INTRODUCTION
In the last two decades, Asia has experienced an increase in inflammatory bowel diseases (IBD) cases, with the highest prevalence in East and South Asia. IBD prevalence in Indonesia was reported to be 1.16%–26.5%. In 2012, the average case in Indonesia for ulcerative colitis was 0.55 per 100,000 people and for Crohn’s disease was 0.33 per 100,000 people. This indicates that IBD is difficult to treat, so the treatment system in Asia must be more aware of and recognize its manifestations and the management of therapy (Dian et al., 2019). Local treatment is preferred for various colon diseases because local therapy is more effective than systemic therapy for conditions such as IBD. After all, it is ideal for minimizing systemic side effects. Therefore, drugs must be manufactured with a specific drug delivery system (Raghuvanshi et al., 2014).
One of the drugs often used to treat colon diseases is dexamethasone, a glucocorticoid class of drugs that are not safe or practical for IBD therapy because of the high side effects associated with its use for a long time (Brunton et al., 2018). However, the colon-targeted drug delivery system (CTDDS) can allow the use of these therapeutic agents because it is in accordance with the use of a controlled drug delivery system to optimize drug utilization by using small amounts of active substances so that the resulting systemic side effects are low (Bruschi, 2015). CTDDS can be achieved through oral or rectal administration (Gulbake and Jain, 2012). Drug delivery via the oral route is the most convenient route. Still, there are some obstacles in the absorption and degradation of the active substance in the upper gastrointestinal tract due to the acidic pH in the stomach, the presence of enzymes, the first-pass effect in the liver, and the intestinal barrier (Mohammed et al., 2017). To minimize systemic absorption and increase treatment locally in the colon, CTDDS has been widely investigated (Amidon et al., 2015; Iswandana et al., 2017a, 2017b, 2018a, 2018b, 2018c).
One of the strategies that have been developed for the targeted colonic delivery systems is a multicoated tablet system. A multicoated tablet system is a tablet coated with a combination of a delivery system based on colonic microflora activity as the first or primary coating and a pH-sensitive system as the second or secondary coating. Polysaccharide polymer was used as the primary coating because colonic microflora enzymes can degrade it to allow the release of drugs in the colon (Prudhviraj et al., 2015). However, polysaccharides can be dissolved in the stomach, so Eudragit was used as a pH-sensitive polymer to prevent drug release in the stomach (Chourasia and Jain, 2004; Gulbake and Jain, 2012; Mehta et al., 2013).
In addition, drug delivery using intestinal microflora is used by probiotics such as Lactobacillus and Bifidobacterium in the formulation. The use of intestinal microflora is carried out due to the limitations of disturbed microflora or sterilization due to antibiotics, common prescriptions for colonic diseases. Based on the research of Ghosh et al. (2010) enough microflora can also degrade polysaccharides, which in this study were chitosan. Hence, the tablet formulation with probiotics gave a better colonic profile than the formulation without probiotics (Ghosh et al., 2010). Previous research has been done on the use of probiotics in tablet formulations. Therefore, this study was conducted to obtain the formulation, characterization, and dissolution profile of dexamethasone multicoated tablets using the probiotics Lactobacillus and Bifidobacterium. The tablets were coated with a polysaccharide such as sodium alginate, chitosan, and xanthan gum as the primary coating and the combination of Eudragit L100 and Eudragit S100 (1:4) as the secondary coating for colon-targeted preparations.
MATERIALS AND METHODS
Materials
The following were used in this study: dexamethasone (Lloyd, Indonesia), dexamethasone Baku Pembanding Farmakope Indonesia (BPFI) (BPOM, Indonesia), chitosan (Bio Chitosan Indonesia), xanthan gum [Deosen Biochemical (Ordos), China], sodium alginate (Shandong Jiejing Group Corporation, China), Avicel® PH 102 (Brataco, Indonesia), Polyvinylpyrrolidone (PVP) K-30 (Sumber Berlian Kimia, Indonesia), talc (Brataco, Indonesia), magnesium stearate (Brataco, Indonesia), Lactobacillus acidophilus (Shandong Zhongke Jiayi Bioengineering, China), Bifidobacterium longum (Shandong Zhongke Jiayi Bioengineering, China), Eudragit L100 (Evonik, Germany), Eudragit S100 (Evonik, Germany), ethanol (RIV Chemicals, Indonesia), isopropyl alcohol (Brataco, Indonesia), triethyl citrate (Jinan Jinbang Chemical, China), chloride acid (Merck, Germany), sodium hydroxide (Merck, Germany), potassium dihydrogen phosphate (Merck, Germany), and Aquadest (Brataco, Indonesia).
