INTRODUCTION
Stilbenes are phenolic compounds having a C6–C2–C6 skeleton and two aromatic rings with hydroxyl groups and are linked by a double-bonded ethylene bridge (Akinwumi et al., 2018; El Khawand et al., 2018). A total of 459 natural stilbenes from 45 plant families and 196 plant species have been identified (Teka et al., 2022). These compounds possess biological activities such as antioxidant, antimicrobial, antidiabetic, antiobesity, cardioprotective, neuroprotective, antineurodegenerative, anti-inflammatory, antiatherosclerosis, antiaging, and anticancer properties (Akinwumi et al., 2018; Teka et al., 2022). Stilbenes are often hydroxylated and/or methoxylated.
Resveratrol is a hydroxylated stilbene and pterostilbene is a methoxylated stilbene with well-studied biological activities and molecular effects (Chan et al., 2019; Tsai et al., 2017). Resveratrol is the most well-known stilbene that is abundant in nature and plays a significant role due to its antioxidant and anti-inflammatory properties (De Filippis et al., 2017). Pterostilbene is structurally related to resveratrol. It is a dimethoxylated derivative of resveratrol with similar biological activities but with more potent antioxidant and anticancer properties (McCormack and McFadden, 2012, 2013). It has better bioavailability due to the two methoxy groups at C3 and C5 that increase lipophilicity (Kapetanovic et al., 2011).
In this article, the chemistry, sources, anticancer and other pharmacological properties, pharmacokinetics, and patents of 3’-hydroxypterostilbene (HPS) and pinostilbene (PS) are reviewed. Despite their essential pharmacological properties, there have been no reviews on these two methoxylated stilbenes. The review of stilbenes that included HPS focused only on their anticancer properties (Tsai et al., 2017). Therefore, this review of HPS and PS is useful for scientists interested in pursuing research on methoxylated stilbenes.
CHEMISTRY
3’-Hydroxypterostilbene
HPS or 3,5-dimethoxy-3’,4’-dihydroxystilbene is a pterostilbene analog (Liu, 2014). HPS was first isolated from the whole plant of Sphaerophysa salsula (Fabaceae) (Ma et al., 2002). HPS has a molecular formula of C16H16O4 and a molecular weight of 272 g/mol. The chemical structure of HPS has a catechol moiety at ring B consisting of two hydroxyl (–OH) groups at C3’ and C4’ (Fig. 1). The two methoxy (–OCH3) groups at C3 and C5 of ring A make this compound more lipophilic with better cellular permeability and bioavailability (Tsai et al., 2017). The chemical structure of HPS is similar to that of pterostilbene except for an –OH group at C3’ at ring B, which is absent in pterostilbene. The two –OH groups at ring B suggest stronger bioactivity than pterostilbene, and the two –OCH3 groups at ring A suggest that it is more lipophilic and has better cellular permeability than resveratrol (Liu, 2014).
Pinostilbene
PS (3-methoxyresveratrol or 3,4’-dihydroxy-5-methoxystilbene) has a molecular formula of C15H14O3 and a molecular weight of 242 g/mol. PS was first isolated from the bark of Pinus sibirica (Tyukavkina et al., 1972). A naturally occurring analog of resveratrol (3,5,4’-trihydroxystilbene), the chemical structure of PS consists of two –OH groups at C3 and C4′ and a –OCH3 group at C5 (Fig. 1). Its structure is similar to that of pterostilbene except for an –OH group at C3 instead of a –OCH3 group at ring A as in pterostilbene.
SOURCES
HPS (≥99%) and PS (≥95%) are commercially available from Sigma-Aldrich. HPS has been isolated from the whole herb of S. salsula (Ma et al., 2002). PS has been reported in the bark of P. sibirica (Tyukavkina et al., 1972), the stem wood of Dracaena loureiri (Likhitwitayawuid et al., 2002), the bark of Soymida febrifuga (Awale et al., 2009), the resin of Dracaena cochinchinensis (Liu et al., 2013), and stem bark of Commiphora africana (Segun et al., 2019).
Besides isolation from natural sources, HPS and PS can be synthesized. HPS was synthesized from 3,4-dihydroxybenzaldehyde in five steps with an overall yield of 33% (Venkateswarlu et al., 2003). The spectral data of the synthesized HPS agreed with that of the natural product and displayed potent antioxidative activity. The process for the manufacture of HPS has been patented by Majeed et al. (2016). HPS is synthesized via ortho-formylation of pterostilbene to obtain 3’-formyl pterostilbene followed by Dakin oxidation of 3′-formyl pterostilbene to yield HPS. PS can be biosynthesized using the recombinant Escherichia coli that harbors an artificial biosynthetic pathway (Kang et al., 2014).
