INTRODUCTION
In the United States, cerebral ischemia is the 5th fact of mortality. Over 800,000 people are affected by the cerebral ischemic stroke every year (Go et al., 2014). Cerebral ischemia is an intense and developing neurological disorder. In cerebral ischemia, early reperfusion of the ischemic brain is found. The risks of reperfusion include serious edema of the brain, hemorrhagic results in the irreversible damage of brain tissue, and related complications leading to death. The most acceptable treatment for the ischemic stroke is the thrombolysis therapy of the occluded cerebral transformation, bruise of the neurovascular system and death of neuronal cells. Neuroprotective agents act on various neuro-pathophysiological mechanistic pathways related to focal cerebral ischemia. This feature leads to investigation of the therapeutic efficacy of different neuroprotective agents against the ischemic stroke (Durukan and Tatlisumak, 2007; Volcho et al., 2018).
In cerebral ischemia, blood clots in cerebral artery decrease blood supply to the brain tissue and disruption in balance of ionic gradients across membranes. Ionic imbalance helps to accumulate calcium and sodium ions and decrease pH in cellular environment. These events are responsible for the change of membrane transport, mitochondrial activity and activating enzymatic processes which is dependent on calcium specially DNA strand separating enzymes. In ischemic brain, excessive generation of free radicals is observed. These free radicals trigger lipid peroxidation leading to cell apoptosis. Principal therapeutic strategy for the treatment of cerebral ischemia is to maintain blood flow for short period of time to preserve neural tissue architecture. In brain ischemia, reperfusion of ischemic brain tissue is a prerequisite to restore its normal activity. Reperfusion makes the secondary damage of brain tissue, called reperfusion injury. Secondary approach is the amelioration of pathophysiological events created by free radicals produced in the damaged ischemic brain tissue. Thus, antioxidants show the significant neuroprotective effect against cerebral ischemia (Lee et al., 2020).
After cerebral ischemic attack, the pathogenesis of brain tissue follows complex pathways towards injury. Activation of local microglia and infiltration of neutrophil from blood to ischemic brain parenchyma are the main causes of the neuroinflammation after cerebral ischemic damage. This activates the transcription factors and produces proinflammatory markers (Lakhan et al., 2009; Muir et al., 2007; Yi et al., 2007). Pro-inflammatory mediators damage neurons, glial cells, axons, and capillaries and cause secondary brain damage after cerebral ischemia (Del Zoppo, 2010).
Bacoside –A, asiatic acid and kaempferol are widely used by ayurvedic physicians for their curative properties against neurodegenerative disorder. Bacopa monnieri leaf contains the bacoside-A. This phytocompound is triterpenoid saponin in nature. Bacoside-A combines the metabolites like bacopasaponin C, jujubogenin isomer of bacopasaponin C, bacoside A3 and bacopaside II. Bacosides-A has a neuroprotective activity (Sekhar et al., 2019) and anti-inflammatory activity (Madhu et al., 2019). Bacoside-A reduces oxidative stress and cancerous property of cells (Bhardwaj et al., 2018). Asiatic acid shows the neuroprotective activity (Krishnamurthy et al., 2009). Centella asiatica leaf contains asiatic acid which is chemically a pentacyclic triterpenoid. Asiatic acid is a neuroprotective agent that prevents inflammation (Park et al., 2017). Convolvulus pluricaulis leaf contains a flavonoid kaempferol. Kaempferol reduces oxidative stress and inflammation of neuronal cells (Yu et al., 2013). Anti-inflammatory activity of the kaempferol was studied in vitro in glial cells and lipopolysaccharide induced inflammatory animal models (Cheng et al., 2018; Park et al., 2011; Yang et al., 2019).
In this article, the antioxidant and neuroprotective activity of both three compounds were investigated by evaluating different enzyme markers related activity against reactive oxygen species (ROS) responsible for the damage of the tissue and for their application in synthesizing drugs toward the treatment of focal cerebral ischemia. To evaluate the possible efficacy of the agents different parameters like neurobehavioral functions, infarct volume, and neuropathological changes related to glial cells (microglia and astrocytes) have been investigated.
MATERIALS AND METHODS
Materials
Bacoside-A (90%, Sigma Aldrich), asiatic acid (97%, Sigma aldrich), kaempferol (97%, Sigma Aldrich), ET-1 (97% Sigma-aldrich), 2,3,5-triphenyl tetrazolium chloride (98% Sigma Aldrich), OX-42 monoclonal antibody (Novus biologicals, USA) (microglial marker), Glial fibrillary acidic protein (GFAP) monoclonal antibody (Proteintech, USA) (Astrocyte marker), HRP-conjugated Goat-antimouse IgG secondary antibody (G-bioscience, USA), Epinephrine (Sigma aldrich, USA), triton X-100 (Merck), DTNB (5,5′-Dithiobis-(2-nitrobenzoic acid) (Sigma aldrich, USA), o-cresolpthalein complexone (Sigma Aldrich, USA), thiobarbituric acid (98%, LOBA CHEMIE)
METHODS
Animal housing and maintenance
In this study, male Wistar rats (200–250 g) were used. Animals were maintained in departmental animal house facility having temperature (25°C ± 2°C) and humidity (45%–50%) and 12 hours light and dark cycle. All the animal experimentations were performed as per recommendation of Institutional Animal Ethics Committee, Department Of Zoology, University Of Kalyani.
