INTRODUCTION
Metabolic disorders like diabetes affected 463 million individuals globally till 2019 and this figure is predicted to climb to 578 million by 2030. This figure is again expected to climb to 700 million by 2045. Those living in high-income countries are more likely to be affected, as are those living in metropolitan areas. One out of every two diabetics does not know they have the disease. According to current predictions, there will be 374 million people with impaired glucose tolerance in 2019, 454 million by 2030, and 548 million in the year 2045 (Saeedi et al., 2019). Anti-diabetic medications having enhanced glycemic control and lesser harmful effects have been the subject of numerous clinical investigations. Recent licensing of sodium-glucose cotransporter-2 inhibitors (SGLT-2) for use in the therapy of diabetes mellitus type 2 (DMT2) either in single or in the combined form alongside existing diabetic medications has increased their visibility in the medical community (Kalra, 2014). Remogliflozin etabonate (RGF), pro-drug of RGF with the chemical term [5-methyl-4-(4-(1-methylethoxy)benzyl)-1-(1-methylethyl)-1H-pyrazol-3-yl 6-O-(ethoxycarbonyl)-b-D-glucopyranoside] is classed as SGLT2 inhibitor. The kidneys’ ability to reabsorb glucose is mostly dependent on a transporter called SGLT2. Often used in conjunction with other drugs, it can be helpful in controlling DMT2 when used additionally with controlled food intake and workout (Dharmalingam et al., 2020; Tammisetty et al., 2021). Vildagliptin (VGT) also recognized as (S)-1-[N-(3-hydroxy-1-adamantyl) glycyl] pyrrolidine-2-carbonitrile, an example of a competitive, reversible dipeptidyl peptidase-4 inhibitor. By blocking this enzyme, the collapse of glucagon-like peptide-1 (GLP-1) and glucose reliant insulinotropic polypeptide (GIP) is postponed. The discharge of insulin is stimulated by GLP-1 and GIP, which prevent glucagon from being secreted by pancreatic beta cells. All of these actions work together to reduce glycogenolysis in the liver and boost insulin production (Boovizhikannan and Palanirajan, 2013; Keating, 2014). A biguanide antidiabetic, metformin hydrochloride (MTH) is also known by its chemical name, 1,1-dimethyl biguanide hydrochloride. This medication is prescribed most often to individuals who are overweight and suffering from DMT2. They do not stimulate insulin secretion but rather have an anti-diabetic effect when some insulin is already present. Possible methods of action include postponing glucose absorption in the gastrointestinal tract, boosting insulin sensitivity, allowing glucose to enter cells, and inhibiting gluconeogenesis in the liver (Indian Pharmacopoeia, 2018; Martindale, 2009; Sen et al., 2016; The Merck Index, 2001). The structures of the three analytes are displayed in Figure 1.
Patients with diabetes typically require a combination of drugs to control their blood sugar levels. The newer combination of RGF, VGT, and MTH was launched in October 2021 by Glenmark Pharmaceuticals Ltd., India; it works through three synergistic mechanisms to help control glycemic levels in people with diabetes (type 2) who may benefit from consuming RGF and VGT together with MTH. Remo® MV 500 is a fixed-dose combination of RGF, VGT, and MTH as tablets that lower glycemia in individuals with type 2 diabetes when used in conjunction with a healthy diet and frequent exercise (Glenmark Pharmaceuticals Ltd, 2021; Hussey et al., 2013; Khaladkar et al., 2022). From ultraviolet (UV) spectrophotometry (Attimarad et al., 2021a, 2021b, 2021c; Banik et al., 2015; Itigimatha et al., 2022; Kumari and Khansili, 2020; Patel and Desai, 2022; Sen et al., 2016, 2022) to HPLC (Attimarad et al., 2022a, 2022b, 2022c; Boovizhikannan and Palanirajan, 2013; Itigimatha et al., 2022; Patel and Desai, 2022; Shah et al., 2020; Shakoor et al., 2020; Tammisetty et al., 2021), and HPTLC (Bendale et al., 2018; El-Kimary et al., 2016; Modi et al., 2013; Patil et al., 2020; Shah et al., 2021; Thomas et al., 2011) the literature review uncovered a wide variety of systematic approaches for the evaluation of RGF, VGT, and MTH alone or in conjunction with different analytes. However, only two research articles reported (Attimarad et al., 2022c; Patel and Desai, 2022) simultaneous estimation of all three drugs under study. But reported methods show some shortcoming in various aspects like range, sensitivity, specificity, etc. Therefore, the proposed methods justify their existence and have been compared with the reported methods (Table 6). The proposed procedures have been validated according to International Conference on Harmonization (ICH) criteria cover a broad concentration series with increased sensitivity, and are relatively simple in terms of standard and sample preparation. Analytical methods based on UV spectroscopy are considered simple, rapid, and inexpensive; they are ideal for determining the quality of commonly prescribed medications. Direct UV spectroscopic methods have the advantage of being simple to use and interpret, but they suffer from the fundamental limitation of being susceptible to the influence of multicomponent formulations and formulation additives (Attimarad et al., 2012). Derivative and ratio derivative UV spectroscopic techniques were therefore devised in addition to the simultaneous equation method (SEM) to counteract these effects, and they were validated as suitable for the simultaneous estimation of RGF, VGT, and MTH without hindrances.
 | Figure 1. Chemical constructions of RGF; VGT and MTH. [Click here to view] |
MATERIALS AND METHODS
Raw materials and reagents
Throughout the study, researchers relied on the RGF reference standard (99.6% w/w), procured from Glenmark Pharmaceuticals Ltd., Sinnar, Nasik, India. Dalton PharmaChem in Vadodara, Gujarat, India, was the source for the VGT (99.25% w/w) and MTH (99.42% w/w). Loba Chemie Pvt. Ltd., Mumbai, India supplied all diluents, raw materials, and excipients (specificity evaluation) made use in the research.
Instruments
The entire study used a Double beam UV visible spectrophotometer (UV-1800 with UV Probe), Shimadzu Corporation, Kyoto, Japan with a Quartz (1 cm) sample container that was essentially the same as the one used in the original instrument. For the purpose of determining precise masses, we used an electronic balance (Adventurer Pro AVG264C), Ohaus Corporation, Pine Brook, NJ.
Preparation of standard solution
Each drug’s stock solution (RGF, VGT, and MTH) was made by adding 10 mg of reference analytes by weight to a 100 ml standard flask. Reference analytes were reduced to a concentration of 100 μg/ml using methanol by filling the container up to its capacity. The concentration was further lowered by diluting the solution with methanol.
