INTRODUCTION
Antibiotic requirements have lately increased. The global burden of bacterial illnesses is very high, aggravated by antibiotic resistance. Antibiotic resistance leads to failed infection treatment, which can ultimately lead to death (WHO, 2017). Thus, research related to the development of new drugs must continue.
One potential source is red ginger, a traditional medicinal plant that the community has long used for treatment (Germplasm Resources Information Network et al., 2017). Ginger comes from the flowering plant Zingiber officinale of the Zingiberaceae family. The rhizome of red ginger was reported to contain sesquiterpenes, such as beta-bisabolene and zingiberene, zingerone, shogaols, and gingerols, with [6]-gingerol as the significant pungent compound primarily (An et al., 2016). On the other hand, Wang et al. (2020) reported that ginger essential oil could inhibit the growth of Staphylococcus aureus and Escherichia coli with a minimum inhibitory concentration of 1.0 and 2.0 mg/ml, respectively.
Endophytic fungi live on plant tissue with a beneficial symbiotic relationship without causing any adverse (Schulz et al., 2002). It has been known that endophytic fungi are essential sources of bioactive secondary metabolites, such as antimicrobial compounds (Schulz et al., 2002; Strobel, 2003a, 2003b). It was hypothesized that 51% of bioactive metabolite taken from endophytic fungus had not been found before (Schulz et al., 2002). Endophytic fungi can create bioactive secondary metabolites with anticancer and antimicrobial effects, including alkaloids, phenolic acids, quinones, steroids, saponins, tannins, and terpenoids (Gouda et al., 2016).
Research reports on fungi originating from red ginger plants are still very limited in number. To investigate the endophytic fungus as a source of antimicrobial compounds, this study will report fungus isolated from the red ginger plant and explore its antimicrobial activity against S. aureus, E. coli, and Candida albicans.
MATERIALS AND METHODS
Identification of sample material
Stems, rhizomes, and leaves of red ginger (Zingiber officinale Linn. var. rubrum) were fresh from Mukomuko Regency, Bengkulu Province, Indonesia. Geographically, it is located at 101°01?15.1?–101°51?29.6? east longitude and 02°16?32.0?–03°07?46.0? south latitude. The sample has been identified in the Herbarium of Universitas Andalas, Padang, Indonesia.
Sample preparation
Sterilization of samples was accomplished through two methods: direct planting and pouring. In direct planting, sample pieces were sequentially sterilized by dipping them in 70% ethanol for 1 minute to sterilize the surface, drying them with a sterile cotton cloth (or rinsing them with distilled water) waiting until the ethanol evaporated. The cleavage surface of each plant organ was put facing the sabouraud dextrose agar (SDA) media. The SDA (g/l) composition is peptone from casein 5.0; peptone from meat 5.0; D(+)glucose 40.0; agar-agar 15.0 (European Pharmacopeia 5.6, 2006). The samples were cultured at 25°C for 5–7 days (room temperature). Each sample used in the pouring method was cleaned with distilled water and cut into little pieces, and then a sample of 10 g was collected and placed in the Erlenmeyer, followed by 100 ml of distilled water. After that, it was diluted to a concentration of 10−6, inoculated on SDA medium, and incubated for 5–7 days at a temperature of 27°C–29°C. Colonies that differ from other colonies in terms of their shapes and colors are known as isolates. Purification was performed until pure isolates were obtained (Kjer et al., 2010).
Cultivation of pure fungi isolates in the medium of rice and extract preparation
The colony of fungus growing in a Petri dish was cut into small pieces and then put into solid rice media in a 1,000 ml Erlenmeyer flask at room temperature. The cultivation process could be done when the fungal is overgrown on the rice after 4–6 weeks. The fungus develops rapidly to cover the rice’s entire top surface. After the maximum growth of the fungus, the cultivation results were extracted using ethyl acetate (EtOAc) solvent. This solvent was used to obtain both semipolar and nonpolar compounds that might be responsible for antimicrobial activity. The fungus’s EtOAc extract was evaporated using a rotary evaporator.