Induction of focal cerebral ischemia
Anesthesia of rats was done by intraperitoneal injection of sodium pentobarbital (30 mg/kg body weight). The animal’s body temperature was 37°C throughout the handling procedure. During the time of surgery, marking of three points along the anterior -posterior axis of the bregma on cranium was done. Three points were drilled. 10 μg ET-1 was dissolved in 10-μl sterile normal saline (0.9%). First, ET-1 (1 μg/per site) was delivered into cortex by intracerebral injection using Hamilton® syringe (Sigma Aldrich, USA) (Horie et al., 2008). ET-1 was injected into the cortex at the rate of 0.8 μl/minute and the needle was kept in-situ for another 5 minutes before slowly removing it. The coordinates of intracerebal injection were measured from the rat atlas of Paxion and Watson (1998). Measurement of intracerebral injection coordinates from bregma and the distance from the surface of brain was
Rat (Cortex position) (Triple injection): AP 0, ML +2.5, DV −2.3; AP +2.3, ML +2.5, DV −2.3; AP +0.7, ML +3.8, DV −2.3 (AP: antero-posterior; ML: midlateral ; DV: dorsoventral)
The similar surgical procedures were done in the sham-operated group without the intracerebral delivery of ET-1.
Examination of neurological deficits
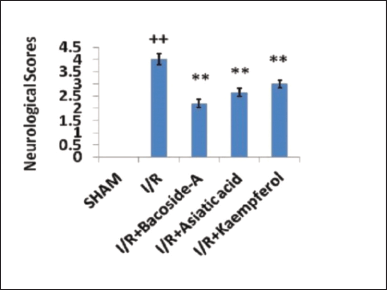
To evaluate the ischemic effect, neurobehavioral testing was performed in each rat after 24 hours of intracerebral injection of ET-1. The neurological deficit after focal cerebral ischemia (indicated as neurological score) was evaluated by a neurobehavioral experiment on 5 point scale where “0” means no neurological deficits; “1” means failure to extend left forepaw fully; “2” means circling to the left; “3” means failing to the left; and “4” means inability to walk spontaneously combined with depressed levels of consciousness (Peng et al., 2007). Neurological scores were evaluated in the ischemic rats after the treatment of bacoside-A, asiatic acid and kaempferol.
Drug treatment
Bacoside-A, asiatic acid, and kaempferol was formulated in a solution of normal saline and 0.5% sodium carboxy-methylcellulose (CMC-Na). The preparation was delivered by oral gavage at dose 50 mg/kg body weight once in a day up to 7 days after ischemia induction. The study on dose course of three phytochemicals showed significant positive effect at 50 mg/kg body weight in (data not shown). A hormetic effect of phytochemicals was observed at the dose of 75 mg/kg of body weight (within the limit of LD50 value). Sham-operated group and focal cerebral ischemia (I/R) group received the 0.5% CMC-Na in saline.
Division of rats was into five groups which have six animals in each group according to neuro-behavioral testing—(i) Sham-operated group (ii) Cerebral ischemia (I/R) group (iii) Cerebral ischemia (I/R) group + Bacoside-A (iv) Cerebral ischemia (I/R) group + Asiatic acid (v) Cerebral ischemia (I/R) + Kaempferol.
Determination of infarct volume
After neurological assessments, the rats of sham, I/R, I/R + bacoside-A, I/R + asiatic acid, and I/R + kaempferol group were decapitated. Dissection of brain tissue was followed coronally into 2-mm slices with the help of a razor blade. The sections were stained by 0.05% 2, 3, 5-tripenyltetrazolium chloride in phosphate buffer saline (PBS) at 37°C for 45 minutes in water bath. After staining, the slices were washed in PBS three times (1 minutes each). Then the slices were kept in 4% formaldehyde at 25°C at 24 hours before the image was captured. In the sections, the red area indicates the normal architecture and the white or pale area identified as the infarct region. The image J/Fiji processing system was applied to estimate the infarct area in every section (Schindelin et al., 2012). Infarct volume ratio = (total of infract area/the total of sections area) * 100%.
Immunohistochemical staining
After anesthesia, perfusion of rat through the heart was done by phosphate buffer saline and then 4% paraformaldehyde. After the collection of brain, the coronal sections of the bregma region in the ipsilateral ischemic hemisphere were used in the immunohistochemistry. Endogenous peroxide activity was blocked by the washing of the sections with 3% hydrogen peroxide. Antigen retrieval was done by sodium citrate buffer (pH 6.0). In the blocking step, incubation of sections was done by using 5% bovine serum albumin. Then the incubation of the sections were done at 4°C overnight with the OX-42 and GFAP primary antibodies. Primary antibodies were recognized by goat-anti mouse IgG secondary antibody. Then the 3,3′-diaminobenzidine tetrahydrochloride was applied and the counterstain of nuclei was done by hematoxylin. Observation of brain sections were done under light microscope.
For the semiquantitative analysis of the immunohistochemical result, three sections from each brain sample each having three microscopic fields from the ischemic boundary zone in cerebral cortex, were digitalized under 40×objective (Chu et al., 2006). Expressions of proteins were quantified by measuring the integrated optical density (IOD) of immuno-positive cells with the help of an image analysis system (Image J/Fijji).