Preparation of combined standard solution
A sequence of a combination of reference solutions (RGF + VGT + MTH, RGF + VGT, RGF + MTH, VGT + MTH) was made by shifting the right quantity of RGF, VGT, and MTH reference solutions (0.25–1.5 ml) into 10 ml standard flasks one at a time and filling them with methanol until they reached the required concentration (2.5–15 μg/ml).
Sample solution preparation
Powdered tablets comprising 10 mg of RGF, 5 mg of VGT, and 50 mg of MTH were measured out and placed in a 50 ml standard bottle. The measured amount of pure RGF (40 mg) and VGT (45 mg) was put into the 50 ml standard flask. The standard bottle was occupied with 30 ml of methanol and agitated for 10 minutes. To reach 50 ml, methanol was poured, and filtration of the mixture was performed using Whatman filter paper 41. One milliliter of the aforementioned solution was transferred to a 10-ml bottle, and the remaining 9 ml were filled with methanol. Further, 1 ml of the subsequent solution was transferred to a volumetric bottle (10 ml) and the remaining space was occupied with the same solvent to produce optimum dilution (10 μg/ml of RGF, VGT, and MTH).
Procedure/method
Simultaneous equation method
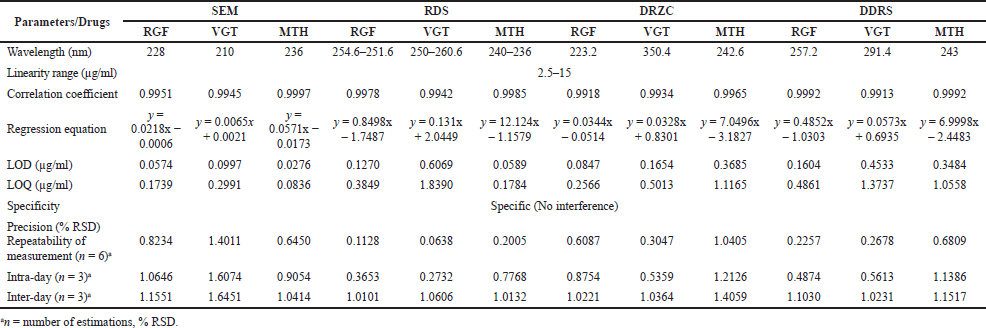
The mentioned methodology was used to estimate RGF, VGT, and MTH in the formulation of tablets. The UV region of 200–400 nm was used to record the UV spectra of each standard analyte under investigation. The approximation of the suggested analytes in the tablet required the selection of an acceptable wavelength, which was done using the overlapping UV spectra. RGF, VGT, and MTH’s overlaid zero-order spectra showed a maximum absorbance at 228, 210, and 236 nm, respectively (Fig. 2). Absorptivity of each medication was then computed and summarized (Table 1). Using the formula, the amount of medication in the formulation was calculated.
The amounts of RGF, VGT, and MTH that are progressively present in the sample solutions are represented in the equation above by Cx, Cy, and Cz.
A1, A2, and A3 are the sample’s absorbances at 228, 210, and 236 nm, respectively. RGF’s absorptivity at 228, 210, and 236 nm is represented by ax1, ax2, and ax3, respectively. The absorptivity of VGT at 228, 210, and 236 nm is represented by ay1, ay2, and ay3. Absorptivity of MTH at 228, 210, and 236 nm is represented by az1, az2, and az3, respectively (Beckett and Stenlake, 2005; Kalyani and Rao, 2018) as presented in Table 1.
Ratio difference spectroscopic approach (RDS)
The ratio spectra were acquired by taking the total of the VGT and MTH absorption spectra (2.5 μg/ml of both as a double divisor) and dividing it by the UV absorption band of several concentrations of RGF and the combined three analytes. The ratio spectrum’s amplitude at 251.6 nm was then deducted from the spectrum’s amplitude at 254.6 nm. Additionally, a standard curve was created using the relationship between amplitude difference and concentration. RGF and MTH (1 μg/ml) were used as a double divisor to divide the UV spectra of analyte solutions produced with varied dilutions of VGT and the triple drug combination, and ratio spectra were also recorded. Later, the amplitude at 260.6 nm was subtracted from the ratio spectrum’s peak amplitude at 250 nm. Also, a calibration graph was formed by plotting the variation in amplitude versus the concentration. To get the ratio spectra, the absorption bands of different amounts of MTH and the ternary mixture were recorded and then divided by the whole absorption band of RGF and VGT solutions (2.5 μg/ml each) as a divisor. Afterward, the ratio spectrum’s peak amplitude at 236 nm was subtracted from the peak amplitude at 240 nm. Also, a linear graph was built by taking the variation in amplitude against the concentration (Abdelrahman and Abdelwahab, 2014; Darwish et al., 2016; Eissa and Abou Al Alamein, 2018; Erk, 2001; Lotfy et al., 2013; Millership et al., 2005; Zaazaa et al., 2015).
Derivative ratio spectrum-zero crossing method (DRZC)
First, the response of RGF, VGT, and MTH, as well as the three drugs together in the UV region were saved at dissimilar concentrations (linearity series). Afterward, the ratio spectra were made by a division of the absorption band of RGF, MTH, and a combined mixture of 3 drugs with VGT by a reference spectrum of 2.5 μg/ml of VGT. After that, the ratio spectra were turned into the first derivative, which made the first-order ratio spectra. The amplitudes were found to be proportional to the ternary mixture concentration of RGF and MTH at 223.2 nm (zero-crossing spot for MTH) and 242.6 nm (zero-crossing spot for RGF) in the first derivative of ratio spectra. To make calibration graphs, the amplitudes of the first derivative ratio spectra were taken versus increasing concentrations of standard RGF and MTH, with standard VGT used as a divisor. The linear graph thus obtained can be used to find out the amount of RGF and MTH. In the same way, the ratio spectra were made by dividing the saved spectra of VGT, MTH, and their ternary mixture with RGF by reference spectra of 2.5 μg/ml RGF. Also, the first derivatives of the ratio spectra were documented. Signals at 350.4 nm (the zero-crossing point for MTH) in the first derivative of the ratio spectra represent VGT. The calibration graphs for VGT were made by figuring out the first derivative ratio spectra with regard to increasing concentrations of reference VGT using reference RGF as a divisor. This method can be used for the estimation of VGT (Alvi et al., 2013; Dinç and Onur, 1998;).