Formulation of dexamethasone core tablets
Table 1 shows the formulation of dexamethasone tablets. Dexamethasone tablets were formulated by the wet granulation method. Dexamethasone, probiotics, and tableting excipients were milled, respectively. The probiotics L. acidophilus and B. longum were mixed until homogeneous, and the amount was divided into two for use before and after granulation. Dexamethasone, half a quantity of probiotics, and Avicel® PH 102 were mixed until homogeneous. The 9% w/w PVP binder solution was mixed with the powder to form a wet mass. The wet mass was sieved using sieve no. 8 to form wet granules. The wet granules were dried at 40°C for 2 hours. The dry granules were sieved using sieve no.18, mixed with half the quantity of probiotics, and a mixture of magnesium stearate and talc (1:2) as a lubricant. The dried granules were compressed to form tablets weight 100 mg using a single punch tablet press (Erweka EP-1, Germany) (Ghosh et al., 2010; Pooja et al., 2011).
Coating of dexamethasone tablets with the primary coating
Table 2 shows the formula for the primary coating. Dexamethasone tablets were coated by a spray coating method using three different polymers, namely, sodium alginate (A), chitosan (C), and xanthan gum (X). About 250 tablets were coated in a coating pan (Erweka DKM, Germany) at 18 rpm. The primary coating solution was sprayed using a spray gun (Meiji F-75G, Japan) and dried using a dryer (Dyson, USA) (Farheen et al., 2011; Raju et al., 2011).
Coating of dexamethasone tablets with a secondary coating
Tablets coated with a primary coating were coated again with a solution of Eudragit L100 and Eudragit S100 (1:4) in isopropyl alcohol (IPA) using 20% w/w triethyl citrate as a plasticizer. Tablets were coated using a primary coating method (Dasankoppa et al., 2012). The formula for the secondary coating can be seen in Table 3.
Characterization of granules
A tapped-bulk density tester measured the dexamethasone granules’ bulk density and tapped density (Erweka, Germany). A total of 50 g of granules was weighed accurately and then poured into a graduated cylinder, and the initial volume (V0) was measured. The density tester was set for 100 taps. After that, it was measured the final volume (Vf). The bulk density and tapped density were calculated using the following formulas (Dangi et al., 2013):
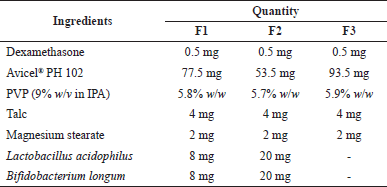 | Table 1. Formula of core tablets. [Click here to view] |
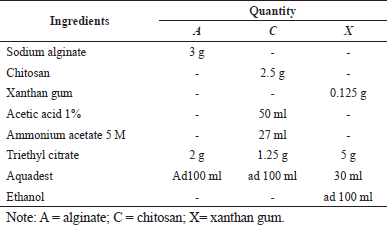 | Table 2. Primary coating formula. [Click here to view] |
ρbulk = W/V0
ρtapped = W/Vf?.
Compressibility index and Hausner’s ratio
The compressibility index of the dexamethasone granules was measured using the following formula:
Compressibility index = (ρtapped−ρbulk)/ρtapped × 100%.
Hausner’s ratio was measured using the following formula:
Hausner’s ratio = ρtapped / ρbulk.
Angle of repose
The angle of repose was measured using the funnel method. A total of 30 g of granules was weighed and then poured into the funnel. The granules will flow through the funnel to form a conical pile. The height (h) and radius (r) were measured, and the angle of repose was calculated using the following formula Angle of repose = tan−1 (height/r) (Dangi et al., 2013).
Flow rate
The flow rate of the dexamethasone granules was measured using a flowmeter (Erweka, Germany). A total of 50 g of granules was weighed accurately and then poured into a flowmeter. Turn on the flowmeter, then wait until all the granules have flown. Record the time when all the granules have flown.
Moisture content of granules
The moisture content of granules was measured by moisture balance (Adam AMB 50, USA). A total of 5 g of granules was weighed accurately and then put into the moisture balance. The temperature of moisture balance was set at 105°C (Crouter and Briens, 2014).
Organoleptic properties of granules
The organoleptic test of granules was carried out by observing the granules’ size, aroma, and color.