ANTICANCER PROPERTIES
3’-Hydroxypterostilbene
The apoptotic activity of HPS was evaluated in different leukemia cell lines. In HL60, K562, and HUT78 drug-sensitive leukemia cells and in HL60-R and K562-ADR multidrug-resistant (MDR) leukemia cells, the HPS concentration capable of inhibiting 50% cell growth (IC50) was 0.8, 0.8, 0.6, 0.9, and 1.2 μM, respectively (Tolomeo et al., 2005). For the same leukemia cell lines, the HPS concentration capable of inducing 50% cell apoptosis (AC50) was 1.0, 3.0, 0.7, 5.0, and 3.5 μM, respectively. HPS was much stronger than pterostilbene, especially against MDR leukemia cells. For example, against HL60-R cells, the IC50 and AC50 values of HPS were 44 and 17 times stronger than those of pterostilbene. In addition, HPS exhibited low toxicity in normal stem cells. Against colony-forming units-granulocyte macrophage normal bone marrow cells, HPS was not cytotoxic with an IC50 value of 50 μM (Tolomeo et al., 2005).
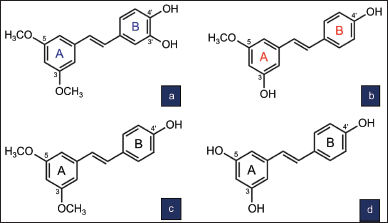 | Figure 1. Chemical structure of HPS (a) and PS (b) in comparison with pterostilbene (c) and resveratrol (d) [Click here to view] |
HPS inhibited the growth of COLO 205, HCT-116, and HT-29 colon cancer cells with IC50 values of 9, 40, and 71 μM, respectively (Cheng et al., 2014; Pan et al., 2015). Cytotoxicity of HPS was more potent than pterostilbene with IC50 values of 33, 47, and 81 μM, respectively. HPS was cytotoxic toward HCT 116 colon, MDA-MB-231 breast, PC-3 prostate, and HepG2 liver cancer cells with IC50 values of 7.6, 18, 23, and 8.4 μg/ml, respectively (Takemoto et al., 2015). The anticancer effects and mechanisms of HPS have been reported in colon cancer cells and leukemia cells, including colon and skin tumors (Table 1). Against colon cancer cells (COLO 205, HCT-116, and HT-29), cytotoxic activities involved apoptosis and autophagy, accompanied by activation of caspase-3, downregulation of cyclooxygenase- (COX-) 2, matrix metallopeptidase- (MMP-) 9, vascular endothelial growth factor (VEGF), and cyclin D1, and inhibition of phosphatidylinositol 3-kinase (PI3K)/Akt, mammalian target of rapamycin (mTOR), and mitogen-activated protein kinase (MAPK) pathways (Cheng et al., 2014; Pan et al., 2015). Inhibition of leukemia cells (K562, HL60-R, and K562-ADR) involved triggering of the intrinsic apoptotic pathway via disruption of mitochondrial membrane potential and suppression of caspase inhibitors (Z-VAD-fmk and Z-LEHD-fmk) (Takemoto et al., 2015). Amelioration of colon tumors in mice involved inhibition of IL-6/signal transducer and activator of transcription 3 (STAT3) signaling and inhibition of the expression of inflammatory enzymes and β-catenin signaling (Lai et al., 2017). Suppression of skin tumors in mice included protection against inflammation in mouse skin papilloma and suppression of p38 and signal transducer and STAT3 signaling pathways (Lee et al., 2021).
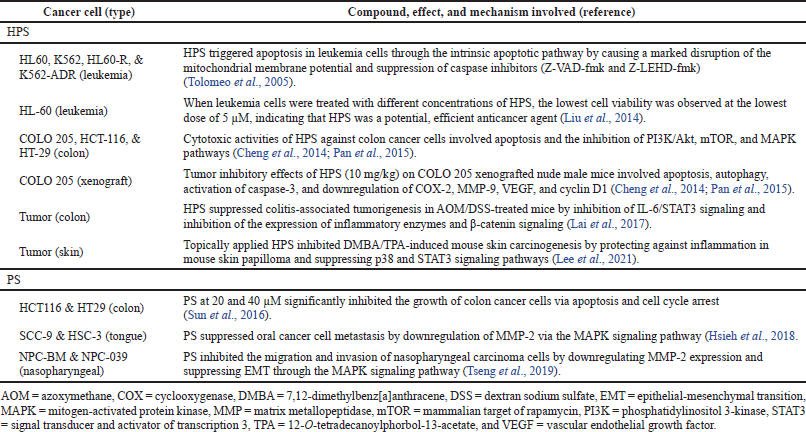 | Table 1. Anticancer properties of HPS and PS. [Click here to view] |
Related to HPS anticancer properties is its ability to inhibit histone deacetylase (HD). At 50 μg/ml, HPS significantly inhibited HD activity compared to trichostatin A, the positive control (Takemoto et al., 2015). HD inhibitors are a relatively new class of anticancer agents that play important roles in inducing apoptosis and cell cycle arrest in cancer cells (Kim and Bae, 2011). Several ongoing clinical trials are testing HD inhibitors for use as anticancer drugs (alone or in combination with other ant-cancer drugs). The molecular mechanisms underlying HD inhibitors in cancer patients, such as HPS, are not fully understood.