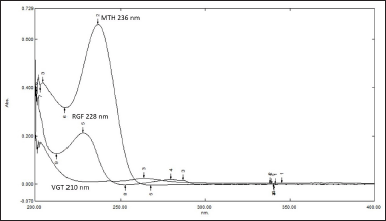 | Figure 2. Overlain UV spectra of RGF, VGT and MTH (12.5 μg/ml). [Click here to view] |
 | Table 1. Average absorptivity values (RGF, VGT and MTH) at various wavelengths using the SEM method. [Click here to view] |
Double divisor ratio spectra derivative method (DDRS)
The ratio spectra were made by taking the spectra of analyte solutions with different strengths of RGF and the combination of three analytes and dividing it by the total absorption band of VGT and MTH (2.5 μg/ml) as the divisor. After that, the first derivatives of ratio spectra were made. The amount of RGF was then calculated at 257.2 nm in the selected spectral zone. The UV spectra of different amounts of VGT and the ternary combination were documented and divided by RGF and MTH (1 μg/ml each in methanol) to get ratio spectra, which were also recorded.
Then, the ratio spectra 1st derivative was recorded, and the signals at 291.4 nm were used to estimate VGT. To obtain the ratio spectra, UV spectra of analyte solutions using different MTH concentrations, and the mixture of three analytes were recorded and division of these spectra using the sum of the absorption spectra of RGF and VGT (2.5 μg/ml) solutions as the divisor was performed. The first derivative of these ratio spectra was then plotted and the quantity of MTH was found out by utilizing the first derivative signals at 243 nm (Dinç et al., 2002a, 2002b; Gohel et al., 2014; Hajian and Soltaninezhad, 2013; Omer and Fakhre, 2020; Youssef and Maher, 2008).
Assessment of sample solution
The solution was made and diluted, as covered in the earlier section. For SEM, the extent of the analyte was determined by simultaneously calculating a formula based on the absorbances of sample solutions at various wavelengths and standard absorptivity values (Table 1). Peak amplitude was measured for the remaining approaches (RDS, DRZC, and DDRS), and regression equations were employed in order to quantify the extent of the analyte.
Validation of spectroscopic methods
The planned methodologies were authenticated by referring to the regulations of the “International Conference on Harmonization” and some of the reported research articles (Attimarad et al., 2021a, 2022a; Banik et al., 2015; ICH, 2005; Kumari and Khansili, 2020; Sen et al., 2015, 2016, 2022).
Specificity
Tablet excipients included in the formulation were tested for potential interactions with the medication’s active ingredient. Each of the tablet excipients was mixed together in the correct proportions, diluted by means of methanol, then filtered utilizing Whatman filter paper (41) to produce the final solution. To further investigate the potential chemical interaction between excipients and analytes, UV scanning was used to compare all dummy and standard solutions.
Linearity and range
Using the SEM method, absorbances were recorded at 228, 210, and 236 nm for RGF, VGT, and MTH in methanol to determine linear correlation and range; using the RDS method, the amplitude difference was determined (RGF: 254.6–251.6 nm; VGT: 260.6–250 nm; MTH: 240–236 nm). Both the DRZC (RGF: 223.2 nm; VGT: 350.4 nm; MTH: 242.6 nm) and DDRS (RGF: 257.2 nm; VGT: 291.4 nm; MTH: 243 nm) methods were used to measure the amplitude. Standard analyte absorbance versus concentration calibration plots was created using the SEM method, reference analyte amplitude difference versus concentration calibration plots using the RDS method, and peak amplitude versus concentration calibration plots using the DRZC and DDRS methods. By employing the least-squares method, we were able to calculate the correlation coefficient, slope, and intercept. The results were calculated as the mean of six separate assessments.
Precision
To assess the accuracy of the procedure, tests were executed to examine its repeatability, intra and inter-day precision. Analyzing sample solutions (RGF, VGT, and MTH: 2 and 8 μg/ml) 6 times and determining the % RSD by evaluating the outcome of each of the medications at numerous wavelengths considering the methodology allowed us to evaluate the repeatability of offered approaches. Testing sample solutions in triplicate on the same day at two different concentrations (RGF, VGT, and MTH: 2 and 8 μg/ml) allowed for an evaluation of intra-day precision within the working range. However, the percentage RSD was calculated throughout 3 days of testing using sample solutions with concentrations at the low and high ends of the range (RGF, VGT, and MTH: 2 and 8 μg/ml, respectively) to assess the consistency between days.
Accuracy
Recovery studies were conducted using the conventional standard addition procedure to verify the viability of the anticipated methods. Standard RGF, VGT, and MTH at the 50%, 100%, and 150% level were added to a sample solution that had already been analyzed (RGF, VGT, and MTH: 2, 4, and 6 μg/ml) to produce a comparable quantity, and the solution was reanalyzed using projected methods before the percentage recoveries were calculated. Results from the accuracy research were analyzed using the following formula, which considers the % of reference RGF, VGT, and MTH recuperated from the pharmaceutical product with the help of the below given formula:
% Recovery = (Total amount of analyte found after adding reference analytes – Amount of analytes found before adding reference analytes)/(Amount of reference analytes added) × 100
Limit of detection (LOD) and limit of quantification (LOQ)
Through the use of mentioned parameters, the sensitivity of the suggested approaches were evaluated. The LOD and LOQ of the investigated analytes were assessed using the following formula, which was taken directly from the ICH strategies.
In the above equation σ = The SD of the response, S = Mean of the slope (standard graph)
Solution stability
We kept medication solutions at ambient environmental setup and in the refrigerator (6°C) and compared their responses and spectral pattern to those of freshly prepared solutions at regular intervals to see how well they held up over time, so analyzing their stability.
Statistical comparison by one-way ANOVA
Assay results were compared using one-way ANOVA, Microsoft 365, Microsoft Corporation, USA.
RESULTS AND DISCUSSION
It is anticipated that the projected spectrophotometric approaches would find widespread application in quality control (QC) settings where both cost and turnaround time for assessments are critical. UV spectroscopic methods are often used to study pharmaceutical preparations because they are easy, quick, cheap, and give consistent results. Compared to other ways of analyzing, these spectrophotometric methods are better and have many benefits. But it is hard to analyze all the analytes without first separating them if the UV spectra of the different components overlap. The proposed work comes up with some simpler and cheaper way to analyze RGF, VGT, and MTH concurrently in combined formulation bearing overlaying spectra.