Biochemical estimation
After the killing of animals by cervical dislocation, brains were decapitated. Homogenization of the infarcted area of the cerebral ischemic group and treated groups was done in the solution of phosphate buffer saline (pH 7.0) and ethylene diamine tetraacidic acid (EDTA). The centrifugation of the homogenate was done at 0°C at 1,000 rpm in a cold centrifuge. In the different biochemical experiments, the supernatant of the homogenate was taken.
Superoxide dismutase (SOD)
Measurement of the level of SOD was done according to the principle of Misra and Fridovich (1979) at 25°C. After mixing of supernatant with the carbonate buffer, the solution was kept at 10 minutes. After the addition of epinephrine in this mixture, the measurement of optical density was done at 480 nm on UV-SPECTROSCOPY.
Catalase (CAT)
According to the slightly modified version of Aebi (1984), the level of CAT was quantified. After mixing of supernatant with 100% ethanol, the mixture was kept in a cold condition for 25 minutes. After the addition of triton X-100, tubes were placed at room temperature. In a cuvette, the phosphate buffer saline, the above mixture (supernatant, 100% ethanol, and triton X-100), and hydrogen peroxide in PBS were mixed. UV-spectroscopy was applied to estimate the optical density at 240 nm.
Reduced glutathione (GSH)
The level of GSH was quantified according to the principle of Ellman (1959). The supernatant was mixed with 4% sulphosalisalisylic acid and then centrifugation of the mixture was done at 4°C for 5 minutes at 1,200 rpm. The supernatant was added with DTNB (5, 5′-Dithiobis-(2-nitrobenzoic acid) and estimation of optical density was done at 412 nm with the help of a UV-Visible spectrophotometer.
Estimation of total calcium
Samples were added to the deprotonation buffer in the glass centrifuge tubes. The tubes were kept in water bath for 3 minutes. Centrifugation of tubes was done at 10,000 rpm in hot conditions. The supernatant was kept in autoclaved test tubes, and o-cresolphthalein complexone was poured into every test tube, shaken well, and measured at 570 nm. Calcium chloride solution was used for the preparation of the standard plot (Lorentz, 1982).
Estimation of lipid peroxidation by measuring malondialdehyde (MDA)
Supernatant was added with 8.1% sodium dodecyl sulfate, vortexed, and incubated at 25°C for 10 minutes. After the addition of thiobarbituric acid (0.6%), the solution was kept in a water bath at 100°C for 60 minutes. The samples were kept at 25°C for cooling. After the addition of the mixture of butanol and pyridine (1.5:1), samples were shaken well. The solution was centrifuged at 1,000 rpm for 5 minutes. UV-spectrophotometer was used to measure the optical density of the colored solution at 532 nm against a reference blank (Ohkawa et al., 1979).
Histopathology
Coronal brain sections from the sham group, focal cerebral ischemia (I/R) group and drug administered groups were kept in a fixing solution composed of glacial acetic acid, formaldehyde (4%), and methanol (1:1:8). After sectioning, the paraffin block of brain tissues was prepared. Staining of brain sections was done with eosin and hematoxylin.
Statistical analysis
One-way analysis of variance was used for analyzing the data obtained from the above experiments. Expressing of data was in the form of mean ± SD. The statistical analysis was performed with the help of the origin software (version Pro 8.0).
RESULTS
Effect of bacoside-A, asiatic acid, and kaempferol on neurological deficit
Generally, impaired motor activities were associated with higher neurological deficits. The sham-operated group did not show any signs of neurobehavioral disorder. Therefore the neurological score of the sham-operated group was zero. Considering a five-point scale, the neurological scores in the cerebral ischemic group increased to 4.2 ± 0.23 after 24 hours of ischemia which was caused by ischemic brain injury whereas oral administration of bacoside-A (50 mg/kg), asiatic acid (50 mg/kg), and kaempferol (50 mg/kg) significantly decreased the neurological scores to 2.2 ± 0.16, 2.65 ± 0.17, and 3.01 ± 0.15, respectively, compared with focal cerebral ischemic (I/R) group (Fig. 1). Bacoside-A reduced the neurological deficit most among these three phytochemicals.
Effect of bacoside-A, asiatic acid and kaempferol on infarct volume
Figure 2A depicts the infarct region of the brain in each group after 24 hours of brain ischemia induction. Similar surgical procedures without the intracerebral delivery of ET-1 were followed in sham-operated rats and this group showed no infarct area. In the focal cerebral ischemic rats (I/R), a definite pale stained or white area in the cortex and striatum of the brain was identified as the ischemic area. Figure 2B shows infarct volume of the ischemic group was 37.8% ± 1.75% of the total brain volume. Oral administration of bacoside-A (50 mg/kg), asiatic acid (50 mg/kg), and kaempferol (50 mg/kg) significantly reduced the infarct volume to 31.8% ± 1.30%, 32.3% ± 1.25% and 34.06% ± 1.30%, respectively compared to the focal cerebral ischemic (I/R) group. Bacoside-A is the most effective phytochemical in reduction of the brain infarct volume.