Antimicrobial activity screening
Agar well diffusion was conducted to investigate the antimicrobial activity of EtOAc extract of endophytic fungus from red ginger against S. aureus ATCC 25923, E. coli ATCC 25922, and C. albicans. Petri dishes were prepared by adding Nutrient Agar (Merck®) containing a bacterial suspension (0.5 McFarland). Nutrient agar contains (mass/volume): 0.5% peptone, 0.3% beef extract/yeast extract, 1.5% agar, 0.5% sodium chloride, and pH adjusted to neutral (6.8) at 25°C (77°F) (American Public Health Association et al., 1920). Chloramphenicol disks (Oxoid®) in a concentration of 30 μg/ml and nystatin disks (Oxoid®) in a concentration of 100 unit/disk were used for positive control against bacterial and fungus pathogenic. As a negative control, paper discs were used, which were added with 20 μl dimethyl sulfoxide (DMSO). For 48 hours, fungi were incubated at 25°C–27°C, whereas bacteria were incubated at 37°C for 24 hours. The EtOAc extract was 5 mg/ml (in DMSO). The antimicrobial screening was carried out using the previously described method (Handayani et al., 2018; 2019; 2020; 2021; Handayani and Aminah, 2017; Handayani and Artasasta, 2017; Sandrawati et al., 2020). The presence of bacterial and fungal inhibition activity is indicated by the clear zone on the Petri disk (Bauer, 1959). Triplicate experiments were conducted to test for antimicrobial activity. The mean diameter of the inhibition zone and the standard deviation were calculated for each extract with an inhibition zone more significant than 10 mm.
Determination of the content of secondary metabolites
Secondary metabolites/phytochemical screening for EtOAc extracts of endophytic fungi with antibacterial activity (inhibition zone >10 mm) was performed. This method detects the content of terpenoid, alkaloid, phenolic, and steroid compounds from the extract (Handayani et al., 2019; Tiwari et al., 2011).
Molecular identification
Using the ITS primer, molecular identification was performed. The DNA extraction was performed using the modified Atashpaz et al. (2010) approach. The first BASE, located in Malaysia, received the polymerase chain reaction products for sequencing. The National Center for Biotechnology Information’s MycoBank database and the Basic Local Alignment Search Tool aligned the sequences pairwise.
RESULTS AND DISCUSSION
Bioactive compounds produced by endophytic fungi from sea sponges, mangrove trees, and medicinal plants from West Sumatra, Indonesia, are the focus of our further research. The findings of this study indicate that endophytic fungi can serve as a surrogate source for new drug discovery (Aminah et al., 2020; Artasasta et al., 2017; Handayani et al., 2016; 2018; 2019; 2021, Handayani and Aminah, 2017; Handayani and Artasasta, 2017; Sandrawati et al., 2020). Medicinal plants are one source of the wide variety of endophytic fungi, and these plants’ organs have been found to contain endophytic fungi. Thirty endophytic fungi from the red ginger’s leaves, stems, and rhizomes show antifungal action against the plant’s pathogenic fungus, Fusarium oxysporum, according to Ginting et al. (2013). The living conditions of red ginger in different and unique locations make this plant a host for various types of endophytic fungi, which can produce chemical metabolites with new chemical structures.
This study discovered 10 endophytic fungi from the leaves, stems, and rhizomes of Z. officinale Linn. var. rubrum (Fig. 1). Based on the characteristics of different colonies obtained from red ginger leaves, rhizomes, roots, and stems, isolates of endophytic fungi were obtained. Leaves and stems have almost the same number of endophytic isolates. Endophytic fungi from leaves were designated with codes JMD, stems by JMB, and rhizomes by JMR. The rhizome yielded five fungal strains, a more significant number than the other plant organs studied (Fig. 2).
The findings of the antimicrobial activity screening are displayed in Table 1. The clear zone on the Petri disk indicates the presence of bacterial and fungal inhibition activity. Four fungal extracts were effective against S. aureus, five against E. coli, and two against C. albicans at a concentration of 5%. The two isolates with the highest inhibition diameter of all the fungi extracts were JMR4 and JMD1 (>10 mm). With an inhibition zone of 14.38 ± 1.34 mm for S. aureus and 16.88 ± 0.69 mm for E. coli, the JMR4 had the largest diameter of inhibition (Fig. 3). As a result of its capacity to prevent the growth of Gram-negative and Gram-positive pathogenic bacteria, this fungal extract may be characterized as broad-spectrum (Table 2). JMR4 EtOAc extract exhibited a small impact on C. albicans growth suppression, with an inhibition zone of 7.17 mm.