Simultaneous equation method
The SEM was created and verified for the quantification of RGF, VGT, and MTH in tablet formulation, demonstrating adequate sensitivity and selectivity. The maximum absorbance in the zero-order UV spectra was seen at 228, 210, and 236 nm for RGF, VGT, and MTH, respectively (Fig. 2). As can be seen in Figure 2, the UV spectra of RGF, VGT, and MTH display spectral overlap, which allows for simultaneous estimation of RGF, VGT, and MTH in the ternary mixture. Using a simultaneous equation, the quantity of drugs in the mixture was calculated. Table 1 displays absorptivity data, whereas Table 2 displays the results of the method validation parameters.
RDS method
It has been demonstrated time and again that derivatization of UV spectra results in an increase in both specificity and selectivity of pharmaceuticals in combined dosage form by enhancing the resolution/resolving power of spectra. As a bonus, derivatization eliminates the excipient effects and permits the computation with respect to the existence of another analyte. The ratio spectroscopic method is predicated on the idea that by division of the spectra in combination with one of the spectra of the analyte, one can obtain a spectrum free of interferences from the analyte and the excipient. Using a spectrum that has been selected as a divisor also reduces background noise and scientific errors. Measurements are made in relation to the peaks, which improves precision, sensitivity, and specificity; this is another advantage of the ratio spectra method. Consequently, ratio spectroscopic methods were chosen to develop as it gives superior outcomes in comparison with other UV methods. In order to find the optimal double divisor, various RGF, VGT, and MTH concentrations were tested. In the end, we decided to evaluate the ternary mixture of RGF, VGT, and MTH using 2.5 μg/ml of VGT and MTH, 1 μg/ml of RGF and MTH and 2.5 μg/ml of RGF and VGT. All solutions of double divisors were prepared separately using methanol. The ratio difference spectroscopic approach was accomplished by saving the absorption spectra of solutions produced at several strengths of RGF and of the ternary combination and division of those spectra using UV spectra of 2.5 μg/ml of VGT and MTH, as displayed in Figure 3(A).
 | Table 2. Summarized data of method validation parameters and linear regression. [Click here to view] |
The ratio spectrum’s peak amplitude at 254.6 nm was deducted from the amplitude at 251.6 nm to find the resulting amplitude. In the next step, we plotted linearity graphs by using the values of the amplitude differences and the amount of RGF to determine the correlation coefficient and the regression equation. As can be seen in Figure 3(B), UV spectra were recorded for solutions made up of varying strengths of VGT and the ternary mixture, and then divided using RGF and MTH (1 μg/ml) as divisors, yielding the resultant ratio spectra. The ratio spectrum was then created by deducting the amplitude obtained at 250 nm from the amplitude at 260.6 nm. Linearity graphs were then created by using the values of the amplitude differences and the respective amounts of VGT to determine the regression equation and the corresponding correlation coefficient. Figure 3(C) displays the ratio spectra, achieved by the division of absorption spectra produced at several strengths of MTH and the ternary mixture by absorption band of 2.5 μg/ml of RGF and VGT separately in methanol. The amplitude at 236 nm was then deducted from the ratio spectrum’s peak amplitude at 240 nm. Afterward, correlation coefficients and equations of regression were derived using linearity graphs constructed with a difference in amplitude and the corresponding quantity of MTH.
Divisor and scaling factor optimization for first derivative of ratio spectra
Different experimental parameter settings were optimized to provide the best possible curve of the 1st derivative of ratio spectra. The most critical parameter involved adjusting the divisor and the scaling factor to maximize efficiency. Differing concentrations of RGF, VGT, and MTH were tested in order to find the optimal concentration to use as a divider. The final decision for RGF and MTH quantification was to use a VGT divisor of 2.5 μg/ml. Similarly, for the estimation of VGT, 2.5 μg/ml of RGF was finalized as a divisor. In order to acquire the first derivative of the ratio spectra, the scaling factor was fixed/tuned at four. First derivative spectra at 2, 4, 8, and 10 nm were explored to determine the optimal wavelength. According to the findings, an 8 nm wavelength was the most effective, thus that is the one that was selected and used, scaled up by a factor of 4.
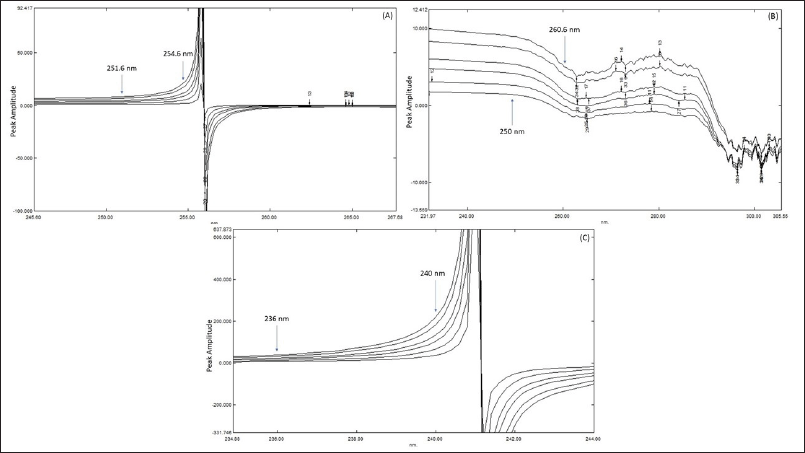 | Figure 3. (A) Overlain ratio spectra of RGF utilizing 2.5 μg/ml (VGT + MTH) as divisor; (B) Overlain ratio spectra of VGT utilizing 1 μg/ml (RGF + MTH) as divisor; (C) Overlain ratio spectra of MTH taking 2.5 μg/ml (RGF + VGT) as divisor. [Click here to view] |
DRZC approach
In this procedure, solutions with varying concentrations of RGF and MTH were scanned and stored between 200 and 400 nm. Utilizing the spectrum of a standard solution containing 2.5 μg/ml VGT, the recorded spectra were subsequently split. Consequently, the ratio spectra were transformed into their first derivative spectra.
By following the analytical signals in the 1st derivative spectra at 223.2 nm for RGF and 242.6 nm for MTH, as displayed in Figures 4A and C, the strengths of RGF and MTH in the ternary combination were able to estimate. Similar ratio spectra were achieved by division of the absorption spectra of VGT and MTH by the spectrum of a standard solution of 2.5 μg/ml RGF. Using a value of Δλ as 8 nm and a scaling factor of 4, the acquired ratio spectra were transformed into their first derivative. These spectra were then analyzed at 350.4 nm to determine the VGT concentration in the ternary combination (Fig. 4B).