Characterization of tablets
Organoleptic test
Organoleptic tests were carried out by observing the tablet’s shape, size, color, surface shape, consistency, and physical tests (Iskandarsyah et al., 2010).
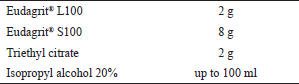 | Table 3. Secondary coating formula [Click here to view] |
Determination of tablet surface morphology
The tablet’s surface morphology was determined with scanning electron microscope (SEM) (Jeol JSM-5310 LV, Japan).
Uniformity of diameter and thickness
The uniformity of tablet size was measured by measuring the diameter and thickness of 20 tablets at random using a vernier caliper (Tricle Brand, China).
Content uniformity test
Content uniformity was carried out by selecting 30 coated tablets randomly, then determining the content of 10 tablets one by one using a method the same as the drug assay method, and then determining the acceptance value (Kementerian Kesehatan, 2020).
Weight variation test
Weight variation was carried out by selecting 30 tablets, then 10 tablets were weighed one by one, and the average weight of the tablets was calculated. The content of each tablet is calculated and expressed as a percent of the label claim based on the assay results. The calculation of the acceptance value of weight variation is the same as the acceptance value of content uniformity (Kementerian Kesehatan, 2020)
Tablet hardness
The hardness of tablets was tested by taking 10 tablets, then placing them one by one on the Hardness Tester (Erweka TBH 28, Germany) in kP units (Dangi et al., 2013)
Friability test
The friability of tablets was determined by weighing 20 tablets, then putting all the tablets into the Friability Tester (Vanguard Pharmaceutical Machinery LIC-2, USA) at 25 rpm for 4 minutes. After the rotation stops, reweigh the entire tablet. Calculate the tablet friability with the following formula (Dangi et al., 2013).
Friability = (Winitial − Wfinal)/Winitial × 100%.
Disintegration test
The disintegration test was carried out by putting one coated tablet in each of the six tubes from the basket of the disintegration tester (Electrolab ED-2, India). At the acid stage, the medium used was a simulated gastric fluid test solution with a temperature of 37°C ± 2°C for 1 hour. If, after 1 hour, no coated tablet shows evidence of disintegration, cracking, or softening, proceed with the buffer stage. At the buffer stage, the medium used was simulated intestinal fluid test solution with a temperature of 37°C ± 2°C for 3 hours (Kementerian Kesehatan, 2020).
Drug content
The calibration curve was made by weighing 10 mg of dexamethasone BPFI, then transferring it into a 100 ml volumetric flask and dissolving it with ethanol-water (2:1) solution to obtain a final solution containing 100 ppm of dexamethasone BPFI. Dilute the standard solution with an ethanol-water (2:1) solution in a 50 ml volumetric flask to obtain 6, 8, 10, 12, 14, 16, and 18 ppm concentrations. Determine the absorption of each concentration using a UV-VIS spectrophotometer (Shimadzu UV-1,800, Japan) at 240 nm, using ethanol-water (2:1) as blank.
An assay of dexamethasone tablets was carried out by accurately weighing 20 tablets of each sample and powdering them to obtain a homogeneous mixture. Then, an amount of powder equivalent to 1.4 mg of dexamethasone was transferred to a 100 ml volumetric flask. Dissolve it in ethanol-water (2:1) solution, then sonicate it for 10 minutes. All samples and standard solution were filtered through a membrane with a pore size of 0.45 μm. Determine the absorption of each sample using a UV-VIS spectrophotometer (Shimadzu UV-1,800, Japan) at 240 nm (Sversut et al., 2015).
In vitro drug release studies
In vitro dissolution study of dexamethasone-coated tablets was carried out using a dissolution tester (Electrolab TDT-08L, India) and dissolution apparatus type I (basket type) at a rotation speed of 100 rpm maintained at 37°C ± 0.5°C. The medium used was 500 ml 0.1 N hydrochloric acid for 2 hours, followed by 500 ml phosphate buffer (pH 7.2) for 3 hours, and finally by 500 ml phosphate buffer (pH 6.8) for 7 hours in anaerobic conditions. Anaerobic conditions were made by preparing a pH 6.8 phosphate buffer using distilled water that had been heated for 30 minutes and bubbled with carbon dioxide (CO2) gas for 10 minutes. A 5 ml of sample was withdrawn at predetermined times of 15, 30, 45, 60, 90, 120, 150, 180, 240, 300, 360, 480, 540, 600, 660, and 720 minutes, and the same volume of fresh medium was replaced. The withdrawn samples were analyzed using a UV-VIS spectrophotometer at 240 nm (Madhu et al., 2012; Pooja et al., 2011; Singh et al., 2015).