Pinostilbene
PS inhibited 26-L5 colon, HeLa cervical, and B16-BL6 melanoma cancer cells with IC50 values of 3.0, 9.4, and 16 μg/ml (Awale et al., 2009). Against MCF-7 breast, A549 lung, PC3 prostate, and HepG2 liver cancer cells, the IC50 values of PS were 29, 42, 38, and 33 μg/ml (Segun et al., 2019). Against PNT2 normal prostate cells, cytotoxicity of PS was very weak at 225 μg/ml. PS significantly reduced the viability of MCF-7 breast cancer cells by 50% at 14 μM and reduced tumor cell migration by 12% (van den Brand et al., 2019). When HT29 and HCT116 colon cancer cells were treated with different concentrations of PS and pterostilbene for 24 and 48 hours, both stilbenes showed similar potency in inhibiting cell growth inducing apoptosis and cell cycle arrest (Sun et al., 2016). Against normal colon cells, neither PS nor pterostilbene showed any growth inhibition up to 40 μM.
The anticancer effects and mechanisms of PS have been reported in colon, tongue, and nasopharyngeal cancer cells (Table 1). PS significantly inhibited the growth of HCT116 and HT29 colon cancer cells via apoptosis and cell cycle arrest (Sun et al., 2016). PS suppressed the metastasis of SCC-9 and HSC-3 oral cancer cells by downregulation of MMP-2 via the MAPK signaling pathway (Hsieh et al., 2018). PS inhibited the migration and invasion of NPC-BM and NPC-039 nasopharyngeal carcinoma cells by downregulating MMP-2 expression and suppressing epithelial-mesenchymal transition (EMT) through the MAPK signaling pathway (Tseng et al., 2019).
An earlier study reported that PS possessed anticancer properties toward childhood acute lymphoblastic leukemia (Katik et al., 2006). In primary lymphoblasts, PS induced apoptosis with an LC50 value of 10 μM. Among the anthracyclines used to treat childhood leukemia, daunorubicin- and doxorubicin-induced apoptosis with response rates of 27% and 23%, respectively. However, a much higher response rate was observed in PS (73%). In daunorubicin-resistant lymphoblasts, PS induced apoptosis with a response rate of 60%.
OTHER PHARMACOLOGICAL PROPERTIES
3’-Hydroxypterostilbene
Other pharmacological properties of HPS include anti-inflammatory, antioxidant, hepatoprotective, anticolitis, antiadipogenesis, sirtuin 1 (SIRT1) modulatory, and α-glucosidase inhibitory activities (Table 2).
Pinostilbene
Other pharmacological properties of PS include anti-inflammatory, cytochrome P 450 (CYP) inhibitory, neuroprotective, antioxidant, antityrosinase, α-glucosidase inhibitory, antiplasmodial, lactase dehydrogenase (LDH) activity release, antimelanogenesis, antiadipogenesis, suppression of hepatic stellate cell (HSC) activation, and tight junction (TJ) protection activities (Table 2).
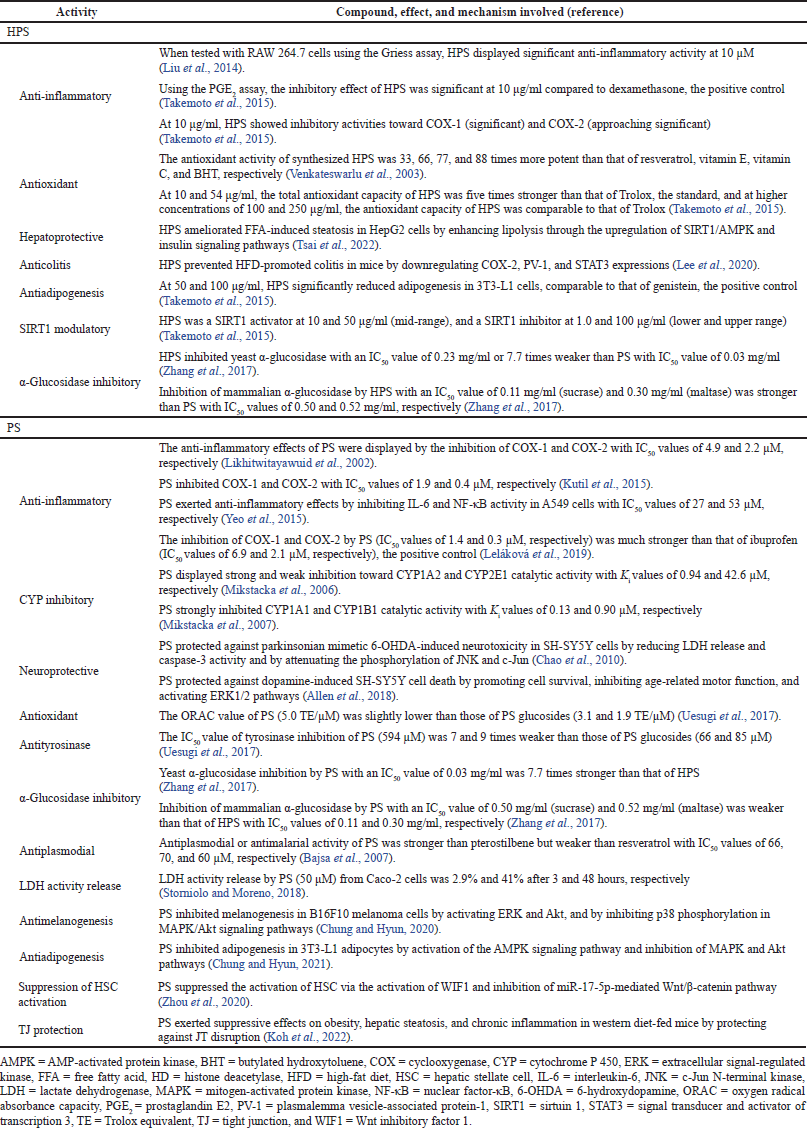 | Table 2. Other pharmacological properties of HPS and PS. [Click here to view] |
Terminologies
The following terminologies used in Tables 1 and 2 are explained for more clarity to readers:
- Apoptosis is the process of programmed cell death that is characterized by distinct changes in morphological characteristics and biochemical mechanisms (Elmore, 2007).
- Xenograft is a widely used model to study anticancer effects where human cancer cells are transplanted under the skin of shaven mice (Richmond and Su, 2008).