DDRS derivative method
First, ternary combinations of RGF, VGT, and MTH were created at varying concentrations, and scanning was done in the UV spectrum (200–400 nm). Then, to obtain the ratio spectra, we divided each spectrum by 2.5 μg/ml VGT and MTH as the denominator. Further, as can be seen in Figure 5(A), the first derivative of ratio spectra was obtained by setting Δλ = 8. A calibration graph was produced by plotting the amplitude at 257.2 nm versus the concentration to estimate the occurrence of RGF. A different set of ratio spectra was collected by splitting ternary mixtures of RGF, VGT, and MTH with 1 μg/ml of RGF & MTH as the double divisor. Figure 5(B) also shows the first derivative of ratio spectra that were achieved with the help of Δλ as 8 and a scaling factor of 4. To evaluate the presence of VGT, we measured the amplitude at 291.4 nm and then plotted this value against the concentration at this wavelength to create a calibration graph. The last bunch of ratio spectra was obtained by division of ternary mixtures of RGF, VGT, and MTH by 2.5 μg/ml of RGF and 2.5 μg/ml of VGT, respectively. Figure 5(C) displays the result of taking the first derivative of ratio spectra with a value equal to Δλ as 8 and a scaling factor of 4. A linear graph was created by putting up the amplitude at 243 nm against the concentration to obtain the MTH concentration.
Method validation
All of the planned methodologies were evaluated in agreement with “International Conference on Harmonization” standards. The next section will elaborate on the results of different validation parameters.
Specificity
Excipients and typical pharmaceuticals did not interact while carrying out the specificity study by observing overlapping spectra of placebo (a blend of reported excipients as per the information from the manufacturer) and medication solutions.
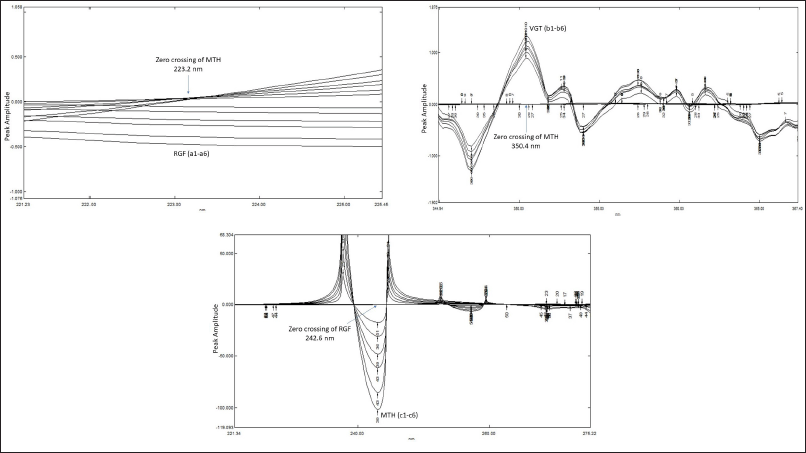 | Figure 4. (A) First derivative ratio spectra of RGF (a1) 2.5, (a2) 5, (a3), 7.5 (a4) 10, (a5) 12.5, (a6) 15 taking 2.5 μg/ml VGT as divisor (Δλ = 8 nm). (B) First derivative ratio spectra of VGT (b1) 2.5, (b2) 5, (b3), 7.5 (b4) 10, (b5) 12.5, (b6) 15 taking 2.5 μg/ml RGF as divisor (Δλ = 8 nm). (C) First derivative ratio spectra of MTH (c1) 2.5, (c2) 5, (c3), 7.5 (c4) 10, (c5) 12.5, (c6) 15 taking 2.5 μg/ml VGT as divisor (Δλ = 8 nm). [Click here to view] |
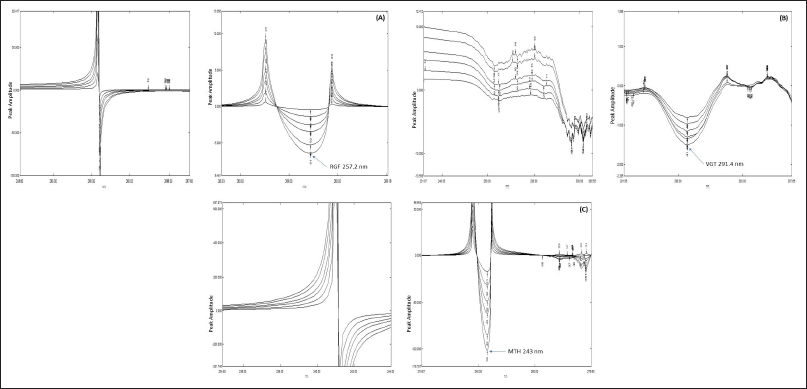 | Figure 5. (A) Ratio spectra and corresponding 1st derivative ratio spectra of RGF using 2.5 μg/ml VGT + 2.5 μg/ml of MTH as divisor at Δλ = 8. (B) Ratio spectra and corresponding 1st derivative ratio spectra of VGT using 1 μg/ml RGF + 1 μg/ml of MTH as divisor at Δλ = 8. (C) Ratio spectra and corresponding 1st derivative ratio spectra of MTH using 2.5 μg/ml RGF + 2.5 μg/ml of VGT as divisor at Δλ = 8. [Click here to view] |
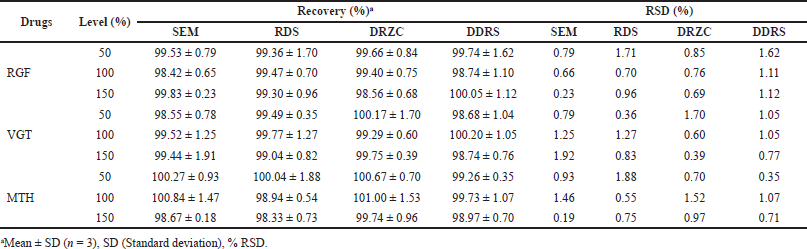 | Table 3. Recovery information of the planned approaches. [Click here to view] |
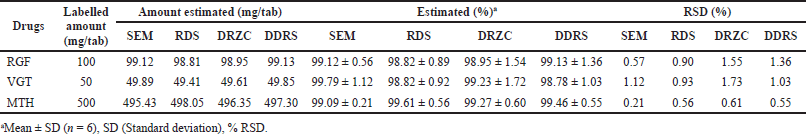 | Table 4. Outcome of formulation evaluation by various approaches. [Click here to view] |
Linearity and range
With the SEM approach, we measured linear association and range by evaluating absorbance at specific wavelengths, while with the RDS method, we measured amplitude difference. The DRZC and DDRS techniques, however, utilized peak amplitude for quantification. All three medicines showed a linear relationship between 2.5 and 15 μg/ml, regardless of the technique used. Every proposed approaches were linear, as supported by the correlation coefficient’s value (Table 2). The results were calculated as the mean of six separate assessments.