RESULT AND DISCUSSION
Characterizations of granules
The formed granules were then evaluated to determine the flow properties before being compressed into tablets. The granules, which can be seen in Figure 1, were white, odorless, and had a spherical shape. The results of the granules moisture content test shown in Table 4 showed that the water content of the F1, F2, and F3 granules was 4.97%, 4.47%, and 4.34%.
The compressibility index and Hausner ratio show the ability of the powder to flow and compress (The United States Pharmacopeial Convention, 2018). The results showed that the flow properties of the granules were good for all formulations. The angle of repose shows the friction between the particles (The United States Pharmacopeial Convention, 2018). The surface of the granule, which is irregular and rough, shows a high angle of repose. Granules with a large particle size will exhibit properties that easily flow and form an angle with a low slope. The results of this study showed that the flow properties of all the granules were a special category. The flow rate affects the flowability of the granules during the compression process. The granules that flow easily during compression produce uniform tablets in weight and size. Based on the results of the flow rate test, the granules that will be compressed were easy to flow, which was indicated by the value of the test, which was under 10 seconds (Aulton, 2002).
 | Figure 1. Granules. [Click here to view] |
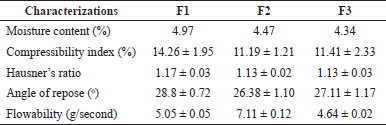 | Table 4. Characterizations of granules. [Click here to view] |
Characterization of core tablets
Core tablets were evaluated by organoleptic tests, tablet surface morphology, size uniformity, weight diversity, hardness, and toughness. Based on the organoleptic test, the tablet has a biconvex shape and a white color. The tablet has a smooth and shiny surface. The biconvex tablet shape makes rolling on the coating pan easier during the coating process (Lachman, 1986). Based on the SEM analysis, the core tablets showed a porous structure. The results of the organoleptic test and the SEM analysis can be seen in Figures 2 and 3.
The size uniformity test was carried out by measuring the diameter and thickness of the tablets using a caliper. The tablets meet the requirements for a tablet diameter of at most three times the thickness of the tablet and not less than one-third of the tablet thickness (Kementerian Kesehatan, 2020). Furthermore, hardness and friability tests are carried out to ensure the strength and durability of the tablets to be coated. The tablet has a hardness of not less than 4 kP and a hardness value of not more than 1% so the tablet is strong enough to be coated (Allen and Ansel, 2014).
Based on the weight variation test, the tablets have a uniform weight. The tablet meets the requirements for an acceptable value of no more than 15.0% (Kementerian Kesehatan, 2020). Even though the tablet contains less than 25% of the active substance by weight, a weight variation test must be carried out because weight gain is one of the essential parameters in the coating process (Merdekawati, 2010). The characterizations of the core tablets can be seen in Table 5.
Characterization of multicoated tablets
Evaluation of multicoated tablets included organoleptic test, tablet surface morphology, size uniformity test, content uniformity test, weight diversity test, friability test, hardness test, disintegration time test, drug content test, and in vitro dissolution profile test. The results of the organoleptic test on multicoated tablets showed that the tablets were biconvex in shape but had different colors and surface textures. Multicoated tablets using sodium alginate have a white color with a smooth and glossy surface. Meanwhile, multicoated chitosan tablets show a yellowish-white color with a rough and uneven surface due to sticking. Multicoated tablets using xanthan gum showed a bright white color with a glossier surface than the core tablet. The results of the organoleptic test of multicoated tablets can be seen in Figure 4. The results of the morphological analysis of multicoated tablets using SEM, which can be seen in Figure 5, showed that the presence of a coating could coat the core tablets. The thickness and diameter increased in multicoated tablets. The tablets meet the requirements for a tablet diameter that is at most three times the thickness of the tablet and not less than one-third of the tablet thickness. The result of the content uniformity test showed that the dexamethasone contained in the multicoated tablets met the specified requirements, which are at least 90.0% and not more than 110.0% of the found on the label. The acceptance value of the content uniformity test also meets the specified requirements because it is less than 15%, indicating that the active pharmaceutical ingredients (APIs) content in the multicoated tablets was uniform. The good flow properties of granules influenced the acceptance requirements of the content uniformity test. The good flow properties of the granules cause the granules to fill the molding space if the tablet is compressed and produce tablets with a uniform API content (Suhery et al., 2016). Meanwhile, the assay showed that all multicoated tablets contained not less than 90.0% dexamethasone and no more than 110.0% of the amount stated on the label that met the requirements.