- Metastasis is the spread of cancer cells to tissues and organs beyond where the tumor originated and led to the formation of new tumors. It is the major cause of death of a patient with cancer (Martin et al., 2013).
- SIRTs are a class of deacetylase enzymes that are linked to metabolic control, apoptosis and cell survival, DNA repair, inflammation, neuroprotection, development, and healthy aging (Villalba and Alcaín, 2012). Modulation of the activities of SIRTs has beneficial effects on human diseases.
- HSC activation causes the formation of proliferative fibrogenic myofibroblasts, which eventually leads to hepatic fibrosis and liver cancer (Tsuchida and Friedman, 2017).
- Intestinal epithelial TJs function as physical intestinal barriers (Lee et al., 2018; Suzuki, 2013). TJs control permeability and regulate the movement of ions, solutes, and water across the intestinal epithelium. The dysfunction of TJs is associated with the initiation and development of intestinal, metabolic, and inflammatory diseases.
- STAT3 belongs to the STAT family of latent cytoplasmic transcription factors that is very transient and tightly regulated (Kamran et al., 2013). When persistently activated, STAT3 plays a central role in tumorigenesis. In tumor cells, blocking STAT3 signaling inhibits tumor growth, angiogenesis, and metastasis without affecting normal cells.
PHARMACOKINETICS
3’-Hydroxypterostilbene
A high-performance liquid chromatographic method was successfully developed and applied to detect and quantify HPS in rat urine and serum samples (Takemoto and Davies, 2009). After intravenous administration of HPS, the predominant compound excreted in the rat urine was HPS glucuronide. Some data on the pharmacokinetic parameters of HPS were as follows: mean half-life in serum and urine was ~0.45/hour and ~1.02/hour, respectively; mean elimination rate constant was ~0.69/hour; mean fraction excreted in the urine unchanged was 17.2%; mean renal clearance was 2.23 l/hour/kg.
In a study on the urinary metabolites of pterostilbene, female mice were administered with pterostilbene in dimethyl sulfoxide by oral gavage (200 mg/kg). Their urine samples were collected after 24 hours, and the metabolites in urine were analyzed using liquid chromatography-mass spectrometry (LC-MS). Results showed that HPS was one of the nine metabolites identified as mono=hydroxylated pterostilbene (Shao et al., 2010).
After ingestion of HPS and pterostilbene (50 mg/kg) separately, the serum of rats was examined (Chen et al., 2017). Rats that ingested HPS revealed the presence of two unidentified metabolites without the parent compound, while rats that ingested pterostilbene had only one metabolite (glucuronide or sulfate conjugate) and the parent compound. These results showed that the biotransformation of HPS might not be glucuronide or sulfate conjugation, unlike pterostilbene (Chen et al., 2017).
In a recent study on the metabolites of pterostilbene incubated with rat, dog, and human hepatocytes for 2 hours, HPS was one of the six primary metabolites identified (Jiang et al., 2021). HPS was formed by hydroxylation at C3’ of ring B of the phenol moiety in all three types of incubated hepatocytes.