Precision
All the predicted methods have great precision as evidenced by the precision experiment results (repeatability, intra, and inter-day changeability) expressed in % RSD guarantee the ICH acceptable constraints (<2) revealed in Table 2.
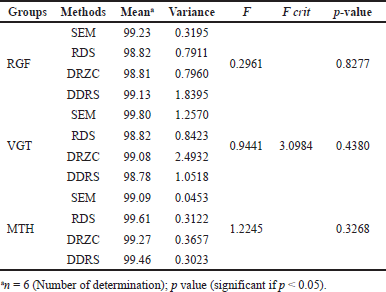 | Table 5. Statistical comparison of assay results utilizing one way ANOVA. [Click here to view] |
Accuracy
The expected procedures’ accuracy was determined using the standard addition approach for recovering analytes. The studies’ recovery rates ranged from 97% to 103% across all drugs and methods, proving the efficacy of the established approaches (Table 3).
LOD and LOQ
The level of responsiveness of the predicted procedures was demonstrated by the low values of LOD and LOQ (Table 2).
Solution stability
The solution remained unchanged for 2 days at room temperature and for 10 days when chilled to 6°C.
Estimation of RGF, VGT, and MTH in tablet dosage form
Assessments of RGF, VGT, and MTH were conducted with the help of the planned methods. The data set was statistically validated after six replicate measurements, with results ranging from 97% to 102% for all the analytes. Thus, the developed methods can be employed for the concurrent evaluation of RGF, VGT, and MTH in combined tablet (Table 4).
Statistical comparison by one-way ANOVA
An examination of the data from the assays was done using statistical methods to determine the impact of each of the four approaches that were projected. One-way ANOVA was employed to compare the statistical significance of the four different approaches. The significance level for all tests was set at p ? 0.05. Table 5 demonstrates the outcomes of the one-way ANOVA and it was found that the created procedures differed little from each other.
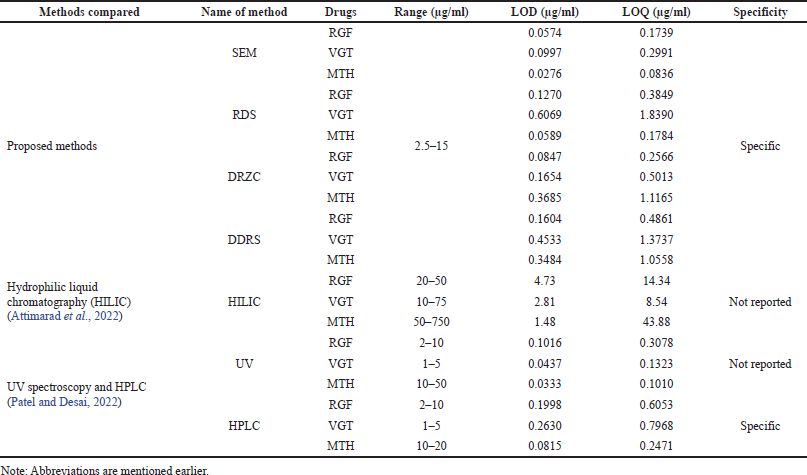 | Table 6. Comparison of proposed methods with reported methods. [Click here to view] |
Proposed methods explain the estimation of RGF, VGT, and MTH in the ternary admixture which was compared with two recently published research articles (Attimarad et al., 2022c; Patel and Desai, 2022) where authors elaborated simultaneous estimation of all three drugs under study. But reported methods show some shortcomings in various aspects like range, sensitivity, specificity, etc. Therefore, the proposed methods justify their existence and have been compared with the reported methods (Table 6). The HILIC method (Attimarad et al., 2022c) suffers from low sensitivity in terms of range, LOD, and LOQ; did not adopt specificity parameter which is very crucial for an analytical method when compared with proposed methods. Whereas another published article (Patel and Desai, 2022) did not opt for a specificity parameter, wavelength selection is not appropriate and mismatched throughout the article (UV method). In HPLC method calibration range of metformin is mismatched throughout the manuscript along with many other flaws. Therefore, the proposed methods are found to be superior when compared with the reported methods.
CONCLUSION
For the concurrent evaluation of RGF, VGT, and MTH in the ternary admixture, four spectroscopic methodologies were proposed: SEM, RDS, DRZC, and DDRS. In accordance with ICH guidelines, the established procedures were verified. The suggested procedures worked reliably, were straightforward to implement, quickly responded to user input, were easy to replicate, and cost effective. Moreover, all of the proposed UV-spectrophotometric methods require fewer steps in sample preparation and provide a wider concentration range with acceptable sensitivity and found to be superior when compared with reported methods. In the QC segment, where time and money are of the essence, the proposed spectrophotometric techniques are held to be reliable. Furthermore, these methods are considered more cost-effective than other analytical procedures because they do not call for costly solvents or intricate instruments. As a result, traditional QC research of RGF, VGT, and MTH in combined dosage forms (tablet) can make use of all existing methodologies.
ACKNOWLEDGMENT
The authors acknowledge Sumandeep Vidyapeeth Deemed to be University, Piparia, Waghodia, Vadodara, Gujarat, India, for their assistance in funding and providing access to necessary materials for this study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Available via https://www.prnewswire.com/in/news-releases/glenmark-becomes-the-first-company-to-launch-remogliflozin-vildagliptin-metformin-fixed-dose-combination-at-an-affordable-price-for-adults-with-type-2-diabetes-in-india-878771225.html.