 | Figure 1. The pictures of red ginger (Z. officinale var. rubrum): (A) leaves, (B) stems, and (C) rhizomes. [Click here to view] |
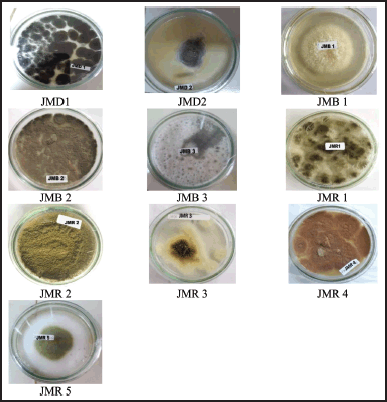 | Figure 2. The pictures of isolated fungal endophytes from Z. officinale var. rubrum growing on SDA. [Click here to view] |
Furthermore, the phytochemical screening of extracts was tested for the JMR4 and JMD1 extracts with the highest antimicrobial activity. This screen observes the color changes after The JMR4 extract produced a positive red color after being tested with the Wilstater test. In the alkaloid content test, JMR4 and JMD1 gave positive results forming a white precipitate after being given Meyer’s reagent. In the phenolic identification test, a blue-black color is formed after phenol reacts with FeCl3. Only JMD1 shows a positive result for the phenol test. The Lieberman–Burchard reagent is used to identify terpenoids and steroids, which gives a green-blue color indicating the presence of steroids. In contrast, a violet ring indicates the presence of terpenoids. The identification of terpenoids and steroids in the EtOAc extract of endophytic fungi JMR4 and JMD1 were positive for the presence of terpenoids with the formation of a violet ring (Table 3).
Macroscopically, the colony of JMR4 shows brown with fading margins, large rounded, flat surfaces, and edges. JMR4 was seen under a microscope, revealing vehicles, conidiophores, and spherical conidia (Fig. 4). Molecular identification showed that JMR4 had identical to Aspergillus terreus with sequence identities of 100%. The phylogenetic tree was constrained using the neighbor-joining method with a bootstrap value of 1.000 (Fig. 5). JMR4 is clustered and identic with Aspergillus terreus EUR1 with accession number MF590163.1. The Kimura two-parameter model shows no genetic difference between JMR4 and A. terreus EUR1 (Table 4). An overview to summarize the experimental design of this research is shown in Figure 6.
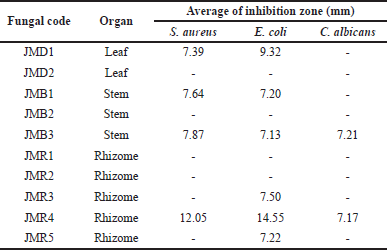 | Table 1. Antimicrobial activity of endophytic fungi from Z. officinale var. rubrum plant against the pathogenic microbe. [Click here to view] |
 | Figure 3. The agar plate pictures of the inhibition zone of fungal isolate JMR4 extract against the growth of (A) S. aureus and (B) E. coli. [Click here to view] |
 | Table 2. Antimicrobial activity of fungal endophyte JMR4 and JMD1. [Click here to view] |
The fungus A. terreus was shown to have tetracyclic acid A, a member of the sesquiterpene antibiotic family having antibacterial and anticancer properties. (Goutam et al., 2017; Hirota et al., 1982). Lovastatin, a medication used to treat high cholesterol in humans, is produced by the fungus A. terreus. This fungus makes more than just lovastatin, and it also makes sulochrins, terretonins, asterriquinones, and butyrolactones, all bioactive compounds (Chen et al., 2018). Liu et al. (2018) discovered three novel compounds: a prenylated tryptophan derivative, luteoride E, a butenolide derivative, versicolactone G, and linear aliphatic alcohol (3E,7E)-4,8-dimethyl-undecane-3,7-diene-1,11-diol and nine known compounds from the coral-associated fungus A. terreus. Versicolactone G had a significant α-glucosidase inhibitory activity with an IC50 value of 104.8 ± 9.5 μM, less than the positive control acarbose (IC50 = 154.7 ± 9.5 μM). Furthermore, practically all substances reported considerable anti-inflammatory efficacy against nitric oxide production, with IC50 values ranging from 5.48 to 29.34 μM. The discovery of bioactive compounds produced by A. terreus has shown the great potential of endophytic fungus for producing structurally unique and pharmacologically active natural products.