Pinostilbene
The pharmacokinetics of PS was studied by intravenous injection (5 or 10 mg/kg) into Sprague-Dawley rats (Chen et al., 2016). LC–MS/MS method was developed to determine and quantify PS in the rat plasma. Results showed that PS displayed rapid clearance of 129 or 107 ml/minute/kg and extremely short mean transit time of 6.2 or 8.5 minutes. The bioavailability of PS was limited but highly erratic. It was inferred that stilbenes with meta-hydroxyl group(s) may be associated with metabolic instability and subsequently more rapid clearance and lower oral bioavailability.
CD-1 mice were fed a diet containing pterostilbene for 3 weeks, and their colon content was examined (Sun et al., 2016). PS was found to be a major metabolite of pterostilbene in the colon. The quantity of PS in the colon content and mucosa was relatively high, comparable to the amount of pterostilbene. PS was likely formed by the demethylation of pterostilbene by gut microbiota demethylases. Therefore, PS is a colonic metabolite of pterostilbene. An earlier study also found that PS (identified as monodemethylated pterostilbene) was one of the nine metabolites in the urine of mice after an oral gavage of pterostilbene (Shao et al., 2010).
PS was another primary metabolite formed by pterostilbene incubated with rat, dog, and human hepatocytes (Jiang et al., 2021). PS was formed by the demethylation of pterostilbene incubated in all three types of hepatocytes. An earlier study reported that PS was among the 21 compounds identified in the rat urine after oral administration of the resin from D. cochinchinensis (Liu et al., 2013).
PATENTS
3’-Hydroxypterostilbene
A patent on HPS was filed by Majeed and Nagabhushanam from New Jersey, US, and by Ganjihal from Bangalore, India, as inventors, and by Sami Labs Ltd. in Bangalore, India, as the assignee (Majeed et al., 2016). The United States patent US 9,458,075 B1, dated October 2016, was entitled “process for the manufacture of HPS.” The invention disclosed a novel and high-yielding novel scheme for synthesizing HPS. Using pterostilbene as the starting material, the invention uses favorable reactants and reagents, is cost-effective and industrially scalable, involves minimal reaction steps, is economically viable, and produces a high yield (60%–70%) of HPS. Concurrently, another patent was filed by Majeed as the inventor and by Nagabhushanam as the assignee (Majeed and Nagabhushanam, 2016). The World Intellectual Property Organization patent WO 2016/032925, dated March 2016, was entitled “3-hydroxypterostilbene and therapeutic applications thereof.” The invention discloses the therapeutic potential of HPS in colon cancer and prostate cancer, specifically, the apoptotic and autophagy properties of HPS in controlling colon and prostate tumors.
Pinostilbene
A patent on PS was filed by Hong, Kang, Heo, and Lee from Daejeon, Korea, and by Ahn from Seoul, Korea, as inventors, and by the Korea Research Institute of Bioscience and Biotechnology in Daejeon, Korea, as the assignee (Hong et al., 2018). The patent WO 2018/008979 A1, dated November 2018, was entitled “recombinant vector for producing PS or pterostilbene.” A method was developed to produce PS or pterostilbene using a recombinant vector. The single vector system is economical and capable of mass production via a single microbial metabolic pathway. PS or pterostilbene produced possesses anticancer, anti-inflammatory, antihyperlipidemic, and antioxidant effects and thus can be beneficially used in the pharmaceutical industry.
CONCLUSION
Methoxylated stilbenes such as pterostilbene are well studied but not the lesser known HPS and PS reviewed in this article. Both HPS and PS have promising prospects for further research. The cellular and molecular mechanisms underlying the effects of HPS and PS on various types of cancer cells would yield exciting results. Further research on their structural modifications is needed to synthesize novel derivatives with enhanced anticancer properties. The effects of methoxylation and hydroxylation on HPS and PS, together with studies on their pharmacokinetics and structure-activity relationships, would be interesting. Clinical research on HPS and PS is warranted to evaluate their chemopreventive efficacy and safety when used alone or in combination with other chemotherapy agents.