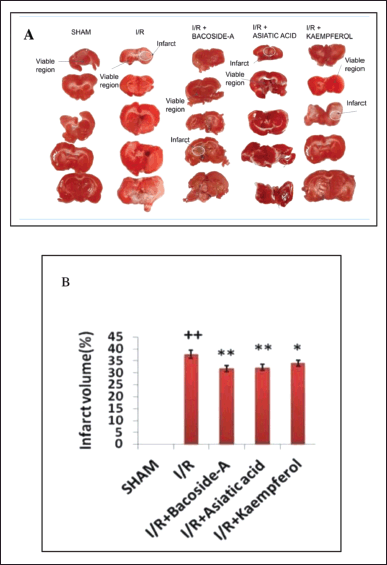 | Figure 1. Effect of bacoside-A, asiatic acid and kaempferol on neurological deficit in endothelin-1 induced focal cerebral ischemia of rat. The data were expressed as mean ± SD (n = 6 each group). *p < 0.05, **p < 0.01 compared with the focal cerebral ischemic control (I/R) group, ++p < 0.01 compared with the sham-operated group. [Click here to view] |
Alteration of pathology of microglia and astrocytes of brain tissue
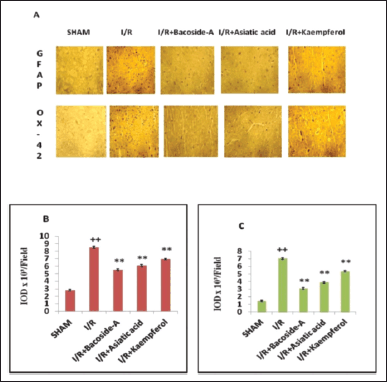
Neuropathological alterations of microglia and astrocytes of the brain were identified by immunohistochemistry using GFAP and OX-42. Glial cells (microglia and astrocytes) were activated and neuroinflammation was found after cerebral ischemic damage. In our result, the cerebral cortex of the sham-operated group showed few numbers of GFAP and OX-42-immunopositive cells (Fig. 3A). But, the numbers of GFAP and OX-42 immunopositive cells were increased in the ischemic penumbra of focal cerebral ischemic (I/R) rat. The numbers of those immunopositive cells were significantly decreased by treatment with bacoside-A, asiatic acid and kaempferol treatment (Fig. 3B and C).
 | Figure 2. Bacoside-A, asiatic acid, and kaempferol on attenuation of cerebral infarct volume in endothelin-1 induced brain of ischemic rat. (A) Picture of coronal brain sections stained with 2,3,5-triphenyltetrazolium chloride. (B) Infarct area presented with respect to the percentage of total brain area. The data were expressed as mean ± SD (n = 3 each group). *p < 0.05, **p < 0.01, compared with the focal cerebral ischemic control (I/R) group, ++p < 0.01 compared with the sham-operated group. [Click here to view] |
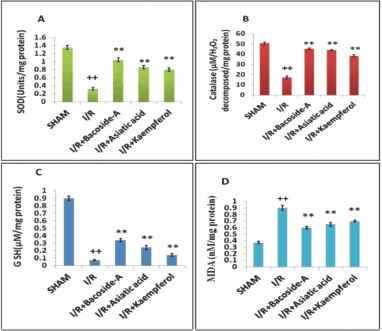
Effect of bacoside-A, asiatic acid, and kaempferol on the levels of SOD, CAT, GSH, and MDA in the brain tissue
In this study, the potency of bacoside-A, asiatic acid, and kaempferol on SOD, CAT, GSH, and MDA levels in the brain tissue of ET-1-induced ischemia-reperfusion rats was investigated. The content of SOD in the ischemic (I/R) rat brain was decreased. Treatment with bacoside-A (50 mg/kg), asiatic acid (50 mg/kg) and kaempferol (50 mg/kg) enhanced the SOD levels significantly (Fig. 4A). Ischemic control (I/R) group showed a significantly decreased level of CAT in comparison with the sham-operated group. The level of CAT was increased significantly in the group treated with bacoside-A (50 mg/kg), asiatic acid (50 mg/kg), and kaempferol (50 mg/kg) as compared with the ischemic control group (Fig. 4B). The ischemic control group (I/R) showed a significantly decreased level of GSH in the ischemic region of brain in comparison with the sham-operated group. The level of GSH was increased significantly in the group treated with bacoside-A (50 mg/kg), asiatic acid (50 mg/kg) and kaempferol (50 mg/kg.) against the focal cerebral ischemic (I/R) rat (Fig. 4C). Focal cerebral ischemic (I/R) showed the increased amount of brain MDA in comparison with the sham-operated group. In the bacoside-A (50 mg/kg), asiatic acid (50 mg/kg) and kaempferol (50 mg/kg) treated groups had significantly decreased level of brain MDA in comparison with the cerebral ischemia group (Fig. 4D).