 | Table 3. Phytochemical screening of EtOAc extract of JMR4 and JMD1. [Click here to view] |
 | Figure 4. Macroscopic (A) and microscopic (B) pictures of fungal isolate JMR4: (1) conidia, (2) vesicles, and (3) conidiophore. [Click here to view] |
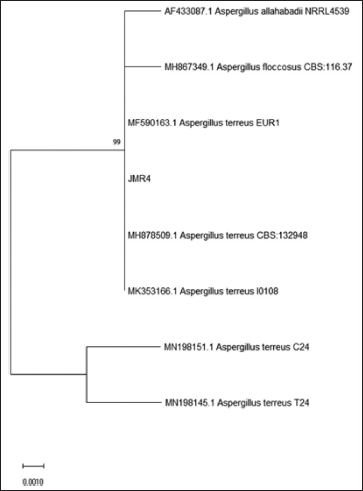 | Figure 5. The phylogenetic tree inferred using the neighbor-joining method of ITS sequence of fungus JMR4 derived from Z. officinale and its allied taxa. [Click here to view] |
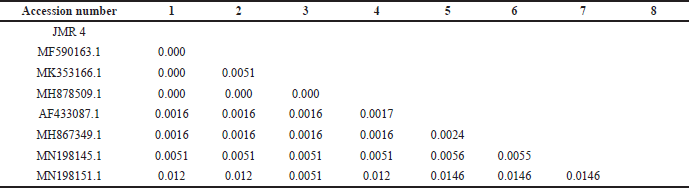 | Table 4. Estimation of evolutionary divergence between sequences using the Kimura two-parameter model. [Click here to view] |
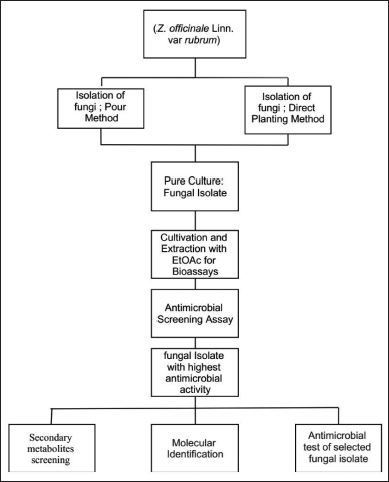 | Figure 6. The experimental design of endophytic fungus isolation from Z. officinale. [Click here to view] |
CONCLUSION
Endophytic fungus is an important source of secondary metabolites with fascinating bioactivities and diverse chemical structures. As a result of this study, 10 fungi from red ginger (Z. officinale Linn. var. rubrum) have been successfully isolated. Based on the results of antimicrobial activity screening, the fungal strain JMR4 has a high ability to suppress the development of pathogenic bacteria such as S. aureus and E. coli. The JMR4 is identical to A. terreus. It is a big challenge to isolate the bioactive chemicals from this fungus for further studies and to develop new antibiotic candidates.
ACKNOWLEDGMENTS
We thank BOPTN of Universitas Andalas, Padang, Indonesia, for financial support, in the project Indonesian Collaborative Research (RKI) 16 PTNBH Scheme A (Host), T/3/UN.16.17/PT.01.03/KO-RKI-A (Host)/2022.
AUTHORS’ CONTRIBUTIONS
All authors contributed significantly to the concept and design, data gathering, data analysis and interpretation, and paper revision.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
ETHICAL APPROVAL
The authors did no studies with human participants or animals in this article. In this investigation, no formal consent was sought.
DATA AVAILABILITY
This study article contains all of the data that was generated and examined.
PUBLISHER’S NOTE
Regarding jurisdictional claims in published institutional affiliation, this publication remains neutral.
REFERENCES
American Public Health Association, American Chemical Society, Association of Official Agricultural Chemists. Standard methods for the examination of water and sewage. American Public Health Association, Harvard University, Cambridge, MA, 1920.
Aminah I, Putra AE, Arbain D, Handayani D. Antibacterial potential of fungi derived extracts of marine sponge Acanthostrongylophora ingens. AACL Bioflux, 2020; 13:1118.
An K, Zhao D, Wang Z, Wu J, Xu Y, Xiao G. Comparison of different drying methods on Chinese ginger (Roscoe): changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem, 2016; 197:1292–300. CrossRef
Artasasta MA, Yanwirasti, Djamaan A, Handayani D. Cytotoxic activity screening of ethyl acetate fungal extracts derived from the marine sponge Neopetrosia chaliniformis AR-01. J App Pharm Sci, 2017; 7(12):174–8.
Atashpaz S, Khani S, Barzegari A, Barar J, Vahed S, Azarbaijani R, Omidi Y. A robust universal method for extraction of genomic DNA from bacterial species. Microbiology, 2010; 79(4):538–42. CrossRef
Bauer AW. Single-disk antibiotic-sensitivity testing of Staphylococci. AMA Arch Intern Med, 1959; 104(2):208. CrossRef
Chen GY, Ruan BH, Yang YB, Wang Q, Li XZ, Luo N, Yang XQ, Zhao LX. Secondary metabolites of the fungus Aspergillus terreus. Chem Nat Compd, 2018: 54(2):415–8; doi:10.1007/s10600-018-2366-3. CrossRef
European Pharmacopeia 5.6. Chapter 2.6.13 B (harmonized method). Council of Europe, 2006.