FINANCIAL SUPPORT
The Lead and Sole Author declares that the funds for the publication of this review article in JAPS (Article Processing Charges) are from World’s Top 2% Scientist Research Grant, CERVIE, UCSI University (Grant Code: T2S-2023/004). The author is grateful for the financial support.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Akinwumi BC, Bordun KA, Anderson HD. Biological activities of stilbenoids. Int J Mol Sci, 2018; 19(3):792–817. CrossRef
Allen EN, Potdar S, Tapias V, Parmar M, Mizuno CS, Rimando A, Cavanaugh JE. Resveratrol and pinostilbene confer neuroprotection against aging-related deficits through an ERK1/2-dependent mechanism. J Nutr Biochem, 2018; 54:77–86. CrossRef
Awale S, Miyamoto T, Linn TZ, Li F, Win NN, Tezuka Y, Esumi H, Kadota S. Cytotoxic constituents of Soymida febrifuga from Myanmar. J Nat Prod, 2009; 72(9):1631–6. CrossRef
Bajsa J, Singh K, Nanayakkara D, Duke SO, Rimando AM, Evidente A, Tekwani BL. A survey of synthetic and natural phytotoxic compounds and phytoalexins as potential antimalarial compounds. Biol Pharm Bull, 2007; 30(9):1740–4. CrossRef
Chan EWC, Wong CW, Tan YH, Foo JP, Wong SK, Chan HT. Resveratrol and pterostilbene: a comparative overview of their chemistry, biosynthesis, plant sources and pharmacological properties. J Appl Pharm Sci, 2019; 9(7):124–9. CrossRef
Chao J, Li H, Cheng KW, Yu MS, Chang RC, Wang M. Protective effects of pinostilbene, a resveratrol methylated derivative, against 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. J Nutr Biochem, 2010; 21(6):482–9. CrossRef
Chen W, Yeo SC, Chuang XF, Lin HS. Determination of pinostilbene in rat plasma by LC–MS/MS: application to a pharmacokinetic study. J Pharm and Biomed Anal, 2016; 120:316–21. CrossRef
Chen YK, Tsai HY, Ho CT. Pharmacokinetic studies of pterostilbene and 3’-hydroxypterostilbene in rats. Nutr Food Sci, 2017; 7(4 Suppl):50.
Cheng TC, Lai CS, Chung MC, Kalyanam N, Majeed M, Ho CT, Ho YS, Pan MH. Potent anti-cancer effect of 3’-hydroxypterostilbene in human colon xenograft tumours. PLoS One, 2014; 9(11):e111814. CrossRef
Chung YC, Hyun CG. Inhibitory effects of pinostilbene hydrate on melanogenesis in B16F10 melanoma cells via ERK and p38 signaling pathways. Int J Mol Sci, 2020; 21(13):4732–43. CrossRef
Chung YC, Hyun CG. Inhibitory effects of pinostilbene on adipogenesis in 3T3-L1 adipocytes: a study of possible mechanisms. Int J Mol Sci, 2021; 22(24):13446–61. CrossRef
De Filippis B, Ammazzalorso A, Fantacuzzi M, Giampietro L, Maccallini C, Amoroso R. Anticancer activity of stilbene-based derivatives. ChemMedChem, 2017; 12(8):558–70. CrossRef
El Khawand T, Courtois A, Valls J, Richard T, Krisa S. A review of dietary stilbenes: sources and bioavailability. Phytochem Rev, 2018; 17(5):1007–29. CrossRef
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol, 2007; 35(4):495–516. CrossRef
Hong YS, Kang SY, Heo KT, Lee JK, Ahn SY. Recombinant vector for producing pinostilbene or pterostilbene. World Intellectual Property Organization Patent, WO 2018/008979 A1, November 2018.
Hsieh MJ, Chin MC, Lin CC, His YT, Lo YS, Chuang YC, Chen MK. Pinostilbene hydrate suppresses human oral cancer cell metastasis by downregulation of matrix metalloproteinase-2 through the mitogen-activated protein kinase signaling pathway. Cell Physiol Biochem, 2018; 50(3):911–23. CrossRef
Jiang L, Yin R, Zheng Q, He C, Chang H. High-resolution mass spectrometry-based methodology for the identification of the metabolites of pterostilbene produced by rat, dog and human hepatocytes. Biomed Chromatogr, 2021; 35(9):e5138. CrossRef
Kamran MZ, Patil P, Gude RP. Role of STAT3 in cancer metastasis and translational advances. BioMed Res Int, 2013; 2013:15. CrossRef
Kang SY, Lee JK, Choi O, Kim CY, Jang JH, Hwang BY, Hong YS. Biosynthesis of methylated resveratrol analogues through the construction of an artificial biosynthetic pathway in E. coli. BMC Biotechnol, 2014; 14(1):67–78. CrossRef
Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analogue, pterostilbene, in rats. Cancer Chemother Pharmacol, 2011; 68(3):593–601. CrossRef
Katik B, Selig A, Velder J, Shults EE, Wieder T, Henze G, Schmalz HG, Prokop A. New pinostilbene analogues overcome anthracycline resistance in acute lymphoplastic leukemia ex vivo. Blood, 2006; 108(11):4403. CrossRef
Kim HJ, Bae SC. Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am J Translat Res, 2011; 3(2):166–79.