 | Figure 3. Alteration of pathology of microglia and astrocytes of brain tissue after treatment with bacoside-A, asiatic acid, and kaempferol in endothelin-1 induced focal cerebral ischemic rat. (A) Images of GFAP—stained astrocytes and OX-42 stained microglia in the pneumbra position of ischemic cortex. (B, C) The IOD quantified the level of GFAP and OX-42 by using the amount of the immune-stained positive cells. The data are expressed as mean ± SD (n = 3 each group). **p < 0.01, compared with the focal cerebral ischemic control (I/R) group, ++p < 0.01 compared with the sham-operated group. [Click here to view] |
 | Figure 4. Bacoside-A, asiatic acid, and kaempferol activity on levels of (A) SOD (B) CAT (C) reduced glutathione (D) MDA in ischemic rat brain. The data are expressed as mean ± SD (n = 3 each group). *p < 0.05, **p < 0.01 compared with the focal cerebral ischemic control (I/R) group, ++p < 0.01 compared with the sham-operated group. [Click here to view] |
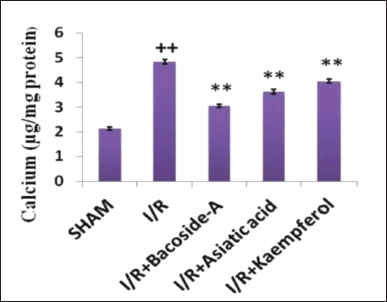
 | Figure 5. Changes of calcium level after treatment with bacoside-A, asiatic acid, and kaempferol of the focal cerebral ischemic rat. The data are expressed as mean ± SD (n = 3 each group). *p < 0.05, **p < 0.01 compared with the focal cerebral ischemic control (I/R) group, ++p < 0.01 compared with the sham-operated group. [Click here to view] |
Effect of bacoside-A, asiatic acid, and kaempferol on calcium levels
The effect of bacoside-A (50 mg/kg), asiatic acid (50 mg/kg), and kaempferol (50 mg/kg) on brain calcium levels in different groups has been estimated. The ischemic control (I/R) group showed a significantly increased level of brain total calcium in comparison with the sham-operated group. Reversal of the increased calcium level was found in the group that received the bacoside-A, asiatic acid, and kaempferol in comparison with the cerebral ischemia group (Fig. 5).
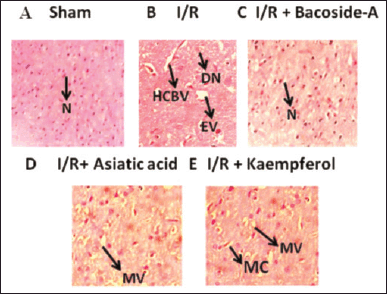 | Figure 6. Histopathological structure of brain tissue after treatment with bacoside-A, asiatic acid and kaempferol of ET-1-induced focal cerebral ischemic rat. N: neuronal cell; HCBV: Hypo perfusion-induced marked congestion of blood vessels; DN: excessive degeneration of neuronal cells; EV: excessive vaculation and necrosis of neural cells; MC: mild congestion; MV: mild vaculations. The magnification of the microscopic images is ×400. [Click here to view] |
Effect of bacoside-A, asiatic acid, and kaempferol on histopathological studies
The brain section of the sham-operated group showed the normal brain histological structure and nerve cells (N) containing viable nucleus (Fig. 6). Hypo perfusion-induced marked congestion of blood vessels (HCBV), increased degeneration of neuronal cells (DN), increased excessive vaculation (EV) and neuronal necrosis were found in cerebral ischemic (I/R) group. Groups treated with kaempferol (50 mg/kg) (I/R + Kaempferol) showed mild congestion (MC) and mild vaculations (MV). Groups treated with asiatic acid (50 mg/kg) (I/R + Asiatic acid) showed only MV, whereas the bacoside-A (50 mg/kg) (I/R + Bacoside-A) treated group showed neuronal cells (N) with a normal nucleus. So, treatment with bacoside-A totally reversed the necrosis and congestion of neurons and congestion of blood vessels in comparison with the ischemic control (I/R) group.
DISCUSSION
ET-1 is a potent vasoconstrictor, so it constricts the cerebral artery and as a result the supply of blood to brain tissue is blocked. Infarction induced by blocking of the arteries in brain is observed in the 60% brain stroke affected people. Therefore, ET-1 induced brain injury is considered as the equivalent pathological condition to brain stroke which is assessed by measuring neurological score. ET-1-induced neurological score was four times higher in ischemic brain in comparison to the sham-operated group.
Bacoside-A is the main component of the plant B. monnieri. The nootropic and neuroprotective activity of bacosides have already been reported (Anand et al., 2012; Janani et al., 2009 and 2010; Kamkaew et al., 2011; Shahid et al., 2016; Sharath et al., 2010). Bacoside-A maintains ionic balance in the cells of brain after exposing the rat model in cigarette smoke (Anbarasi et al., 2006). Asiatic acid is the major compound of the plant C. asiatica. Asiatic acid showed neuroprotective activity in mice model too (Krishnamurthy et al., 2009). Another phytochemical, kaempferol is the active compound of C. pluricaulis and has low water solubility that can be improved by additional sugar molecule (Yu et al., 2013). Previous evidences have shown the attenuation of lipopolysaccharide-induced inflammation by kaempferol in cultured BV2 microglial cells (Park et al., 2011). Intravenous administration of kaempferol- 3-O rutinoside decreases brain infarct volume and neurological deficit scores in ischemic rats (Li et al., 2006). Our research evaluated the comparative neuroprotective activity of bacoside-A, asiatic acid and kaempferol in ET-1 induced ischemic brain injury. It has been observed in our study that treatment with bacoside-A (50 mg/kg), asiatic acid (50 mg/kg), and kaempferol (50 mg/kg) significantly attenuated neurological deficits and infarct volume in comparison with the cerebral ischemia group.
Several reports informed that brain ischemia-reperfusion injury damages all types of brain cells such as neurons and axons and blood-brain barrier components e.g. microglia, astrocytes and oligodendritic cells. Glial activation after focal cerebral ischemic injury kills the brain tissue through the inflammation of neurons (Xu et al., 2020). Therefore, the efficacy of the bacoside-A, asiatic acid and kaempferol on definite glial cell markers after focal cerebral ischemia was investigated by the process of immunohistochemistry of OX-42 and GFAP. We found that the OX-42 and GFAP levels were decreased markedly in the ischemic region of brain after the administration of bacoside-A, asiatic acid, and kaempferol. Our results informed that the anti-inflammatory and neuroprotective activity of bacoside-A, asiatic acid, and kaempferol inhibits the glial activation. Research data showed that the oxidative stress can be found in the ischemic area of brain (Jelinek et al., 2021). Our research result showed that the decreased levels of oxidative stress markers, e.g., SOD, CAT, and GSH, and increased levels of MDA were found in the ET-1-induced focal cerebral ischemic tissue. Hydrogen peroxide is formed by the SOD from superoxide radicals. Hydrogen peroxide is dissociated into oxygen and water with the help of CAT and glutathione peroxidase and this prevents the generation of hydroxyl free radicals (Kurutas, 2016). In the different stages of metabolic cycle, these enzymes act in a co-operative fashion against free radicals. When the antioxidant properties of the tissues could not act properly, the oxidative stress of cells and lipid peroxidation in the membrane were found (Parikh et al., 2003). In the ischemic region of brain, activation of inducible nitric oxide synthase produces a high amount of nitric oxide. This nitric oxide can react with superoxide molecules to produce peroxynitrile. Peroxynitrile hydroxylates the aromatic residue of amino acids and nucleotide in the cytoplasm and nucleus of the cells (Beckman et al., 1992; Moreno and Pyror, 1992). For this, internal mechanisms of cell deregulate and neuronal loss is found after focal cerebral ischemic damage. In the ischemic region, amount of MDA increases and amounts of GSH, CAT and SOD decrease. In this study, GSH level was depleted in focal cerebral ischemic rat (I/R). In brain, the most important endogenous antioxidant weapon is GSH. The scavanging of lipid peroxide and hydrogen peroxide is done by this enzyme (Fujii et al., 2022). After ischemic repurfusion injury, level of GSH in brain cells is depleted by the formation of cysteine from GSH, mixture of disulfide and decline production of GSH (Shivakumar et al., 1995). High amount of ROS leads to the peroxidation of membrane lipid. This event accumulates MDA in cell (Ayala et al., 2014). Peroxidation of lipid is occurred by damage of brain tissue associated with free radicals. The membrane phospholipids polyunsaturated fatty acid (PUFA) peroxidation resumes till the consumption of substrate by antioxidants. Product of lipid peroxidation mainly the MDA level is most important marker of focal cerebral ischemic damage (Awooda, 2019). Many research reports revealed that the infarct area, clinical symptoms and patient’s character are associated with the increased level of MDA (Bolokadze et al., 2004; Gariballa et al., 2002; Polidori et al., 2002; Re et al., 1997; Sharpe et al., 1994). Increased MDA level in ischemic rat indicates ROS mediated reperfusion injury. Our data showed that the elevation of lipid peroxidation level and depletion of antioxidant markers namely SOD, CAT and GSH in focal cerebral ischemic groups are found which are supported by other research reports of cerebrovascular disorders (Farbiszewski et al., 1995; Jenner, 1994; Viglino et al., 1998). Increased calcium level in cytosol indicates the elevation of oxidative damage to brain tissue. In the present study, bacoside-A, asiatic acid, and kaempferol inhibited oxidative stress in the ET-1-induced focal cerebral ischemic tissue of rats.
Histopathological studies revealed that the sham group contains neuronal cells (N) with normal nucleus. Cerebral ischemic (I/R) group showed marked HCBV, excessive DN, excessive EV, and histopathological necrosis in brain tissue. But bacoside – the treated group showed only neuronal cells (N) with normal nucleus. Asiatic-acid-treated group showed MV and kaempferol treated group showed the MV and MC blood vessels in the brain tissue.
CONCLUSION
Induction of focal cerebral ischemia was done in rats by the intracerebal injection of ET-1. ET-1 produces blood clot in middle cerebral artery and reduces the oxygen supply to brain tissue. This results the severe damage of brain tissue. Oral administration is the most suitable approach for the treatment of focal cerebral ischemia. After treatment with three different phytochemicals such as bacoside-A, asiatic acid, and kaempferol significantly improved the damaged brain tissues. Bacosides-A improved neurological deficit and the infarct volume better than the other two phytochemicals. Bacoside-A repaired more damaged astrocytes and glial cells. Bacoside-A increased the SOD, CAT, GSH levels and decreased the MDA and calcium levels most among all three phytochemicals. Beside this, histopathology study revealed that the bacoside-A improved the cytoarchitecture of damaged brain tissue mostly. But asiatic acid and kaempferol treatment did not improve the damaged brain tissue remarkably. From the above experiments, it can be concluded that bacoside-A is most effective neuroprotective antioxidant out of the three phytochemicals against ET-1-induced focal cerebral ischemic brain injury.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the Central Council for Research in Ayurvedic Sciences (CCRAS), Ministry of AYUSH, Govt. of India; University Grants Comission, New Delhi and University Of Kalyani for supporting this research.