Abdelrahman MM, Abdelwahab NS. Superior spectrophotometric method for determination of a ternary mixture with overlapping spectra. Anal Methods, 2014; 6(2):509–14. CrossRef
Alvi SN, Patel MN, Kathiriya PB, Patel BA, Parmar SJ. Simultaneous determination of prasugrel and aspirin by second order and ratio first order derivative ultraviolet spectrophotometry. J Spectrosc, 2013; 2013:705363. CrossRef
Attimarad M, Al-Dhubiab BE, Alhaider IA, Nair AB, Sree-harsha N, Mueen AK Simultaneous determination of moxifloxacin and cefixime by first and ratio first derivative ultraviolet spectrophotometry. Chem Cent J, 2012; 6(1):105. CrossRef
Attimarad M, Nair AB, Nagaraja S, Aldhubiab BE, Venugopala KN, Pottathil S. Smart UV derivative spectrophotometric methods for simultaneous determination of metformin and remogliflozin: development, validation and application to the formulation. Indian J Pharm Educ Res, 2021c; 55(1s):s293–302. CrossRef
Attimarad M, Nair AB, Sreeharsha N, Al-Dhubiab BE, Venugopala KN, Shinu P. Development and validation of green UV derivative spectrophotometric methods for simultaneous determination metformin and remogliflozin from formulation: evaluation of greenness. Int J Environ Res Public Health, 2021b; 18:448. CrossRef
Attimarad M, Venugopala KN, Al-Dhubiab BE, Elgorashe RE, Shafi S. Development of ecofriendly derivative spectrophotometric methods for the simultaneous quantitative analysis of remogliflozin and vildagliptin from formulation. Molecules, 2021a; 26(20):6160. CrossRef
Attimarad M, Venugopala KN, Chohan MS, David M, Molina EI, Sreeharsha N, Nair AB, Tratrat C, Altaysan AI, Balgoname AA. An experimental design approach to quantitative expression for quality control of a multicomponent antidiabetic formulation by the HILIC method. Molecules, 2022c; 27(10):3135. CrossRef
Attimarad M, Venugopala KN, Islam MM, Shafi S, Altaysan AI. Rapid simultaneous quantitative analysis of hypoglycemic agents by RP HPLC: development, validation and application to medicine. Indian J Pharm Educ Res, 2022a; 56(2):564–72. CrossRef
Attimarad M, Venugopala KN, Nair AB, Sreeharsha N, Deb PK. Experimental design approach for quantitative expressions of simultaneous quantification of two binary formulations containing remogliflozin and gliptins by RP-HPLC. Separations, 2022b; 9(2):23. CrossRef
Banik S, Karmakar P, Miah MA. Development and validation of a UV-spectrophotometric method for determination of vildagliptin and linagliptin in bulk and pharmaceutical dosage forms. Bangladesh Pharm J, 2015; 18(2):163–8. CrossRef
Beckett AH, Stenlake JB. Instrumental methods in the development and use of medicines. Practical pharmaceutical chemistry (Part-2), 4th edition. CBS Publishers and Distributors, New Delhi, India, pp 1–3, 275–99, 2005.
Bendale AR, Singh RP, Vidyasagar G. Development and validation stability indicating HPTLC method for determination of vildagliptin and metformin hydrochloride in the pharmaceutical dosage forms. Int J Appl Pharm, 2018; 10(1):36–45. CrossRef
Boovizhikannan T, Palanirajan VK. RP-HPLC determination of vildagliptin in pure and in tablet formulation. J Pharm Res, 2013; 7(1):113–6. CrossRef
Darwish HW, Bakheit AH, Naguib IA. Comparative study of novel ratio spectra and isoabsorptive point based spectrophotometric methods: application on a binary mixture of ascorbic acid and rutin. J Anal Methods Chem, 2016; 2016:2828647. CrossRef
Dharmalingam M, Aravind SR, Thacker H, Paramesh S, Mohan B, Chawla M, Asirvatham A, Goyal R, Shembalkar J, Balamurugan R, Kadam P, Alva H, Kodgule R, Tandon M, Vaidyanathan S, Pendse A, Gaikwad R, Katare S, Suryawanshi S, Barkate H. Efficacy and safety of remogliflozin etabonate, a new sodium glucose co-transporter-2 inhibitor, in patients with type 2 diabetes mellitus: a 24-week, randomized, double-blind, active-controlled trial. Drugs, 2020; 80(6):587–600. CrossRef
Dinç E, Baydan E, Kanbur M, Onur F. Spectrophotometric multicomponent determination of sunset yellow, tartrazine and allura red in soft drink powder by double divisor-ratio spectra derivative, inverse least-squares and principal component regression methods. Talanta, 2002b; 58(3):579–94. CrossRef
Dinç E, Onur F. Application of a new spectrophotometric method for the analysis of a ternary mixture containing metamizol, paracetamol and caffeine in tablets. Anal Chim Acta, 1998; 359(1–2):93–106. CrossRef
Dinç E, Palab?y?k IM, Üstünda? O, Yurtsever F, Onur F. Simultaneous spectrophotometric determination of chlorphenoxamine hydrochloride and caffeine in a pharmaceutical preparation using first derivative of the ratio spectra and chemometric methods. J Pharm Biomed Anal, 2002a; 28(3–4):591–600. CrossRef
Eissa MS, Abou Al Alamein AM. Innovative spectrophotometric methods for simultaneous estimation of the novel two-drug combination: sacubitril/valsartan through two manipulation approaches and a comparative statistical study. Spectrochim Acta A Mol Biomol Spectrosc, 2018; 193:365–74. CrossRef
El-Kimary EI, Hamdy DA, Mourad SS, Barary MA. HPTLC determination of three gliptins in binary mixtures with metformin. J Chromatogr Sci, 2016; 54(1):79–87. CrossRef
Erk N. Derivative ratio spectrophotometry and differential derivative spectrophotometric determination of isoniazid and pyridoxine hydrochloride in dosage forms. Spectrosc Lett, 2001; 34(6):745–61. CrossRef
Gohel RV, Parmar SJ, Patel BA. Development and validation of double divisor-ratio spectra derivative spectrophotometric method for simultaneous estimation of olmesartan medoxomil, amlodipine besylate and hydrochlorthiazide in tablet dosage form. Int J Pharm Tech Res, 2014; 6:1518–25.
Hajian R, Soltaninezhad A. The spectrophotometric multicomponent analysis of a ternary mixture of paracetamol, aspirin, and caffeine by the double divisor-ratio spectra derivative method. J Spectrosc, 2013; 2013:405210. CrossRef
Hussey EK, Kapur A, O’Connor-Semmes R, Tao W, Rafferty B, Polli JW, James Jr CD, Dobbins RL. Safety, pharmacokinetics and pharmacodynamics of remogliflozin etabonate, a novel SGLT2 inhibitor, and metformin when co-administered in subjects with type 2 diabetes mellitus. BMC Pharmacol Toxicol, 2013; 14:25. CrossRef
Indian pharmacopoeia. Government of India, Ministry of Health and Family Welfare. The Indian Pharmacopoeia Commission, Ghaziabad, India, Vol. 2, pp 2544–8, 2018.