Koh YC, Lin SJ, Nagabhushanam K, Ho CT, Pan MH. The anti-obesity and anti-inflammatory capabilities of pterostilbene and its colonic metabolite pinostilbene protect against tight junction disruption from western diet feeding. Mol Nutr Food Res, 2022; 66:2200146. CrossRef
Kutil Z, Kvasnicova M, Temml V, Schuster D, Marsik P, Cusimamani EF, Lou JD, Vanek T, Landa P. Effect of dietary stilbenes on 5-lipoxygenase and cyclooxygenases activities in vitro. Int J Food Prop, 2015; 18(7):1471–7. CrossRef
Lai CS, Yang G, Li S, Lee PS, Wang BN, Chung MC, Nagabhushanam K, Ho CT, Pan MH. 3′-Hydroxypterostilbene suppresses colitis-associated tumorigenesis by inhibition of IL-6/STAT3 signaling in mice. J Agric Food Chem, 2017; 65(44):9655–64. CrossRef
Lee B, Moon KM, Kim CY. Tight junction in the intestinal epithelium: its association with diseases and regulation by phytochemicals. J Immunol Res, 2018; 2018:11. CrossRef
Lee PS, Chiou YS, Chou PY, Nagabhushanam K, Ho CT, Pan MH. 3′-Hydroxypterostilbene inhibits 7,12-dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced mouse skin carcinogenesis. Phytomedicine, 2021; 81:153432. CrossRef
Lee PS, Chiou YS, Nagabhushanam K, Ho CT, Pan MH. 3’-Hydroxypterostilbene potently alleviates obesity exacerbated colitis in mice. J Agric Food Chem, 2020; 68(19):5365–74. CrossRef
Leláková V, Šmejkal K, Jakubczyk K, Veselý O, Landa P, Václavík J, Bobá? P, Pížová H, Temml V, Steinacher T, Schuster D. Parallel in vitro and in silico investigations into anti-inflammatory effects of non-prenylated stilbenoids. Food Chem, 2019; 285:431–40. CrossRef
Likhitwitayawuid K, Sawasdee K, Kirtikara K. Flavonoids and stilbenoids with COX-1 and COX-2 inhibitory activity from Dracaena loureiri. Planta Med, 2002; 68(9):841–3. CrossRef
Liu, B. Identification of oxidative products of pterostilbene and 3?-hydroxypterostilbene in vitro and evaluation of anti-inflammatory and anti-cancer cell proliferative activity. Master’s Thesis, Rutgers University-Graduate School, New Brunswick, Canada, 2014.
Liu F, Wu B, Xiao W, Wang Z, Sun Z, Yin J, Huang C. Identification of the major components of Resina Draconis extract and their metabolites in rat urine by LC-ESI-MSn. Chromatographia, 2013; 76(17):1131–9. CrossRef
Ma ZJ, Li X, Li N, Wang JH. Stilbenes from Sphaerophysa salsula. Fitoterapia, 2002; 73(4):313−5. CrossRef
Majeed M, Nagabhushanam K, Ganjihal SS. Process for the manufacture of 3’-hydroxypterostilbene. United States Patent, US 9,458,075 B1, October 2016.
Majeed M, Nagabhushanam K. 3-Hydroxypterostilbene and therapeutic applications thereof. World Intellectual Property Organization (WIPO) patent, WO 2016/032925, March 2016.
Martin TA, Ye L, Sanders AJ, Lane J, Jiang WG. Cancer invasion and metastasis: molecular and cellular perspective. In: Rahul Jandial (Ed.). Metastatic cancer: clinical and biological perspectives, Landes Bioscience, Austin, TX, 2013.
McCormack D, McFadden D. Pterostilbene and cancer: current review. J Surg Res, 2012; 173(2):53–61. CrossRef
McCormack D, McFadden D. A review of pterostilbene antioxidant activity and disease modification. Oxid Med Cell Longev, 2013; 2013:15. CrossRef
Mikstacka R, Przybylska D, Rimando AM, Baer-Dubowska W. Inhibition of human recombinant cytochromes P450 CYP1A1 and CYP1B1 by trans-resveratrol methyl ethers. Mol Nutr Food Res, 2007; 51(5):517–24. CrossRef
Mikstacka RR, Rimando AM, Szalaty KA, Stasik KA, Baer-Dubowska WA. Effect of natural analogues of trans-resveratrol on cytochromes P4501A2 and 2E1 catalytic activities. Xenobiotica, 2006; 36(4):269–85. CrossRef
Pan MH, Ho YS, Ho CT. Potent anti-cancer effect of 3’-hydroxypterostilbene in human colon xenograft tumors. Toxicol Lett, 2015; 2(238):S238. CrossRef
Richmond A, Su Y. Mouse xenograft models vs. GEM models for human cancer therapeutics. Dis Model Mech, 2008; 1(2–3):78–82. CrossRef
Segun PA, Ogbole OO, Ismail FM, Nahar L, Evans AR, Ajaiyeoba EO, Sarker SD. Resveratrol derivatives from Commiphora africana (A. Rich.) Endl. display cytotoxicity and selectivity against several human cancer cell lines. Phytother Res, 2019; 33(1):159–66. CrossRef