AUTHOR’S CONTRIBUTION
The experimental design was framed by AG and DN and executed by AG and NK. Data were processed by AG and NK. The manuscript was written and edited by the corresponding author and co authors.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ETHICAL APPROVAL
Animal experimentations were performed as per recommendation of IAEC, Department Of Zoology, University Of Kalyani. The ethical approval certificate no. KU/03-09-21/02 dated 03.09.2021.
DATA AVAILABILITY
All data produced and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aebi H. Catalase. Meth Enzymol, 1984; 105:125–6.
Anand T, Phani KG, Pandareesh MD, Swamy MS, Khanum F, Bawa AS. Effect of bacoside extract from Bacopa monniera on physical fatigue induced by forced swimming. Phytother Res, 2012; 26(4):587.
Anbarasi K, Vani G, Balakrishna K, Devi CS. Effect of bacoside A on brain antioxidant status in cigarette smoke exposed rats. Life Sci, 2006; 78(12):1378–84.
Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-Nonenal. Oxdid Med Cell Longev, 2014; 2014:360438.
Awooda HA. Pathophysiology of cerebral ischemia: role of oxidative/nitrosative stress. J Biosci Med, 2019; 7(3):20–8.
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA, 1992; 87:1620–4.
Bhardwaj P, Jain CK, Mathur A. Comparative evaluation of four triterpenoid glycoside saponins of bacoside A in alleviating sub-cellular oxidative stress of N2a neuroblastoma cells. J Pharm Pharmacol, 2018; 70(11):1531–40.
Bolokadze N, Lobjanidze I, Momtselidze N, Solomonia R, Shakarishvili R. Blood rheological properties and lipid peroxidation in cerebral and systemic circulation of neurocritical patients. Clin Hemorheol Microcir, 2004; 30:99–105.
Cheng X, Yang YL, Yang H, Wang YH, Du GH. Kaempferol alleviates LPS-induced neuroinflammation and BBB dysfunction in mice via inhibiting HMGB1 re-lease and down-regulating TLR4/MyD88 pathway. Int Immunopharmacol, 2018; 56:29–35.
Chu K, Lee ST, Koo JS, Jung KH, Kim EH, Sinn DI, Kim JM, Ko SY, Kim SJ, Song EC, Kim M, Roh JK. Peroxisome proliferator—activated receptor-gamma-agonist, rosiglitazone, promotes angio-genesis after focal cerebral ischemia. Brain Res, 2006; 1093:208–18.
Del Zoppo GJ. The neurovascular unit in the setting of stroke. J Intern Med, 2010; 267(2):156–71.
Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav, 2007; 87(1):179–97.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys, 1959; 82:70–7.
Farbiszewski R, Bielawski K, Bielawska A, Sobaniec W. Spermine protects in vivo the anti-oxidant enzymes in transiently hypoperfused rat brain. Acta Neurobiol Expermentalis, 1995; 55:253–8.
Fujii J, Takujiro H, Tsukasa O. Superoxide radicals in the execution of cell death. Antioxidants, 2022; 11(3):501.
Gariballa SE, Hutchin TP, Sinclair AJ. Antioxidant capacity after acute ischemic stroke. QJM, 2002; 95:685–90.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, MohlerIII ER, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey GK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation, 2014; 129:e28–92.
Horie N, Maag AL, Hamilton SA, Shichinohe H, Bliss TM, Steinberg GK. Mouse model of focal cerebral ischemia using endothelin-1. J Neurosci Methods, 2008; 173(2):286–90.
Janani P, Sivakumari K, Geetha A, Ravisankar B, Parthasarathy C. Chemopreventive effect of bacoside A on N-nitrosodiethylamine-induced hepatocarcinogenesis in rats. J Cancer Res Clin Oncol, 2010; 136(5):759–70.
Janani P, Sivakumari K, Parthasarathy C. Hepatoprotectiveactivity of bacoside A against N-nitrosodiethylamine-induced liver toxicity in adult rats. Cell Biol Toxicol, 2009; 25(5):425–34.
Jelinek M, Jurajda M, Duris K. Oxidative stress in the brain: basic concepts and treatment strategies in stroke. Antioxidants, 2021; 10(12):1886.
Jenner P. Oxidative damage in neurodegenerating diseases. Lancet, 1994; 344:796–8.
Kamkaew N, Scholfield CN, Ingkaninan K, Maneesai P, Parkington HC, Tare M, Chootip K. Bacopa monnieri and its constituents is hypotensive in anaesthetized rats and vasodilator in various artery types. J. Ethnopharmacol, 2011; 137(1):790–5.
Krishnamurthy RG, Senut MC, Zemke D, Min J, Frenkel MB, Greenberg EJ, Yu SW, Ahn N, Goudreau J, Kassab M, Panickar KS, Majid A. Asiatic acid, a pentacyclic triterpene from Centella asiatica, is neuroprotective in a mouse model of focal cerebral ischemia. J Neurosci Res, 2009; 87(11):2541–50.
Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J, 2016; 15:71.
Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med, 2009; 7:97.
Lee KH, Cha M, Lee BH. Neuroprotective effect of antioxidants in the brain. Int J Mol Sci, 2020; 21(19):7152.
Li R, Guo M, Zhang G, Xu X, Li Q. Nicotiflorin reduces cerebral ischemic damage and upregulates endothelial nitric oxide synthase in primarily cultured rat cerebral blood vessel endothelial cells. J Ethnopharmacol, 2006; 107:143–50.
Lorentz K. Improved determination of serum calcium with orthocresolpthalein complex one. Clin Chem Acta, 1982; 126:327–33.
Madhu K, Prakash T, Maya S. Bacoside-A inhibits inflammatory cytokines and chemokine in experimental autoimmune encephalomyelitis. Biomed Pharmacother, 2019; 109:1339–45.
Misra HP, Fridovich J. The role of superoxide anion in the autooxidation of epinephrine and simple assay for superoxide dismutase. J Biol Chem, 1979; 247:3170–5.
Moreno JJ, Pryor WA. Inactivation of alpha 1-proteinase inhibitor by peroxynitrite. Chem Res Toxicol, 1992; 5:425–31.
Muir KW, Tyrrell P, Sattar N, Warburton E. Inflammation and ischemic stroke. Curr Opin Neurol, 2007; 20:334–42.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animals and tissue by thiobarbituric acid reaction. Ana Biochem, 1979; 95:351–8.
Parikh V, Mohammad Khan M, Mahadik SP. Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res, 2003; 37:43–51.
Park SE, Sapkota K, Kim S, Kim H, Kim SJ. Kaempferol acts through mi-togen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. Br J Pharmacol, 2011; 164:1008–25.
Park JH, Seo YH, Jang JH, Jeong CH, Lee S, Park B. Asiatic acid attenuates methamphetamine-induced neuroinflammation and neurotoxicity through blocking of NF-kB/STAT3/ERK and mitochondria-mediated apoptosis pathway. J Neuroinflammation, 2017; 14(1):240.
Paxion G, Watson C. The rat brain in stereotaxic coordinates. 4th edition, Academic Press, San Diego, CA, 1998.
Peng HY, Du JR, Zhang GY, Kuang X, Liu YX. Effect of Z-ligustilide against permanent focal ischemic damage in rats. Biol Pharm Bull, 2007; 30:309–12.
Polidori MC, Cherubini A, Stahl W, Senin U, Sies H. Plasma carotenoid and malondialdehyde levels ischemic stroke patients: relationship to early outcome. Free Radic Res, 2002; 36:265–8.
Re G, Azzimondi G, Lanzarini C, Bassein L, Vaona I. Plasma lipoperoxidative markers in ischaemic stroke suggest brain embolism. Eur J Emerg Med, 1997; 4:5–9.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods, 2012; 9:676–82.
Sekhar VC, Viswanathan G, Baby S. Insights into the molecular aspects of neuroprotective bacoside A and bacopaside l. Curr Neuropharmacol, 2019; 17(5):438–46.
Shahid M, Subhan F, Ullah I, Ali G, Alam J, Shah R. Beneficial effects of Bacopa monnieri extract on opioid induced toxicity. Heliyon, 2016; 2(2):e00068.
Sharath R, Harish BG, Krishna V, Sathyanarayana BN, Swamy HM. Wound healing and protease inhibition activity of bacoside-A, isolated from Bacopa monnieri wettest. Phytother Res, 2010; 24(8):1217–22.
Sharpe PC, Mulholland C, Trinick T. Ascorbate and malondialdehyde in stroke patients. Ir J Med Sci, 1994; 163:488–91.
Shivakumar BR, Kolluri SV, Ravindranath V. Glutathione and protein thiol homeostasis in brain during reperfusion after cerebral ischemia. J Pharmacol Exp Ther, 1995; 274:1167–73.
Viglino P, Scara M, Rotilio G, Rigo A. A kinectic study of the reactions between H2O2 and Cu, Zn superoxide dismutase, evidence for an electrostatic control of the reaction rate. Biochem Biophys Acta, 1998; 952:77–82.
Volcho KP, Laev SS, Ashraf GM, Aliev G, Salakhutdinov NF. Application of monoterpenoids and their derivatives for treatment of neurodegenerative disorders. Curr Med Chem, 2018; 25(39):5327–46.
Xu S, Lu J, Shao A, Zhang JH. Glial cells: role of the immune response in ischemic stroke. Front Immunol, 2020; 11:294.
Yang YL, Cheng X, Li WH, Liu M, Wang YH, Du GH. Kaempferol attenuates LPS-induced striatum injury in mice involving chen-neuroinflammation, maintaining BBB integrity, and down-regulating the HMGB1/TLR4 pathway. Int J Mol Sci, 2019; 20:491.
Yi JH, Park SW, Kapadia R, Vemuganti R. Role of transcription factors in mediating post ischemic cerebral inflammation and brain damage. Neuro Chem Int, 2007; 50:1014–27.
Yu L, Chen C, Wang LF, Kuang X, Liu K, Zhang H, Du JR. Neuroprotective effect of kaempferol glycosides against brain injury and neuroinflammation by inhibiting the activation of NF-κB and STAT3 in transient focal stroke. PLoS One, 2013; 8(2):e55839.