Ginting RCB, Sukarno N, Widyastuti U, Darusman LK, Kanaya S. Diversity of endophytic fungi from red ginger (Zingiber officinale Rosc.) plant and their inhibitory effect to Fusarium oxysporum plant pathogenic fungi. HAYATI J Biosci, 2013; 20(3):127–37. CrossRef
Gouda S, Das G, Sen SK, Shin H-S, Patra JK. Endophytes: a treasure house of bioactive compounds of medicinal importance. Front Microbiol, 2016; 7; doi:10.3389/fmicb.2016.01538 CrossRef
Goutam J, Sharma G, Tiwari VK, Mishra A, Kharwar RN, Ramaraj V, Koch B. Isolation and characterization of “Terrein” an antimicrobial and antitumor compound from endophytic fungus Aspergillus terreus (JAS-2) associated from Achyranthus aspera Varanasi, India. Front Microbiol, 2017; 8:1334. CrossRef
Germplasm Resources Information Network (GRIN), Agricultural Research Service (ARS), United States Department of Agriculture (USDA). 2017.
Handayani D, Ornando R, Rustini. Antimicrobial activity screening of symbiotic fungi from marine sponge Petrosia nigrans collected from south coast of West Sumatera, Indonesia. Int J Pharmacogn Phytochem Res, 2016; 8(4):623–6.
Handayani D, Artasasta MA. Antibacterial and cytotoxic activities screening of symbiotic fungi extract isolated from marine sponge Neopetrosia chaliniformis AR-01. J App Pharm Sci, 2017; 7 (05):066–9.
Handayani D, Ananda N, Artasasta MA, Rustini R, Fadriyanti O, Tallei TE. Antimicrobial activity screening of endophytic fungi extracts isolated from brown algae Padina sp. J App Pharm Sci, 2019; 9(3):9–13. CrossRef
Handayani D, Aminah I. Antibacterial and cytotoxic activities of ethyl acetate extract of symbiotic fungi from West Sumatra marine sponge Acanthrongylophora ingens. J App Pharm Sci, 2017; 7(2):237–40.
Handayani D, Rasyid W, Rustini, Tallei TE, Hertiani T. Cytotoxic activity screening of fungal extracts derived from the West Sumatran marine sponge Haliclona fascigera to several human cell lines: Hela, WiDr, T47D, and Vero. J Appl Pharm Sci, 2018; 8(1):55–8.
Handayani D, Artasasta MA, Safirna N, Ayuni DF, Tallei TE, Hertiani T. Fungal isolates from marine sponge Chelonaplysilla sp.: diversity, antimicrobial and cytotoxic activities. Biodiversitas, 2020; 21(5):1954; doi:10.13057/biodiv/d210523 CrossRef
Handayani D, Artasasta MA, Mutia D, Atikah N, Rustini, Tallei TE. Antimicrobial and cytotoxic activities screening fungal secondary metabolites isolated from marine sponge Callyspongia sp. AACL Bioflux, 2021; 14(1):249.
Hirota A, Nakagawa M, Sakai H, Isogai A. Terrecyclic acid A, a new antibiotic from Aspergillus terreus. II. Structure of terrecyclic acid A. J Antibiot, 1982; 35(7):783–7. CrossRef
Kjer J, Debbab A, Aly AH, Proksch P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat Protoc, 2010; 5(3):479–90. CrossRef
Liu M, Sun W, Wang J, He Y, Zhang J, Li F, Zhang Y. Bioactive secondary metabolites from the marine-associated fungus Aspergillus terreus. Bio Chem, 2018; 80:525–30. CrossRef
Sandrawati N, Hati SP, Yunita F, Putra AE, Ismed F, Tallei TE, Hertiani T, Handayani D. Antimicrobial and cytotoxic activities of marine sponge-derived fungal extracts isolated from Dactylospongia sp. J Appl Pharm Sci, 2020; 10(04):028–33. CrossRef
Schulz B, Boyle C, Draeger S, Römmert A-K, Krohn K. Endophytic fungi: a source of novel biologically active secondary metabolites. Mycol Res, 2002; 106(9):996–1004. CrossRef
Strobel GA. Endophytes as sources of bioactive products. Microbes Infect, 2003a; 5(6):535–44. CrossRef
Strobel GA, Daisy B, Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev, 2003b; 67:491–502. CrossRef
Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci, 2011; 1(1):98–106.
Wang X, Shen Y, Thakur K, Han J, Zhang JG, Hu F, Wei Z-J. Antibacterial activity and mechanism of ginger essential oil against Escherichia coli and Staphylococcus aureus. Molecules, 2020; 25(17):3955. CrossRef
WHO. WHO publishes the list of bacteria for which new antibiotics are urgently needed. 2017. Available via https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed