INTRODUCTION
Caffeine (1,3,7-trimethylxanthine) is a natural alkaloid used as a component of beverages, medicines, and dietary supplements. It has been shown that caffeine has a behavioral and mental effect on humans and animals, similar to the impact of typical psychomotor stimulants (amphetamine, cocaine) (Garrett and Griffiths, 1997), as well as increased blood pressure in doses in caffeinated beverages and medicines (McMullen et al., 2011). Caffeine can cause weight loss (Tabrizi et al., 2019), which is often included in dietary supplements for weight loss. Controlling and labeling the caffeine content of dietary supplements are necessary to reduce the health risks for consumers of such products. Since weight loss products can also contain vitamins, plant extracts, and excipients, increasing the selectivity of the caffeine determination method is an important task. In addition, modern methods must be safe for the environment and analysts.
A large number of methods, including high-performance liquid chromatography (HPLC) (Fajara and Susanti, 2017; Gliszczy?ska-?wig?o and Rybicka, 2015; Rahim et al., 2014; Srdjenovic et al., 2008; Tzanavaras and Themelis, 2007; Zuo et al., 2002), gas chromatography (Sereshti and Samadi, 2014), capillary electrophoresis (Regan and Shakalisava, 2005), ultraviolet (UV) spectroscopy (Belay et al., 2008), and electrochemical method (Amare and Admassie, 2012), are used for assay of the caffeine content of coffee, tea, and caffeinated beverages.
Capillary electrophoresis allows rapid separation of caffeine from its counterparts. Still, the usual relative SD of the peak areas of successive injections is 3%–4% (Regan and Shakalisava, 2005), which significantly impairs the metrological characteristics of the method.
Direct UV spectrophotometry is a nonselective method; thus, the determination of caffeine involves using time-consuming sample preparation using toxic solvents—dichloromethane or chloroform (Belay et al., 2008).
As seen from the inspection of methods used for caffeine assay, the HPLC method is most often used. Almost all such methods use octadecylsilyl stationary phase (SiO2-C18) in combination with traditional reversed-phase HPLC solvents—methanol and acetonitrile (Coura et al., 2021; Fajara and Susanti, 2017; Fekry et al., 2022; Gliszczy?ska-?wig?o and Rybicka, 2015; Palur et al., 2020; Rahim et al., 2014; Srdjenovic et al., 2008; Tzanavaras and Themelis, 2007; Zuo et al., 2002). This means that these methods do not meet the requirements for environmental safety because of toxic solvents. Ethanol is one of the safest solvents to use in liquid chromatography (P?otka et al., 2013); however, it has certain limitations due to the higher system pressure and optical transparency of ethanol in the detection range of 190–220 nm (Yabré et al., 2018).
This study aims to develop an environmental friendly, selective method for determining caffeine in dietary supplements for weight loss, and establish chromatographic parameters for separating caffeine from other components of nutritional supplements for weight loss.
MATERIALS AND METHODS
The following items were used in this study.
Objects
Pills XLS DUO Slim & Shape, batch A814 (XLS)
Ingredients: cocoa butter, 100 mg; green tea, 100 mg; apple, 50 mg; grapefruit, 50 mg; inulin, 50 mg; artichoke, 50 mg; pineapple, 25 mg; parsley, 20 mg; fennel, 10 mg; blackcurrant, 10 mg; excipients.
Instant coffee drink, Light Energy Drive, batch 11120 coffee light (CL)
Ingredients: organic instant coffee, 3,580 mg; L-carnitine tartrate, 68 mg; green tea extract, 68 mg; garcinia extract, 68 mg; pineapple extract, 68 mg; guarana extract, 68 mg; senna extract, 46, 8 mg; vitamin premix [vitamin A (beta-carotene), 4,400 IU; vitamin C, 1,332 mg; biotin, 4 mg; vitamin B1, 1,332 mg; vitamin B2, 1,332 mg; vitamin B6, 2,668 mg; vitamin B12, 5.2 mcg; nicotinic acid, 13.2 mg].
Certified reference materials
Caffeine produced by Supelco, cat. number: PHR1009; theophylline produced by Supelco, cat. number: PHR1023; theobromine, produced by Supelco, cat. number: 42993.
Reagents
Water for chromatography, R obtained from installation Simplicity UV, Millipore, USA; ethanol 96% (V/V) Lux, manufacturer—State Enterprise “UKRSPYRT,” Ukraine.