 | Figure 2. Core tablets. [Click here to view] |
 | Figure 3. Surface of core tablet (SEM, with magnification of 200×). [Click here to view] |
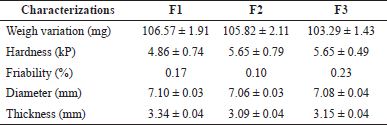 | Table 5. Characterizations of the core tablet. [Click here to view] |
 | Figure 4. Macroscopic appearance of multicoated tablets: (A) sodium alginate; (C) chitosan; (X) xanthan gum. [Click here to view] |
 | Figure 5. Surface of tablet coated with (A) alginate, (C (chitosan) and (X) xanthan gum (SEM, with magnification of 200×). [Click here to view] |
One parameter that needs to be considered in the tablet coating process is the increase in weight—the increase in the weight of the primary coating by 6.5%, 5%, and 7% (w/w) of alginate, chitosan, and xanthan gum, respectively. Meanwhile, the tablet weight increased by 8% (w/w) when the tablets were recoated with the secondary coating. An increase in tablet weight indicates that the coating solution effectively hits the tablet surface to form a coating layer (Merawati, 2009). The friability test showed that the multicoated tablets of all formulations met the requirements because it was less than 1%. The presence of the coating made the tablet more resistant to abrasion, even though it slipped during the test. The hardness test showed that the hardness of the multicoated tablets of all formulations was between 10 and 20 kP. The hardness of the multicoated tablets was greater than that of the core tablets. The increase in the multicoated tablet hardness test showed that the coating caused an adhesion bond on the tablet surface that could increase the hardness (Merawati, 2009).
The disintegration test showed that the multicoated tablets of all formulations were not crushed or cracked in the first hour using a gastric fluid medium. Then, the disintegration test showed that the multicoated tablets did not meet the disintegration time requirements for enteric-coated tablets in the intestinal fluid medium for 3 hours because none disintegrated. Multicoated tablets did not disintegrate in both mediums because Eudragit S100 will only dissolve at a pH of more than 7 (Nicholas et al., 2011). Therefore, it is necessary to test the dissolution profile of multicoated tablets to ensure that the drug can be released in the colon using a medium that simulates colonic fluid. The characterizations of multicoated tablets can be seen in Table 6.
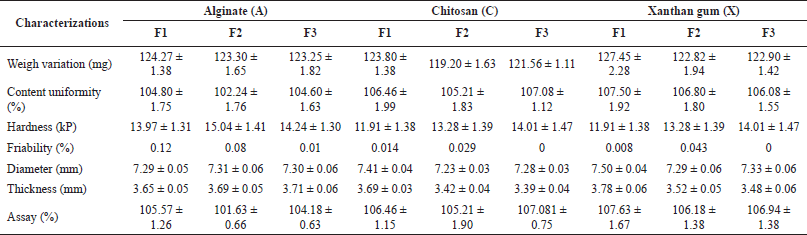 | Table 6. Characterizations of multicoated tablet. [Click here to view] |
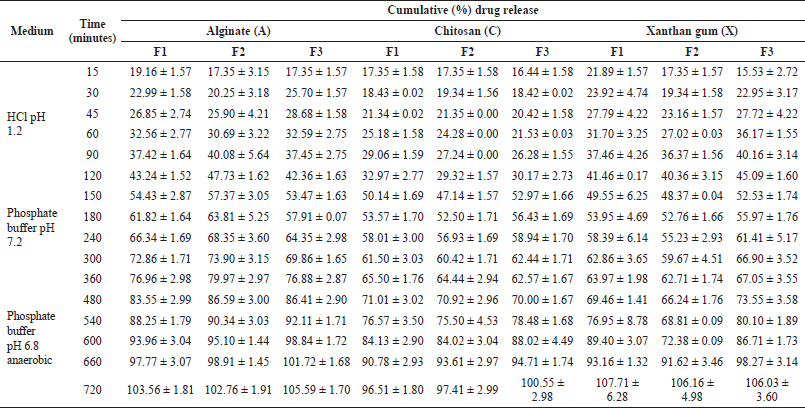 | Table 7. Dissolution profile of multicoated tablet. [Click here to view] |
In vitro drug release studies
In this study, the dissolution tests used 0.1N hydrochloric acid medium at pH 1.2, phosphate buffer at pH 7.2, and phosphate buffer at pH 6.8. Simulating gastric fluid was used during the first 2 hours. Next, the medium was changed to a phosphate buffer with a pH of 7.2 as an artificial small intestinal fluid. Phosphate buffer medium pH 7.2 was used for 3 hours according to humans’ small intestine transit time (Pooja et al., 2011). Phosphate buffer medium pH 6.8, simulating colonic fluid, was used for 7 hours. Colon is an anaerobic environment that contains microbiota in the form of obligately anaerobic bacteria. Compared to the stomach and small intestine, the colon contains up to 1011 bacteria per gram of intestinal contents (Wahlgren et al., 2019). Therefore, to create an anaerobic environment in the dissolution test according to the actual colonic environment, the phosphate buffer medium pH 6.8 flowed with CO2 gas for 10 minutes (Singh et al., 2015). The cumulative percentage of drug release of xanthan gum polymer in 0.1N hydrochloric acid medium from F1 was 41.46% ± 0.17%, F2 was 40.36% ± 3.15%, and F3 was 45.09% ± 1.60%. Multicoated tablets with alginate polymer-drug release in 0.1N hydrochloric acid medium were 43.24% ± 1.52%, 47.73% ± 1.62%, and 42.36% ± 1.63% for F1, F2, and F3. In multicoated tablets with chitosan polymer, the cumulative drug release in 0.1N hydrochloric acid medium for F1 was 32.97%, F2 was 29.32%, and F3 was 30.17%. Targeted colon drug delivery was expected to release the drug minimally in the upper gastrointestinal (Amidon et al., 2015). The drug is not expected to be released more than 10% in the artificial stomach and small intestine medium (Kementerian Kesehatan, 2020). However, all the formulations in this study showed high cumulative drug release in the simulated gastric medium. This could be due to the insufficient thickness of the secondary coating to resist drug release in the acidic medium and an uneven secondary coating layer on the surface of the primary coated tablet. So, the acid medium penetrates the pores of the tablet. As a result, there is an early release of the drug in an acidic medium (Nguyen et al., 2019).
In a phosphate buffer medium with a pH of 6.8, the drug is expected to be released entirely. In this medium, the probiotics in the core tablet were expected to degrade the primary coating layer. The higher the number of probiotics in the tablet, the higher the cumulative percentage of drug release in the colon (Ghosh et al., 2010). Multicoated tablets with xanthan gum, alginate, or chitosan polymers showed results that were not by the initial hypothesis, which stated that the formulation with the highest amount of probiotic content would provide greater release results when compared to other formulations (Ghosh et al., 2010). The effect of probiotics on drug release should be proved by testing the viability of probiotics, both before mixing the powder in the granulation process and after coating the core tablets. The probiotic viability test should be carried out to ensure that the probiotics contained in the preparation are still alive (Klayraung et al., 2009). The viability of probiotics is related to their stability during preparation, which can be affected by temperature, oxygen, and humidity. The dissolution profile of multicoated tablets can be seen in Table 7 and Figure 6.
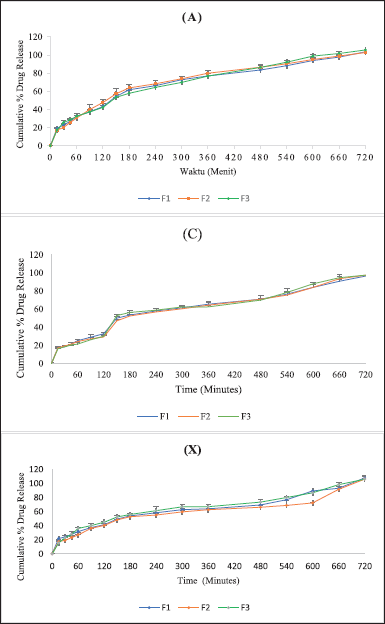 | Figure 6. Cumulative percent of dexamethasone released from colon-targeted multicoated tablets: (A) sodium alginate; (C) chitosan; (X) xanthan gum. [Click here to view] |
CONCLUSION
Based on the results, the characterization of multicoated dexamethasone tablets with probiotics using polysaccharides and pH-sensitive polymers as CTDDS has been obtained. However, according to in vitro dissolution test results, the formulation of multicoated tablets was not optimum to delay drug release in an acidic medium. The presence of probiotics does not affect drug release in tablets coated with sodium alginate (A), chitosan (C), and xanthan gum (X).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work was financially supported by Universitas Indonesia through PUTI Q2 Grant with contract number NKB-1176/UN2.RST/HKP.05.00/2022.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest, financial or otherwise.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Allen LV, Ansel HC. Ansel’s pharmaceutical dosage forms and drug delivery systems. 10th edition, Lippincott Williams & Wilkins, Philadelphia, PA, 2014.
Amidon S, Brown JE, Dave VS. Colon-targeted oral drug delivery systems: design trends and approaches. AAPS PharmSciTech, 2015; 16(4):731–41. CrossRef
Aulton ME. Pharmaceutics the science of dosage form design. 2nd edition, Churchill Livingstone, London, UK, 2002.
Brunton LL, Dandan RH, Knollmann BC. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th edition, McGraw-Hill Education, New York, NY, 2018.
Bruschi ML. Strategies to modify the drug release from pharmaceutical systems. Woodhead Publishing, Cambridge, UK, 2015.
Chourasia MK, Jain SK. Polysaccharides for colon targeted drug delivery. Drug Deliv, 2004; 11(2):129–48. CrossRef
Crouter A, Briens L. The effect of moisture on the flowability of pharmaceutical excipients. AAPS PharmSciTech, 2014; 15(1):65–74. CrossRef
Dangi AA, Ganure AL, Divya J. Formulation and evaluation of colon targeted drug delivery system of levetiracetam using pectin as polymeric carrier. J Appl Pharm Sci, 2013; 3(1):78–87.
Dasankoppa FS, Patwa S, Sholapur H, Arunkumar GR. Formulation and characterization of colon specific drug delivery system of prednisolone. Saudi J Health Sci, 2012; 1(8):143–50. CrossRef
Dian D, Simkoputera J, Wiguna C. Inflammatory bowel disease in young adult [Case Report]. Indones J Gastroenterol Hepatol Dig Endosc, 2019; 20(1):58–62. CrossRef
Farheen F, Elango K, Devi DR, Santhanalakshmi G. Formulation and evaluation of controlled porosity prednisolone osmotic tablets for colon targeting. Res J Pharm Technol, 2011; 4(7):1106–10.
Ghosh PK, Gupta VB, Gondoliya B, Rathore MS. Probiotic-assisted colon-specific delivery of diclofenac sodium from guar gum matrix tablet: in vitro evaluation. Asian J Pharm, 2010; 4(4):173–8. CrossRef
Gulbake A, Jain SK. Chitosan: a potential polymer for colon-specific drug delivery system. Expert Opin Drug Deliv, 2012; 9(6):713–29. CrossRef
Iskandarsyah, Sutriyo, Hayati D. Pengaruh kombinasi hidroksipropil metilselulosa-xanthan gum sebagai matriks pada profil pelepasan tablet teofilin lepas terkendali. Maj Ilmu Kefarmasian, 2010; 7(3):58–70. CrossRef
Iswandana R, Amangkoe E, Isnaini R. Tetrandrine beads using alginate/polyvinyl alcohol and alginate-carboxymethyl cellulose: not ideal as colon-targeted dosage form. J Pharm Negat Results, 2018a; 9(1):14–20. CrossRef
Iswandana R, Mutia MP, Widyaningrum FK. Unsuccessful delivery of tetrandrine from colon-targeted dosage forms comprising alginate/hydroxypropyl methylcellulose and alginate–chitosan beads. Int J Appl Pharm, 2018b; 10(Special Issue 1):396–402. CrossRef
Iswandana R, Putri KS, Dwiputra R, Yanuari T, Sari SP, Djajadisastra J. Formulation of chitosan tripolyphosphate-tetrandrine beads using ionic gelation method: in vitro and in vivo evaluation. Int J Appl Pharm, 2017a; 9(5):109–15. CrossRef
Iswandana R, Putri KS, Sandiata CE, Triani S, Sari SP, Djajadisastra J. Formulation of tetrandrine beads using ionic gelation method Ca-pectinate coated pH-sensitive polymers as colon-targeted dosage form. Asian J Pharm Clin Res, 2017b; 10(10):90–5. CrossRef
Iswandana R, Putri KS, Wulandari FR, Najuda G, Sari SP, Djajadisastra J. Preparation of calcium alginate-tetrandrine beads using ionic gelation method as colon-targeted dosage form. J Appl Pharm Sci, 2018c; 8(5):68–74. CrossRef
Kementerian Kesehatan RI. Farmakope Indonesia Edisi VI. Direktorat Jenderal Kefarmasian dan Alat Kesehatan Republik Indonesia, Jakarta, Indonesia, 2020
Klayraung S, Viernstein H, Okonogi S. Development of tablets containing probiotics: effects of formulation and processing parameters on bacterial viability. Int J Pharm, 2009; 370:54–60. CrossRef
Lachman L, Lieberman HA, Kanig JL. Theory and practice of industrial pharmacy. 3rd edition, Lea & Febiger, Philadelphia, PA, 1986.
Madhu S, Baibhav J, Monika B, Manish G. Formulation and evaluation of colon targeted tablets of mesalazine. J Drug Deliv Ther, 2012; 2(5):24–36. CrossRef
Mehta R, Chawla A, Sharma P, Pawar P. Formulation and in vitro evaluation of Eudragit S?100 coated naproxen matrix tablets for colon?targeted drug delivery system. J Adv Pharm Technol Res, 2013; 4(1):31–41. CrossRef
Merawati M. Formulasi tablet salut lapis tipis serbuk bawang putih (Allium sativum linn.) menggunakan pregel pati singkong hidroksipropil sebagai bahan penyalut. Universitas Indonesia, Depok, Indonesia, 2009.
Merdekawati T. Preparasi dan karakterisasi kitosan-n-asetil tablet salut lapis tipis preparasi dan karakterisasi kitosan-n-asetil. Universitas Indonesia, Depok, Indonesia, 2010.
Mohammed MA, Syeda J, Wasan KM, Wasan EK. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics, 2017; 9(4):53. CrossRef
Nguyen MNU, Tran PHL, Tran TTD. A single-layer film coating for colon-targeted oral delivery. Int J Pharm, 2019; 559:402–9. CrossRef
Nicholas ME, Panaganti S, Prabakaran L, Jayveera KN. Novel colon specific drug delivery system: a review. Int J Pharm Sci Res, 2011; 2(10):2545–61.
Pooja C, Naveen M, Rathore MS, Anurekha PJ, Kumar JA. Probiotic assisted colon targeted drug delivery system: research scope. Asian J Pharm Clin Res, 2011; 4(2):12–5.
Prudhviraj G, Vaidya Y, Singh SK, Yadav AK, Kaur P, Gulati M, Gowthamarajan K. Effect of co-administration of probiotics with polysaccharide based colon targeted delivery systems to optimize site specific drug release. Eur J Pharm Biopharm, 2015; 97:164–72. CrossRef
Raghuvanshi NS, Goswami L, Kothiyal P. Formulation and evaluation of colon targeted dosage form of prednisolone tablet using sterculia gum. J Appl Pharm Res, 2014; 2(3):16–9.
Raju D, Padmavathy J, Saraswathi VS, Saravanan D, Lakshmi IA. Formulation and development of enteric coated tablets of prednisolone as a colon targeted drug delivery. Int J Pharm Sci Res, 2011; 2(3):685–90.
Singh SK, Yadav AK, Prudhviraj G, Gulati M, Kaur P, Vaidya Y. A novel dissolution method for evaluation of polysaccharide based colon spesific delivery system: a suitable alternative to animal sacrifice. Eur J Pharm Sci, 2015; 73:72–80. CrossRef
Suhery WN, Fernando A, Giovanni B. Perbandingan metode granulasi basah dan kempa langsung terhadap sifat fisik dan waktu hancur orally disintegrating tablets (ODTS) piroksikam. J Sains Farm Klin, 2016; 2(2):138–44.
Sversut RA, Vieira JC, Rosa AM, Singh AK, do Amaral MS, Kassab NM. Improved UV spectrophotometric method for precise, efficient and selective determination of dexamethasone in pharmaceutical dosage forms. Orbit Electro J Chem, 2015; 7(1):7–12. CrossRef
The United States Pharmacopeial Convension. The United States pharmacopeia 41st and The National Formulary 36th. United Book Press, Inc, Baltimore, MD, 2018.
Wahlgren M, Axenstrand M, Håkansson Å, Marefati A, Pedersen BL. In vitro methods to study colon release: state of the art and an outlook on new strategies for better in-vitro biorelevant release media. Pharmaceutics, 2019; 11(95):1–24. CrossRef