipase inhibitors: the road voyaged and successes. Life Sci. 2021;271:119115.
36. Matheson J, Zhou XM, Bourgault Z, Le Foll B. Potential of fatty acid amide hydrolase (FAAH), monoacylglycerol lipase (MAGL), and diacylglycerol lipase (DAGL) enzymes as targets for obesity treatment: a narrative review. Pharmaceuticals. 2021;14(12):1316.
37. Liu TT, Liu XT, Chen QX, Shi Y. Lipase inhibitors for obesity: a review. Biomed Pharmacother. 2020;128:110314.
38. Bajes HR, Almasri I, Bustanji Y. Plant products and their inhibitory activity against pancreatic lipase. Rev Bras Farmacogn. 2020;30:321–30.