Equipment
High-performance chromatograph AZURA UHPLC manufactured by KNAUER (Germany), which consists of pump AZURA P 6.1L, autosampler AZURA 3,950, column thermostat AZURA ?? 2.1, and diode array detector AZURA Detector DAD 6.1L. Chromatograms and UV spectra were processed using Open LAB CDS EZCrom Edition software.
Balances
Mettler Toledo XS204, permissible load, 220 g, discreteness, 0.1 mg. Centrifuge: SIGMA, Universal 320 R, Germany. Rotor 1620A, a radius of 99 mm. Ultrasonic cleaner: Daihan, Wuc-A010H, Korea.
Discovery HS C18 250*4,6 5 μm and Discovery F5 250*4,6 5 μm columns were used as a stationary phase in the study.
- Chromatography conditions were as follows:
- Channel A: water for chromatography, R
- Channel ?: ethanol 96% (V/V)
- Column thermostat temperature: 40°C
- Wavelength: 272 nm
- Mobile phase flow rate: 1,0 ml/minute
- Injection volume: 20 μl
Preparation of solutions
Sample averaging and average weight determination were as follows: instant coffee drink Light Energy Drive: 10 sachets; pills XLS DUO Slim & Shape: 10 crushed pills.
Preparation of a solution for determining the suitability of a chromatographic system
Samples of caffeine, theobromine, and theophylline in about 10 mg were placed in a volumetric flask of 500 ml and made up to volume with water.
Preparation of caffeine solutions for determining the linearity of the method
Solutions with five following concentrations were prepared: 5, 8, 10; 12; 15 mcg/ml in water.
Preparation of test sample solutions for the study of precision
To analyze a sample of the test object CL, six samples with a nominal concentration of about 10 mcg/ml. Averaged samples of about 1.2 g were placed in 100 ml flasks, and 70 ml of distilled water was heated to 80°C. Selected samples were filtered through a PTFE(L) membrane filter with a pore size of 0.45 µm and a diameter of 25 mm.
To analyze a sample of the second test object XLS, six samples with a nominal concentration of 10 mcg/ml were prepared. Averaged samples of about 0.5 g were placed in 100 ml flasks; 5 ml of 0.1 M hydrochloric acid solution, 5 ml of ethanol 96% (V/V), and 20 ml of distilled water heated to 80°C were added. Flasks were placed in an ultrasonic cleaner for 30 minutes, after which the solutions were cooled and made up to volume. Afterward, a 5:50 dilution was carried out in 50 ml flasks. Selected samples were filtered through a PTFE(L) membrane filter with a pore size of 0.45 µm and a diameter of 25 mm.
Preparation of test sample solutions to verify the accuracy of the method
Six solutions of both CL and XLS were prepared in the same way for precision. The samples were centrifuged at 5,000 rpm. The supernatant was removed; sample preparation was carried out with the solid residue in the same way as that for the corresponding samples. The samples were then analyzed for caffeine content.
Calculation of capacity factor (k) and efficiency (N)
N and k determined for isocratic elution and linear gradient elution experiments were calculated the same way, using the formula prescribed in Ph.Eur.2.2.46.
RESULTS AND DISCUSSION
Development of the method
Selection of chromatographic column and mobile phase
When ethanol-water mixtures are used as mobile phases, SiO2-C18 is usually used as a corresponding stationary phase. It is known that the retention of compounds on SiO2-C18 depends on the level of dispersion interactions between the adsorbate and the adsorbent. In the case of polar compounds, this retention significantly deteriorates. To improve the retention of compounds of hydrophilic nature, stationary phases with bonded groups of alternative selectivity may be used. In particular, in this study, the SiO2-PFP stationary phase was tested for the retention and separation of purine alkaloids—caffeine, theophylline, and theobromine.
Two commercially available stationary phases Discovery F5 250*4,6 5 μm and Discovery HS C18 250*4,6 5 μm were compared on the caffeine retention with the mobile phases of acetonitrile-water, ethanol-water mixtures in different ratios, as shown in Figure 1.
It was found that there is no significant difference in caffeine retention factors in both stationary phases when using acetonitrile, Figure 1a and 6. In contrast, when using ethanol, the caffeine retention factor (k) on SiO2-PFP significantly exceeds that of SiO2-?18. This can be explained by the fact that ethanol, unlike acetonitrile, does not deactivate alternative interactions, namely, the π-π interaction of the pentafluorophenyl (PFP) group with π-electrons of the analyte (Thevenon-Emeric et al., 1991) and the dipole–dipole interaction of the molecule dipole and the dipole of the PFP group. The difference between ethanol and acetonitrile for reversed-phase HPLC lies in the absence of π-electrons in the ethanol molecule and a much smaller dipole–dipole moment of this solvent.