Shao X, Chen X, Badmaev V, Ho CT, Sang S. Structural identification of mouse urinary metabolites of pterostilbene using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom, 2010; 24(12):1770–8. CrossRef
Storniolo CE, Moreno JJ. Resveratrol analogues with antioxidant activity inhibit intestinal epithelial cancer Caco-2 cell growth by modulating arachidonic acid cascade. J Agric Food Chem, 2018; 67(3):819–28. CrossRef
Sun Y, Wu X, Cai X, Song M, Zheng J, Pan C, Qiu P, Zhang L, Zhou S, Tang Z, Xiao H. Identification of pinostilbene as a major colonic metabolite of pterostilbene and its inhibitory effects on colon cancer cells. Mol Nutr Food Res, 2016; 60(9):1924–32. CrossRef
Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci, 2013; 70(4):631–59. CrossRef
Takemoto JK, Davies NM. A high-performance liquid chromatographic analysis and preliminary pharmacokinetic characterization of 3’-hydroxypterostilbene in rats. Biomed Chromatogr, 2009; 23(10):1086–91. CrossRef
Takemoto JK, Remsberg CM, Davies NM. Pharmacologic activities of 3’-hydroxy pterostilbene: cytotoxic, anti-oxidant, anti-adipogenic, anti-inflammatory, histone deacetylase and sirtuin 1 inhibitory activity. J Pharm Pharm Sci, 2015; 18(4):713–27. CrossRef
Teka T, Lele Z, Xiaoyan G, Li Y, Lifeng H, Xiaohui Y. Stilbenes: source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical application—a comprehensive review. Phytochemistry, 2022; 197:113128. CrossRef
Tolomeo M, Grimaudo S, Di Cristina A, Roberti M, Pizzirani D, Meli M, Dusonchet L, Gebbia N, Abbadessa V, Crosta L, Barucchello R. Pterostilbene and 3’-hydroxy pterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int J Biochem Cell Biol, 2005; 37(8):1709–26. CrossRef
Tsai HY, Ho CT, Chen YK. Biological actions and molecular effects of resveratrol, pterostilbene, and 3’-hydroxypterostilbene. J Food Drug Anal, 2017; 25(1):134–47. CrossRef
Tsai HY, Shih YY, Yeh YT, Huang CH, Liao CA, Hu CY, Nagabhushanam K, Ho CT, Chen YK. Pterostilbene and its derivative 3’-hydroxypterostilbene ameliorated non-alcoholic fatty liver disease through synergistic modulation of the gut microbiota and SIRT1/AMPK signaling pathway. J Agric Food Chem, 2022; 70(16):4966–80. CrossRef
Tseng PY, Liu YT, Lin CC, Chuang YC, Lo YS, Hsi YT, Hsieh MJ, Chen MK. Pinostilbene hydrate inhibits the migration and invasion of human nasopharyngeal carcinoma cells by down-regulating MMP-2 expression and suppressing epithelial–mesenchymal transition through the mitogen-activated protein kinase signaling pathways. Front Oncol, 2019; 9:1364–75. CrossRef
Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol, 2017; 14(7):397–411. CrossRef
Tyukavkina NA, Gromova AS, Lutskii VI, Voronov VK. Hydroxystilbenes from the bark of Pinus sibirica. Chem Nat Compd, 1972; 8(5):570–2. CrossRef
Uesugi D, Hamada H, Shimoda K, Kubota N, Ozaki SI, Nagatani N. Synthesis, oxygen radical absorbance capacity, and tyrosinase inhibitory activity of glycosides of resveratrol, pterostilbene, and pinostilbene. Biosci Biotechnol Biochem, 2017; 81(2):226–30. CrossRef
van den Brand AD, Villevoye J, Nijmeijer SM, van den Berg M, van Duursen MB. Anti-tumor properties of methoxylated analogues of resveratrol in malignant MCF-7 but not in non-tumorigenic MCF-10A mammary epithelial cell lines. Toxicology, 2019; 422:35–43. CrossRef
Venkateswarlu S, Satyanarayana B, Sureshbabu CV, Subbaraju GV. Synthesis and antioxidant activity of 4-[2-(3,5-dimethoxyphenyl)ethenyl]-1,2-benzenediol, a metabolite of Sphaerophysa salsula. Biosci Biotechnol Biochem, 2003; 67(11):2463–6. CrossRef
Villalba JM, Alcaín FJ. Sirtuin activators and inhibitors. Biofactors, 2012; 38(5):349–59. CrossRef
Yeo SC, Fenwick PS, Barnes PJ, Donnelly LE. Anti-inflammatory effects of resveratrol analogues in cellular models of airway inflammation. Eur Respir J, 2015; 46:OA487. CrossRef
Zhang AJ, Rimando AM, Mizuno CS, Mathews ST. α-Glucosidase inhibitory effect of resveratrol and piceatannol. J Nutr Biochem, 2017; 47:86–93. CrossRef
Zhou G, Li C, Zhan Y, Zhang R, Lv B, Geng W, Zheng J. Pinostilbene hydrate suppresses hepatic stellate cell activation via inhibition of miR-17-5p-mediated Wnt/β-catenin pathway. Phytomedicine, 2020; 79:153321. CrossRef