International Conference on Harmonization (ICH). Validation of analytical procedures: text and methodology Q2(R1). ICH, Geneva, Switzerland, 2005.
Itigimatha N, Chadchan KS, Yallur BC, Hadagali MD. Simple and sensitive RP-HPLC and UV spectroscopic methods for the determination of remogliflozin etabonate in pure and pharmaceutical formulations. Turk J Pharm Sci, 2022; 19(2):213–9. CrossRef
Kalra S. Sodium glucose Co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther, 2014; 5(2):355–66. CrossRef
Kalyani L, Rao CVN. Simultaneous spectrophotometric estimation of salbutamol, theophylline and ambroxol three component tablet formulation using simultaneous equation methods. Karbala Int J Mod Sci, 2018; 4(1):171–9. CrossRef
Keating GM. Vildagliptin: a review of its use in type 2 diabetes mellitus. Drugs, 2014; 74(5):587–610. CrossRef
Khaladkar K, Mohan B, Suryawanshi S. Efficacy and safety of a fixed dose combination of remogliflozin etabonate and vildagliptin in patients with type-2 diabetes mellitus: a randomized, active-controlled, double-blind, phase III study. J Assoc Physicians India, 2022; 70(4):11–2.
Kumari B, Khansili A. Analytical method development and validation of UV-visible spectrophotometric method for the estimation of vildagliptin in gastric medium. Drug Res, 2020; 70(09):417–23. CrossRef
Lotfy HM, Hassan NY, Elgizawy SM, Saleh SS. Comparative study of new spectrophotometric methods; an application on pharmaceutical binary mixture of ciprofloxacin hydrochloride and hydrocortisone. J Chil Chem Soc, 2013; 58(3):1892–8. CrossRef
Martindale. The complete drug reference. 36th edition, Pharmaceutical Press (An Imprint of RPS Publishing), London, UK, Vol. I, pp 453–54, 2009.
Millership JS, Parker C, Donnelly D. Ratio spectra derivative spectrophotometry for the determination of furosemide and spironolactone in a capsule formulation. Il Farmaco, 2005; 60(4):333–8. CrossRef
Modi DK, Parejiya PB, Patel BH. A simple and sensitive HPTLC method for simultaneous determination of metformin hydrochloride and sitagliptin phosphate in tablet dosage form. J Chem, 2013; 2013:13956. CrossRef
Omer SA, Fakhre NA. Simultaneous determination of ternary mixture of carboxin, chlorpyrifos, and tebuconazole residues in cabbage samples using three spectrophotometric methods. J Anal Methods Chem, 2020; 2020:4912762. CrossRef
Patel JS, Desai S. Analytical method development and validation for simultaneous estimation of remogliflozin etabonate, vildagliptin and metformin hydrochloride in combined dosage form. Int J Pharm Pharm Res, 2022; 25(2):572–97.
Patil KR, Deshmukh TA, Patil VR. A stability indicating HPTLC method development and validation for analysis of vildagliptin as bulk drug and from its pharmaceutical dosage form. Int J Pharm Sci Res, 2020; 11:2310–6.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R, IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract, 2019; 157:107843. CrossRef
Sen AK, Hinsu DN, Sen DB, Zanwar AS, Maheshwari RA, Chandrakar VR. Analytical method development and validation for simultaneous estimation of teneligliptin hydrobromide hydrate and metformin hydrochloride from its pharmaceutical dosage form by three different UV spectrophotometric methods. J Appl Pharm Sci, 2016; 6(09):157–65. CrossRef
Sen AK, Pandey H, Maheshwari RA, Zanwar AS, Velmurugan R, Sen DB. Novel UV spectroscopic methods for simultaneous assessment of empagliflozin, linagliptin and metformin in ternary mixture. Indian J Pharm Educ Res, 2022; 56(4s):s669–81. CrossRef
Sen DB, Sen AK, Zanwar AS, Balaraman R, Seth AK. Determination of alogliptin benzoate and metformin hydrochloride in tablet dosage form by simultaneous equation and absorption ratio method. Int J Pharm Pharm Sci, 2015; 7(8):380–83.
Shah DA, Gondalia II, Patel VB, Mahajan A, Chhalotiya UK. Stability indicating liquid chromatographic method for the estimation of remogliflozin etabonate. J Chem Metrol, 2020; 14(2):125–32. CrossRef
Shah DA, Gondalia II, Patel VB, Mahajan A, Chhalotiya U, Nagda DC. Stability indicating thin-layer chromatographic method for estimation of antidiabetic drug remogliflozin etabonate. Future J Pharm Sci, 2021; 7:83. CrossRef
Shakoor A, Ahmed M, Ikram R, Hussain S, Tahir A, Jan BM, Adnan A. Stability-indicating RP-HPLC method for simultaneous determination of metformin hydrochloride and vildagliptin in tablet and biological samples. Acta Chromatogr, 2020; 32(1):39–43. CrossRef
Tammisetty MR, Challa BR, Puttagunta SB. A novel analytical method for the simultaneous estimation of remogliflozin and metformin hydrochloride by UPLC/PDA in bulk and formulation application to the estimation of product traces. Turk J Pharm Sci, 2021; 18(3):296–305. CrossRef
The Merck Index. 13th edition, Merck and Co, White House Station, NJ, p 1061, 2001.
Thomas AB, Patil SD, Nanda RK, Kothapalli LP, Bhosle SS, Deshpande AD. Stability-indicating HPTLC method for simultaneous determination of nateglinide and metformin hydrochloride in pharmaceutical dosage form. Saudi Pharm J, 2011; 19(4):221–31. CrossRef
Youssef RM, Maher HM. A new hybrid double divisor ratio spectra method for the analysis of ternary mixtures. Spectrochim Acta A Mol Biomol Spectrosc, 2008; 70(5):1152–66. CrossRef
Zaazaa HE, Elzanfaly ES, Soudi AT, Salem MY. Application of the ratio difference spectrophotometry to the determination of ibuprofen and famotidine in their combined dosage form; comparison with previously published spectrophotometric methods. Spectrochim Acta A Mol Biomol Spectrosc, 2015; 143:251–5. CrossRef