Increased caffeine retention factor while using PFP stationary phase combined with ethanol–water mobile phase can increase the selectivity of separation of purine alkaloids, which are characterized by the high dipole moment of a molecule and the presence of π-electrons.
Selection of chromatographic column temperature
One of the disadvantages of using ethanol as a solvent in mobile phases for chromatography is that its mixtures with water give viscous solutions, which causes increased pressure in the chromatographic system. For example, a water–ethanol 96% 50:50 mixture causes a pressure of 364 bar at a flow of 1.0 ml/minute on a chromatographic column Discovery F5 250 × 4.6 (5 μm), as shown in Figure 2.
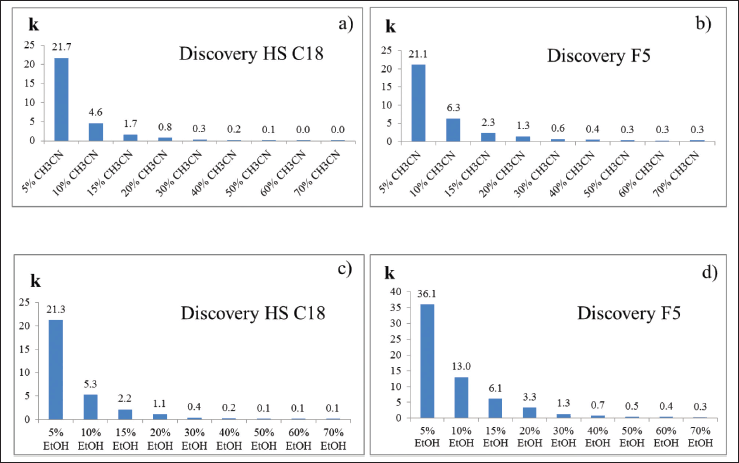 | Figure 1. Diagrams of the retention factor based on the composition of the mobile phase, both stationary phase columns. (a) Caffeine retention factor on Discovery HS C18 depending on the content of acetonitrile in mobile phase. (b) Caffeine retention factor on Discovery PFP depending on the content of acetonitrile in mobile phase. (c) Caffeine retention factor on Discovery HS C18 depending on the content of ethanol in mobile phase. (d) Caffeine retention factor on Discovery PFP depending on the content of ethanol in mobile phase. [Click here to view] |
One of the ways to reduce the pressure in the chromatographic system is to increase the temperature of the chromatographic column (Li and Carr, 1997). The influence of temperature on pressure is established in Figure 3.
Increasing the temperature of the chromatographic column solves the problem of high pressure when using mobile phases based on water–ethanol mixtures. A temperature of 40°C was chosen for chromatography, given that the pressure is significantly reduced at this temperature, and the use of higher temperatures can reduce the service life of the chromatographic column.
Selection of detection wavelength
Detection at the maximum of caffeine absorption in the selected mobile phase is chosen at 272 nm, which coincides with the data from references (Belay et al., 2008).
Selection of gradient program
Gradient elution for samples with a complex matrix is almost mandatory, as it is necessary to wash all matrix components out of the stationary phase to ensure reproducible chromatography of successive injections. For this, the chromatographic characteristics of the caffeine peak were tested under chromatography conditions 1 and 2 (Tables 1 and 2), which differ in the rate of ethanol content increase in the mobile phase (4%/minute and 8%/minute, respectively).
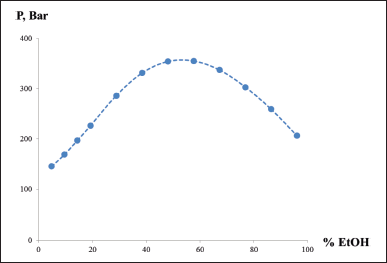 | Figure 2. Relation between the pressure in the chromatographic system and the ethanol–water ratio in the mobile phase. [Click here to view] |
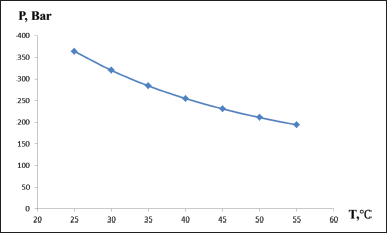 | Figure 3. Pressure versus temperature; water–ethanol 50:50 mixture used as mobile phase. [Click here to view] |
Given that under chromatography conditions 1, the retention factor is higher, and the efficiency of the chromatographic column is higher (Table 3), the separation ability of chromatography conditions 1 will be potentially higher.
Suitability of the chromatographic system
The suitability of the chromatographic system was checked before the validation work. The results are shown in Table 4. Peaks of all structural analogs are sufficiently separated from each other, as seen on the chromatogram in Figure 4.
According to the results of the calculations, this method is suitable, as all of the above conditions are met.
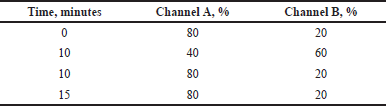 | Table 1. Gradient program 1. [Click here to view] |
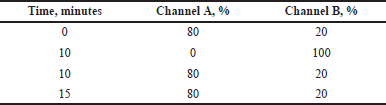 | Table 2. Gradient program 2. [Click here to view] |
 | Table 3. Chromatographic parameters of caffeine peak during chromatography with different gradients. [Click here to view] |
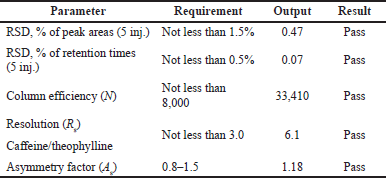 | Table 4. The results of checking the suitability of the chromatographic system. [Click here to view] |
Validation
Specificity
To confirm the specificity of the method, chromatography of a standard sample of caffeine with a concentration of 10 mcg/ml, test sample solutions, and solvent (mobile phase) was performed; the spectral purity of caffeine peaks was determined.
Specificity was based on the fulfillment of such conditions:
1. The retention time and the UV spectrum of the standard sample chromatogram peak coincide with the test sample solutions. Obtained chromatograms are shown in Figures 5–7.
The relative deviation of the retention time for the test samples was calculated. For CL, ? = 0.01%, and for XLS, ? = 0.38%. UV spectra are similar and have a maximum of about 272 nm. The spectral purity of the peaks exceeds 99.9%.
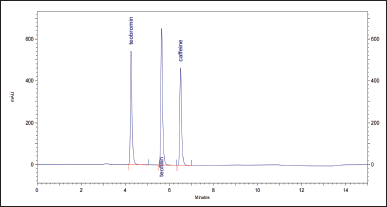 | Figure 4. Chromatogram of the solution is used to check the suitability of the chromatographic system. [Click here to view] |
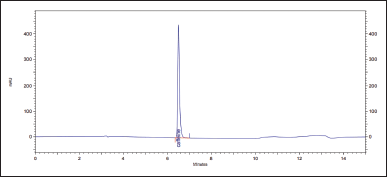 | Figure 5. Chromatogram of a standard sample of caffeine with a concentration of 10 mcg/ml. [Click here to view] |
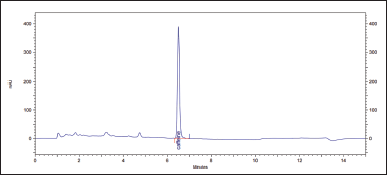 | Figure 6. Chromatogram of the solution of the test sample CL. [Click here to view] |
2. No peaks on the solvent chromatogram could interfere with the assay or identification of caffeine, as shown in Figure 8.
3. Separation from structural analogs (theophylline and theobromine) is shown in Figure 4. All substances are reliably separated from each other.
Linearity
The linearity of the method was examined in the concentration range from 5.0 to 15.0 mcg/ml. Measurements were performed for five solutions of different concentrations. As can be seen from the data in Table 5, the proposed method satisfies all criteria; the method is linear in the studied range of 5.0–15.0 mcg/ml of caffeine concentrations.
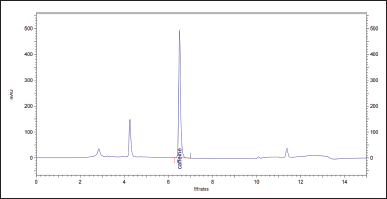 | Figure 7. Chromatogram of the solution of the test sample XLS. [Click here to view] |
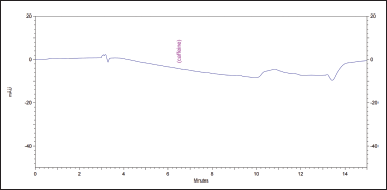 | Figure 8. Chromatogram of the solvent (water). [Click here to view] |
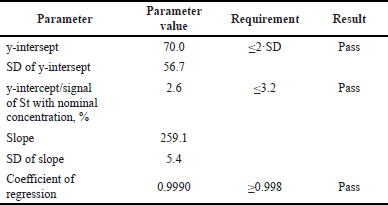 | Table 5. The results of the linearity study. [Click here to view] |
Precision (repeatability and intermediate precision)
1. Verification of precision for the studied object CL
Two series of measurements were performed by two analysts using the same method on different days to examine precision. In each series, six test solutions were used.
As seen in Table 6, the relative standard deviation (RSD) of the analysis results from analysts one and two does not exceed the established criteria, indicating the good reproducibility of the results.
2. Verification of precision for the studied object XLS
As seen in Table 7, the RSD of the analysis results from analysts one and two does not exceed the established criteria, indicating the proper reproducibility of the results.
Stability of solutions over time
A solution of standard caffeine (Std) with a concentration of 10 mcg/ml and test solutions (CL, XLS) were injected into the chromatographic system immediately after preparation and after 1 day of being stored at 8°C. The criterion of the insignificance of change in concentration of the solutions is set at no more than 1.0%.
The analytical solutions were found to be stable for 1 day when stored at 8°C (Table 8).
Accuracy
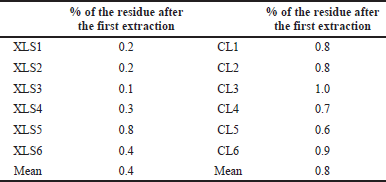
The accuracy was determined by checking the complete extraction from the solid residue. For this, sample preparation for the assay was centrifuged, the supernatant was removed, and the residue was resampled the same way as the test solution.
The amount of caffeine in solid residue does not exceed 2.0% (Table 9). Therefore, the method can be considered correct, and one-step extraction is sufficient for accurate assay, significantly simplifying and speeding up sample preparation.
 | Table 6. Results of the verification of precision of the method for ?L. [Click here to view] |
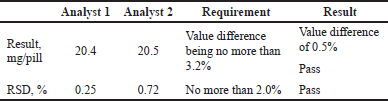 | Table 7. Results of the verification of precision of the method for XLS. [Click here to view] |
 | Table 8. Stability of analytical solutions. [Click here to view] |
 | Table 9. The results of the accuracy study. [Click here to view] |
 | Table 10. Robustness results. [Click here to view] |
Robustness
The method’s robustness in replacing the chromatographic column discovery F5 250*4.6 (5 μm) with Discovery F5 150*4.6 (5 μm) was tested. The change in the geometric parameters of the chromatographic column was taken into account by changing the flow rate from 1.0 to 0.6 ml/minute.
The assay results for XLS dietary supplements under these changes differed by 0.5%, which satisfies the requirements for intermediate precision, as shown in Table 10.
It should be noted that reducing the length of the chromatographic column can significantly reduce the pressure, whereas the separation between caffeine and theophylline changes insignificantly. This approach solves the problem of high pressure when using water–ethanol mixtures as a mobile phase without losing the separation capability of the chromatographic system.
CONCLUSION
The developed chromatographic method using SiO2-PFP stationary phase and the water–ethanol mobile phase is an effective and reliable technique for determining caffeine in dietary supplements for weight loss. The method was validated for two dietary supplements, Light Energy Drive and XLS DUO Slim & Shape. The validation characteristics meet the acceptance criteria, which indicates that this method can be used to analyze multicomponent dietary supplements for caffeine content. Light Energy Drive contains a small amount of caffeine, namely, about 20 mg per single dose, whereas XLS DUO Slim & Shape contains about 165 mg in a single dose. The high caffeine content in this drug can endanger the patient’s mental or physical health if taking 2–3 tablets at a time. Additionally, optimization direction was suggested by replacing the column with a 40% shorter length to decrease pressure build-up in the system. Overall, the proposed method provides a safe and environmental friendly approach to analyzing caffeine in dietary supplements.
ACKNOWLEDGMENTS
The work was held on State Enterprise’s “Central Laboratory for Quality Control of Medicines and Medical Products.” The authors thank laboratory director Roman Markin for their support and all the brave defenders of Ukraine that made finalizing this publication possible.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Amare M, Shimelis S. Polymer modified glassy carbon electrode for the electrochemical determination of caffeine in coffee. Talanta, 2012; 93:122–28. CrossRef
Belay A, Kassahun T, Mesfin R, Araya A. Measurement of caffeine in coffee beans with uv/vis spectrometer. Food Chem, 2008; 108(1):310–15. CrossRef
Coura J, Silva C, Silva E, Valladão S, Duarte M. Development and validation of a fast and simple HPLC-UV method to determine caffeine in guarana (Paullinia cupana) food supplements. Quim Nova, 2021; 44(10):1353–9. CrossRef
Fajara BEP, Susanti H. HPLC determination of caffeine in coffee beverage. IOP Conf Ser Mater Sci Eng, 2017; 259(1):0–6. CrossRef
Fekry R, Kelani K, Fayez Y, Tantawy M. Comparative validated chromatographic methods for the simultaneous determination of caffeine, codeine, paracetamol along with the related compound “p-aminophenol” in tablets. JPC J Planar Chromat Modern TLC, 2022; 35: 51–9. CrossRef
Garrett BE, Griffiths RR. The role of dopamine in the behavioral effects of caffeine in animals and humans. Pharmacol Biochem Behav, 1997; 57(3):533–41. CrossRef
Gliszczy?ska-?wig?o A, Rybicka I. Simultaneous determination of caffeine and water-soluble vitamins in energy drinks by HPLC with photodiode array and fluorescence detection. Food Anal Methods, 2015; 8(1):139–46. CrossRef
Li J, Carr P. Accuracy of emperical corellation for estimating diffusion coefficients in aqueous organic mixtures. Anal Chem, 1997; 69:2530–36. CrossRef
McMullen MK, Whitehouse JM, Shine G, Towell A. Habitual coffee and tea drinkers experienced increases in blood pressure after consuming low to moderate doses of caffeine. Food Funct, 2011; 2(3–4):197–203. CrossRef
Palur K, Archakam SC, Koganti B. Chemometric assisted UV spectrophotometric and RP-HPLC methods for simultaneous determination of paracetamol, diphenhydramine, caffeine and phenylephrine in tablet dosage form. Spectrochim Acta Part A Mol Biomol Spectrosc, 2020; 243:118801. CrossRef
P?otka J, Tobiszewski M, Sulej AM, Kupska M, Górecki T, Namie?nik J. Green chromatography. J Chromatogr A, 2013; 1307:1–20. CrossRef
Rahim AA, Nofrizal S, Saad B. Rapid tea catechins and caffeine determination by HPLC using microwave-assisted extraction and silica monolithic column. Food Chem, 2014; 147:262–68. CrossRef
Regan F, Shakalisava Y. Rapid simultaneous determination of alkylxanthines by CZE and its application in analysis of pharmaceuticals and food samples. Anal Chim Acta, 2005; 540(1):103–10. CrossRef
Sereshti H, Samadi S. A rapid and simple determination of caffeine in teas, coffees and eight beverages. Food Chem, 2014; 158:8–13. CrossRef
Srdjenovic B, Djordjevic-Milic V, Grujic N, Injac R, Lepojevic Z. Simultaneous HPLC determination of caffeine, theobromine, and theophylline in food, drinks, and herbal products. J Chromatogr Sci, 2008; 46(2):144–49. CrossRef
Tabrizi R, Saneei P, Lankarani KB, Akbari M, Kolahdooz F, Esmaillzadeh A, Nadi-Ravandi S, Mazoochi M, Asemi Z. The effects of caffeine intake on weightloss: a systematic review and dos-response meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr, 2019; 59(16):2688–96. CrossRef
Thevenon-Emeric G, Tchapla A, Martin M. Role of π- π interactions in reversed-phase liquid chromatography. J Chromatogr, 1991; 550:267–83. CrossRef
Tzanavaras PD, Themeli DG. Development and validation of a high-throughput high-performance liquid chromatographic assay for the determination of caffeine in food samples using a monolithic column. Anal Chim Acta, 2007; 581(1):89–94. CrossRef
Yabré M, Ferey L, Somé IT, Gaudin K. Greening reversed-phase liquid chromatography methods using alternative solvents for pharmaceutical analysis. Molecules, 2018; 23(5):1065. CrossRef
Zuo Y, Chen H, Deng Y. Simultaneous determination of catechins, caffeine and gallic acids in green, oolong, black and pu-erh teas using HPLC with a photodiode array detector. Talanta, 2002; 57(2):307–16. CrossRef